Published online Mar 28, 2012. doi: 10.3748/wjg.v18.i12.1328
Revised: January 18, 2012
Accepted: February 8, 2012
Published online: March 28, 2012
AIM: To investigate the expression and clinical significance of Wnt member 5a (Wnt5a) and receptor tyrosine kinase-like orphan receptor 2 (Ror2) in hepatocellular carcinoma (HCC).
METHODS: HCC tissues obtained from 85 patients were examined the mRNA expression of Ror2 by real-time reverse transcription polymerase chain reaction and the protein expressions of Wnt5a, Ror2, β-catenin and Ki-67 via immunohistochemical staining. The correlation between protein expression and HCC patients’ clinicopathology data and prognoses were analyzed.
RESULTS: Compared to nontumorous (hepatitis or cirrhotic) tissues, Ror2 mRNA expression was clearly decreased in HCC. Ror2 and Wnt5a protein expressions in the majority of HCC patients (63% and 77%, respectively) was significantly less in tumor tissues, as compared to adjacent nontumorous tissues, and this reduction was correlated with increasing serum α-fetoprotein and tumor stage. In 68% (58/85) of the HCC cases, the expression of β-catenin in tumor tissues was either downregulated in the cellular membrane, upregulated in the cytoplasm, or both. Survival analysis indicated that Wnt5a and Ror2 protein expressions could be regarded as independent prognostic factors for HCC; HCC patients with decreased Wnt5a or Ror2 protein expression had a poorer prognosis than those with elevated Wnt5a and Ror2 expression (P = 0.016, P = 0.007, respectively).
CONCLUSION: Wnt5a and Ror2 may serve as tumor suppressor genes in the development of HCC, and may serve as clinicopathologic biomarkers for prognosis in HCC patients.
- Citation: Geng M, Cao YC, Chen YJ, Jiang H, Bi LQ, Liu XH. Loss of Wnt5a and Ror2 protein in hepatocellular carcinoma associated with poor prognosis. World J Gastroenterol 2012; 18(12): 1328-1338
- URL: https://www.wjgnet.com/1007-9327/full/v18/i12/1328.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i12.1328
Hepatocellular carcinoma (HCC) is one of the most frequently occurring tumors worldwide. It develops mostly in cirrhotic livers, and risk factors include chronic infection by the hepatitis B and C viruses (HBV and HCV), as well as nonviral liver diseases[1,2]. Unfortunately, the cellular mechanisms of hepatocarcinogenesis remain poorly understood. Recent advances have shown that apart from autocrine stimulation by growth factors such as insulin-like growth factor-II and transforming growth factor-α, the dysregulation of at least four different growth regulatory pathways is frequently involved in hepatocarcinogenesis[3,4]. These signaling pathways include the retinoblastoma, the transforming growth factor-β, the tumor protein 53, and the wingless-type murine-mammary-tumor virus integration site family (Wnt). These pathways also interfere with each other at different levels[2,5,6].
The Wnt family of genes encodes a large and diverse group of signaling molecules involved in the patterning, proliferation, and differentiation of a variety of organs and cell types[7,8]. The Wnt ligand binds to its receptor Frizzled and the low-density lipoprotein receptor-related proteins (Lrp) 5 and 6 to activate the canonical Wnt/β-catenin signaling pathway, or functions through β-catenin-independent (noncanonical) pathways which include the planar cell polarity and Wnt/Ca2+ pathways[9]. Wnt ligands are typically classified into canonical and non-canonical Wnts by the pathways they work through[9-11].
The Wnt member 5a (Wnt5a) is one of the most highly investigated non-canonical Wnts and has been implicated in almost all aspects of non-canonical Wnt signaling[12-14]. In terms of cancer developmental research, Wnt5a has lived in the shadow of its better-characterized relatives. This was largely because of its apparent inability to transform cells or signal through the canonical β-catenin pathway that is so important in cancer[15-18]. Recent work with a wide of human tumors has indicated that Wnt5a has a critical role in malignant progression, but there is conflicting evidence as to whether that role is tumor-promoting or tumor-suppressing[17-22]. We have shown that Wnt5a has a tumor suppressing effect in HCC and is probably associated with HBV infection[23,24]. Emerging evidence suggests that the functions of Wnt5a can be drastically altered depending on the availability of key receptors[17,18,25]. It was recently reported that an alternative Wnt receptor, receptor tyrosine kinase-like orphan receptor 2 (Ror2), an orphan tyrosine kinase, mediates Wnt5a-initiated noncanonical signaling and is required for the Wnt5a-mediated inhibition of canonical signaling[25,26].
The Ror2 receptor belongs to the receptor tyrosine kinase superfamily[25]. This large protein family is involved in regulating diverse cellular processes such as the cell cycle, cell migration, proliferation and differentiation[27]. In addition, the Ror2 protein and its homolog Ror1 play essential roles during development. Mutations of the Ror2 receptor, resulting in protein misfolding or premature truncation, have been associated with human diseases such as dominant Brachydactyly type B and recessive Robinow syndrome[28]. Currently, investigations to elucidate the role of Ror2 in cancer have shown paradoxical results, indicating that Ror2 was overexpressed in oral and renal cell cancer and metastatic melanoma, but downregulated in colon cancer[29-31]. These different effects appear to be dependent on the cancer type and signaling pathway[32].
Here, we investigate the expression and clinical significance of Ror2, Wnt5a and β-catenin in HCC.
We collected tumors from 85 consecutive patients who had undergone surgery for HCC at the Jinan Military General Hospital from January 2006 to September 2010. The Ethics Committee of the Jinan Military General Hospital approved the protocol of this study. Among the 85 patients, 55 had serum α-fetoprotein (AFP) ≥ 30 μg/L, and 73 were sera positive for hepatitis B surface antigen (HBsAg). On gross examination, 3 cases had tumor sizes that were ≤ 2 cm, and 82 had tumor sizes > 2 cm (median tumor size, 6.1 cm; range, 1.0-16 cm). Histopathological diagnoses were made according to the pathological classification system of the World Health Organization (2000), and the tumor was staged following the tumor-node-metastasis classification of the International Union Against Cancer[33]. Nine cases were well differentiated; 60 cases were moderately differentiated; and 16 cases were poorly differentiated. In total, 56 HCC cases had liver cirrhosis; 25 cases had chronic hepatitis; and 4 cases had basically normal liver tissue. We also collected 3 cases of lung metastasis. Furthermore, tissues of comparative normal liver obtained during surgery for liver cholelithiasis (n = 3) and HBV-infected liver biopsies (n = 5) were studied.
Nineteen of the 85 cases included chronic (n = 8) and cirrhotic (n = 11) HCC. From these, fresh tissues were obtained immediately after resection, including HCC tumor and adjacent nontumorous liver tissues. In addition, normal liver tissues (n = 3) with no HBV infection were obtained during surgery for liver cholelithiasis. In these 22 cases, one portion of the fresh tissue was snap frozen in liquid nitrogen immediately and stored at -80 °C; the remainder portion was fixed in 10% buffered formalin and embedded in paraffin.
The available patient clinicopathological information included gender, age, serum AFP, serum HBsAg, tumor size, tumor stage, histological grade and cancer-specific survival time.
Total RNA was extracted from 10-mm frozen HCC tissue sections. To isolate the RNA from defined areas containing ≥ 80% tumor cells, all tumors were manually microdissected under direct visual control through a dissecting microscope. Total RNA in the frozen tissues was extracted using Trizol (Invitrogen) following the manufacturer’s recommendations. Total RNA was digested with DNase I (Invitrogen) and was used for the first-strand cDNA reaction. The reaction mixture consisted of 5 μg of DNase I-treated RNA, 1 × reverse transcriptase buffer, 2.5 mmol dNTP mix, 3.5 μmol oligo primer, and 2.5 U/mL MultiScribe™ reverse transcriptase (PE Applied Biosystems). Each sample was handled using the same protocol, with the exception that reverse transcriptase was added to exclude the presence of interference from genomic DNA.
Real-time reverse transcription polymerase chain reaction (qRT-PCR) was carried out using SYBER green dye in a Rotor Gene 3000 Detection System (Corbett Research, Sydney, Australia). Each SYBER green reaction (25 μL) contained one microliter diluted cDNA and 10.5 μL SYBR Green PCR Master Mix, as well as 5 pmol forward and reverse primer (Ror2: forward 5’-AGGTGCCTATGCAAGTTCA-3’, reverse 5’-TGTGCGAGGTTTAAGGTCTA-3’). Samples were activated by incubation at 95 °C for 5 min and denatured at 95 °C for 20 s. This was followed by annealing at 60 °C for 20 s and extension at 72 °C for 20 s, for 40 cycles. In all of the cDNA samples, gene expressions of Ror2 and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (forward 5’-GAAGGTGAAGGTCGGAGTC-3’; reverse 5’-GAAGATGGTGATGGGATTTC-3’), an internal quantitative control, were determined by SYBR green fluorescence using the Rotor-Gene 3000; the ratios of Ror2 to the housekeeping gene GAPDH represented the normalized relative levels of Ror2 expression. A non- template negative control was also included in each experiment. Analyses of all tumor samples were carried out at least twice, and the mean value was calculated.
Immunohistochemical staining was performed on thin sections (4 μm) of paraffin-embedded archival tissue. The samples were dewaxed with xylene/ethanol before antigen retrieval (i.e., pressure cooked for one minute at full pressure, 15 psi, in 0.001 mol/L EDTA buffer, pH 8.0). The primary antibodies used were: Wnt5a (LS-C47384, Lifespan, 1:200), Ror2 (PAB3386, Abnova, 1:200), β-catenin (C19220, BD Transduction Laboratories, 1:400) and Ki-67 (MIB-1, Dako, 1:100). Immuno- histochemical staining of antibodies was done using the Dako Envision Plus System (K5007, Dako). The antibody binding was visualized with 3, 3’-diaminobenzidine tetrahydrochloride (DAB) before brief counterstaining with Mayer’s hematoxylin. For monoclonal antibodies of mouse origin, negative controls were obtained using isotypic mouse immunoglobulin in the same dilution as the primary antibody of concern. All control experiments gave negative results.
Two authors (Cao YC and Jiang H) who had no knowledge of the patients’ clinical status reviewed all of the immunostained sections. Cases with discrepant results were re-evaluated jointly until agreement was reached. For expression of Wnt5a, Ror2 and β-catenin protein, in cases with multiple areas of low intensity that occurred during evaluation of immunostaining, five areas were selected at random and scored.
The degree of immunohistochemical staining was recorded using a semi- quantitative and subjective grading system that considered both the intensity of staining and the proportion of tumor cells that had an unequivocal positive reaction. Grades for stain intensity were: 0: No staining; 1: Weak staining; 2: Positive staining; and 3: Strong staining. For rating stained areas: 0: No staining; 1: Positive staining in < 10% of tumor cells; 2: Positive staining in 10% to 50% of tumor cells; 3: Positive staining in > 50% of tumor cells. The staining index was calculated as the staining intensity multiplied by the positive area.
Ki-67-positive cells were counted by viewing ≥ 200 HCC cells from ≥ 10 randomly selected fields. The percentage of antigen-positive nuclei among the total number of nuclei counted was calculated to obtain the nuclear labeling index (LI).
In the subsequent statistical analysis, the cutoff points for the staining index categories were mainly based on median values, as well as each marker’s frequency distribution curve and the size of the subgroups. Therefore, cytoplasmic Wnt5a and Ror2 and membranous β-catenin staining indices were categorized by their median value as high (> 4) or low (0-4), and the cytoplasmic β-catenin staining index was categorized as high (> 3) or low (0-3). However, nuclear β-catenin expression was categorized based on the absence (staining index = 0) or presence (staining index ≥ 1) of staining. The Ki-67 labeling indices were divided into two groups (LI < 10% and LI ≥ 10%).
To determine the prognostic factor, the outcome of the 82 patients was determined by reviewing their medical charts. The follow-up period ranged from one to 54 mo (average: 31.3 mo; median: 27.0 mo). The end point in the analysis was HCC-related death. The overall and disease-free survival rates were estimated using the Kaplan-Meier method and compared with the log-rank test. The prognostic analysis was carried out with univariate and multivariate Cox regressions models.
The differences in Ror2 mRNA expression between HCC and nontumorous liver tissue was statistically analyzed using Student’s t-test and one-way analysis of variance (ANOVA) for multiple comparisons. The correlations between the clinicopathological parameters and Wnt5a, Ror2 and β-catenin protein expression were analyzed using the χ2 or Fisher’s exact tests.
Pearson’s correlation was used to determine the correlation between mRNA and protein expression, as well as between the expressions of different proteins. All statistical calculations were carried out using SPSS software (for Windows, version 13.0). A significant difference was defined at P < 0.05.
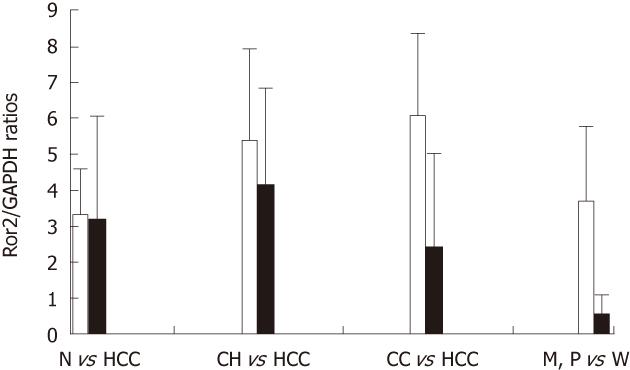
The Ror2 gene (mRNA) expression levels relative to that of GAPHD in normal, HCC, chronic hepatitis, cirrhotic liver and adjacent nontumorous liver tissues are shown in Figure 1. Ror2 mRNA levels were elevated in chronic hepatitis (5.420 ± 5.492, n = 11) and cirrhotic liver tissues (6.128 ± 5.252, n = 8) compared to that of normal (3.381 ± 1.182, n = 3) and HCC (3.189 ± 3.856, n = 19). Based on Student’s t-test, statistically significant differences were found between the Ror2 mRNA levels in HCC vs adjacent nontumorous (chronic hepatitis or cirrhotic) liver tissues (P = 0.029), but not between HCC and normal liver tissues (P = 0.934) or normal and adjacent nontumorous liver tissues (P = 0.094). The Ror2 mRNA level in moderately and poorly differentiated tumor tissues (n = 16) was greater by 7.2-fold (P = 0.014) than the level in well-differentiated tumor tissues (n = 3). No significant differences were found between Ror2 gene expression levels and other clinicopathological findings such as age, serum AFP concentration, tumor size, and HCC tumor stage.
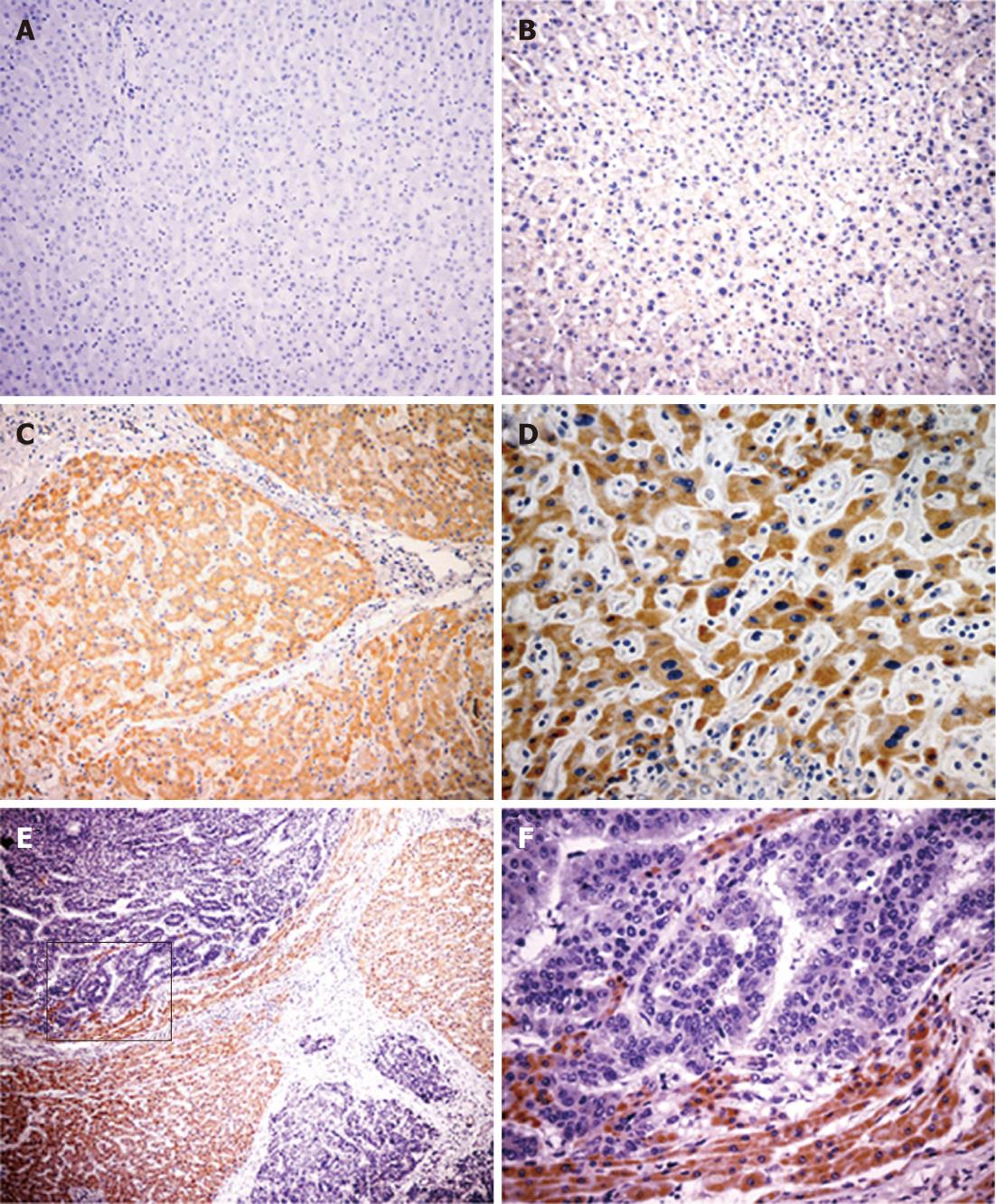
Immunohistochemistry was performed to evaluate Ror2 protein expression in tumor and non-tumorous liver cells. In non-tumorous liver cells and HCC tumor cells, Ror2 protein expression was displayed in the cytoplasm, but in stromal cells Ror2 protein was not observed. In comparative normal liver cells Ror2 was negative or weakly expressed (Figure 2A and B), whereas all chronic hepatitis, cirrhotic, and dysplastic liver cells exhibited positive immunostaining for Ror2 (Figure 2C and D), In 62/85 (72.9%) of the HCCs, Ror2 immunostaining was reduced or absent (Figure 2E and F).
A significant correlation was found between the normalized Ror2 gene expression ratio and the protein expression level in normal, tumor and non-tumorous liver tissues (r = 0.254, P = 0.021). Furthermore, statistical comparisons between Ror2 mRNA expression and patients’ clinicopathological features revealed a significant negative association between Ror2 mRNA and tumor stage (P < 0.001), and between Ror2 mRNA and serum AFP (P < 0.001). However, there were no significant differences between Ror2 protein expression and the other clinicopathological findings in HCC (Table 1).
| Variables | n | Ror2 immunoreactivity | P | |
| Low | High | |||
| Gender | 0.890 | |||
| Male | 77 | 56 | 21 | |
| Female | 8 | 6 | 2 | |
| Age (yr) | 0.725 | |||
| < 53 (median) | 38 | 27 | 11 | |
| ≥ 53 | 47 | 35 | 12 | |
| Serum AFP level (μg/L) | < 0.001 | |||
| < 30 | 30 | 14 | 16 | |
| ≥ 30 | 55 | 48 | 7 | |
| HBsAg | 0.862 | |||
| Positive | 73 | 53 | 20 | |
| Negative | 12 | 9 | 3 | |
| Tumor size (cm) | 0.116 | |||
| ≤ 2 | 3 | 1 | 2 | |
| > 2 | 82 | 61 | 21 | |
| Histological grade | 0.090 | |||
| Well differentiated | 9 | 7 | 2 | |
| Moderately differentiated | 60 | 40 | 20 | |
| Poorly differentiated | 16 | 15 | 1 | |
| Liver cirrhosis | 0.553 | |||
| Present | 56 | 42 | 14 | |
| Absent | 29 | 20 | 9 | |
| T classification | < 0.001 | |||
| T1 | 3 | 1 | 2 | |
| T2 | 30 | 14 | 16 | |
| T3 | 40 | 36 | 4 | |
| T4 | 12 | 11 | 1 | |
| Total | 85 | 62 | 23 | |
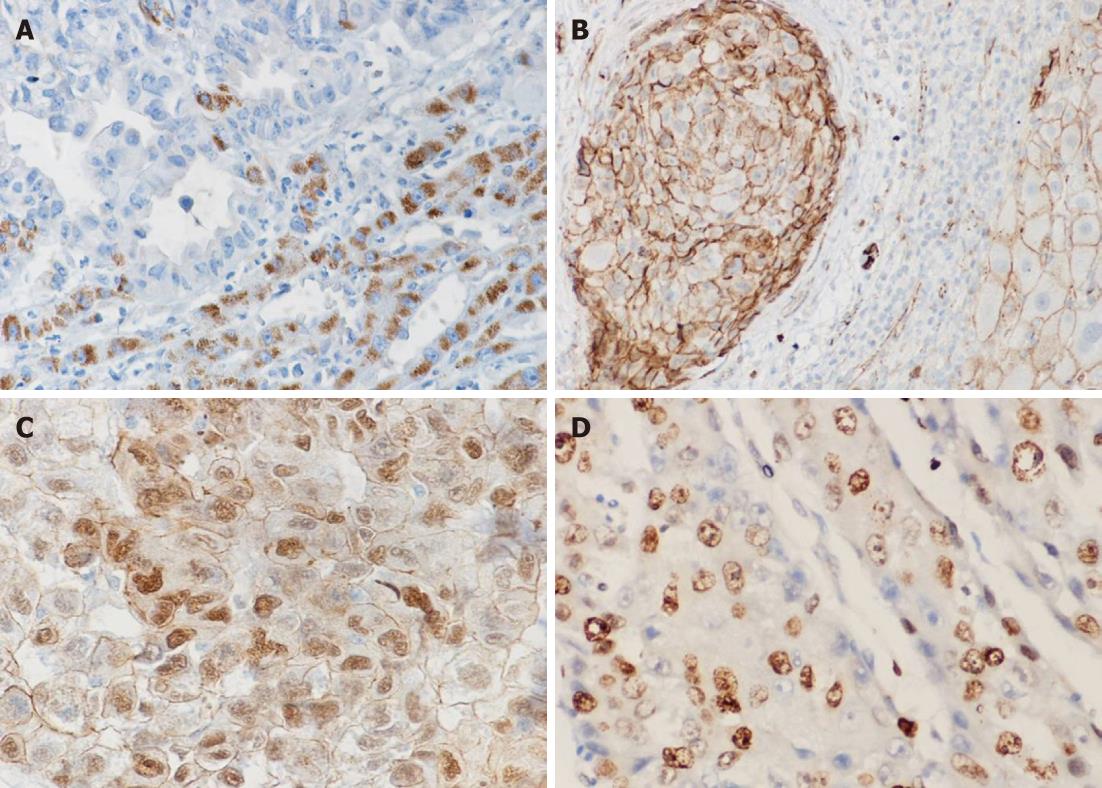
Wnt5a protein expression was observed in the cytoplasm of non-tumorous liver and tumor cells, but nowhere in stromal cells. There was little or no Wnt5a seen in normal liver cells. However, all chronic hepatitis, cirrhosis and dysplastic liver cells exhibited strong positive immunostaining for Wnt5a. In contrast, in 65/85 (76.5%) of HCC patients, Wnt5a immunostaining was reduced or absent compared to the levels in adjacent nontumorous (hepatitis and cirrhotic) tissues (Figure 3A). There was a significant negative correlation between Wnt5a expression and tumor stage (P < 0.001), and between Wnt5a and serum AFP (P = 0.016). However, there were no significant associations between Wnt5a protein expression and the other clinicopathological features of HCC patients.
β-catenin protein expression in hepatocellular carcinoma
In non-neoplastic liver tissue, a thin membranous β-catenin signal delineated the hepatocytes, and strong membranous and pale cytoplasmic staining of bile ductules was observed. As shown in Figure 3B and C, altered expressions of β-catenin were found in 68.2% (58/85) of HCC cases. These alterations included reductions in the cellular membrane, increases in the cytoplasm, or both, and nuclear accumulation (in 7%, 6/85). However, no evidence of altered β-catenin expression was found in cirrhotic nodules or dysplastic liver cells in adjacent noncancerous liver tissue. In tumor tissues, altered β-catenin expression was significantly associated with a worsening histopathological tumor grade (P = 0.041) and was not significantly associated with the other clinicopathological parameters.
Correlations among the protein expressions of Wnt5a, Ror2 andβ-catenin
Associations among the protein expression levels of Wnt5a, Ror2 and β-catenin are shown in Table 2. Low cytoplasmic Wnt5a expression was positively associated with low cytoplasmic Ror2 expression (r = 0.411, P < 0.001) and abnormal β-catenin expression (r = 0.254, P = 0.019) in HCC tissue. Similarly, there was a statistically significant correlation between low cytoplasmic Ror2 expression and abnormal β-catenin expression (r = 0.267, P = 0.014).
| Wnt5a | β-catenin | ||||||
| Variable | n | Low | High | P-value | n | A | P-value |
| Ror2 | |||||||
| Low | 62 | 54 | 8 | < 0.001 | 15 | 47 | 0.014 |
| High | 23 | 11 | 12 | 12 | 11 | ||
| β-catenin | |||||||
| N | 27 | 16 | 11 | 0.019 | |||
| A | 58 | 48 | 10 | ||||
To investigate the biological functions of proteins in HCC, the Ki-67 LI was assessed in relation to Ror2, Wnt5a and β-catenin status. A strong correlation between a high Ki-67 LI and the reductive loss of Ror2 (r = -0.344, P = 0.002), or Wnt5a (r = -0.278, P = 0.010), but not β-catenin (r = 0.095, P = 0.386) was found (Figure 3D).
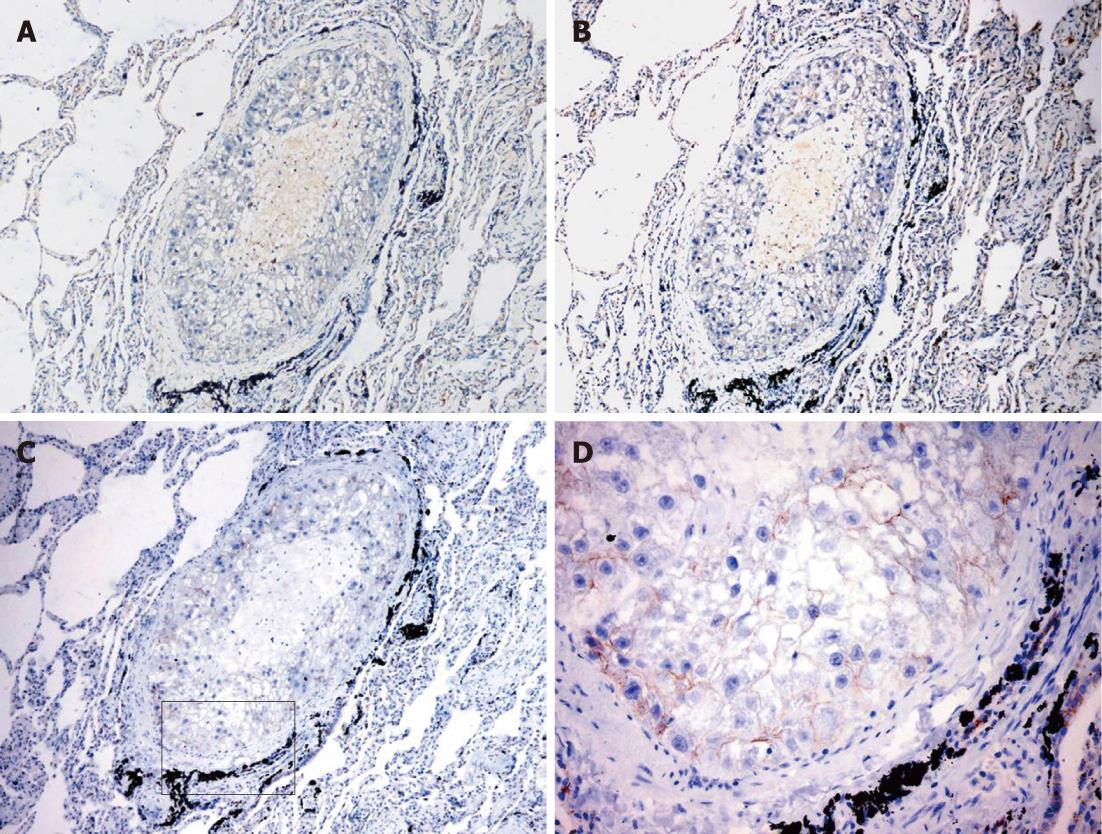
A previous study reported that Wnt5a and Ror2 were expressed predominantly in metastatic but not primary lesions of metastatic melanoma, suggesting that Wnt5a and Ror2 might be closely correlated with tumor invasiveness and metastasis[31,34]. To determine whether a similar phenomenon occurs in the metastasis of HCC, three cases of lung metastasis of HCC were included in this study. Immunohistochemical analysis showed that Wnt5a and Ror2 were not expressed in either primary or metastatic lesions (Figure 4A and B), whereas β-catenin- positive staining were detected in the cellular membrane (Figure 4C and D). The Ki-67 LI in tumor tissues was 10%.
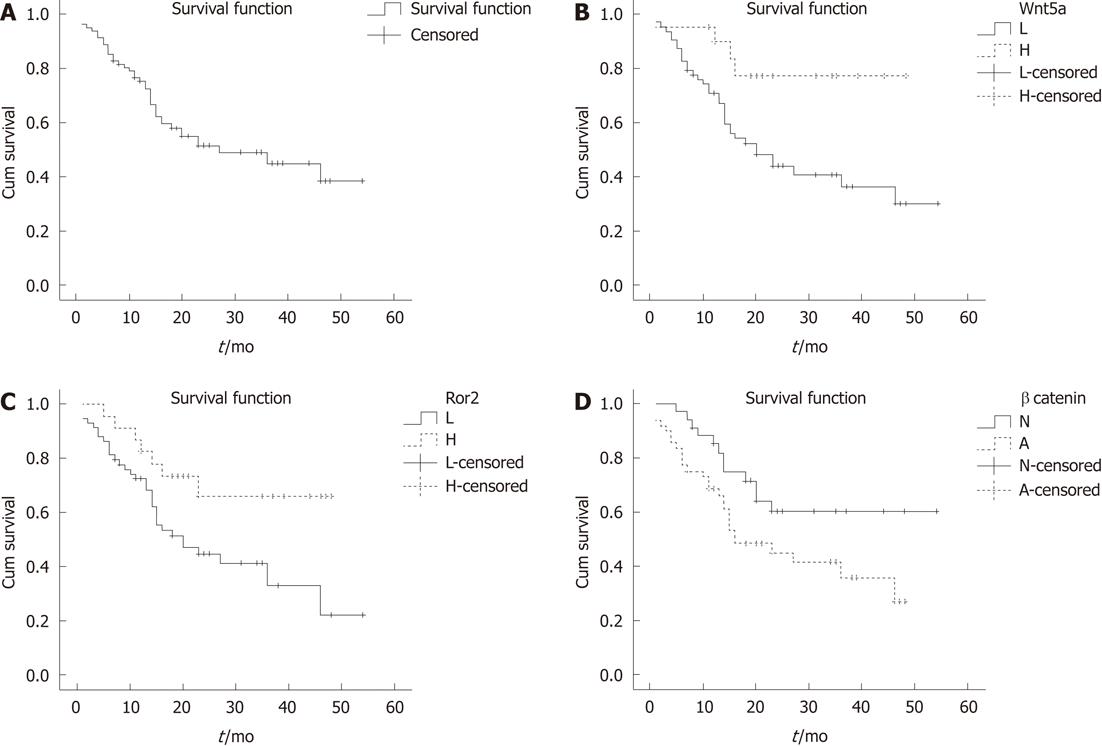
The median follow-up was 27.0 mo for survivors (range, 1-54 mo). Three patients were lost to follow-up after surgery and were excluded from the survival analyses. The overall survival curve for the remaining 82 HCC cases is shown in Figure 5A. The estimated 1- and 3-year overall rate of survival was 75% and 44%, respectively. Kaplan-Meier analysis was used to compare the survival rates of HCC patients with tumors expressing low or high levels of Wnt5a and Ror2 and normal or abnormal β-catenin (Figure 5B-D).
In a univariate Cox proportional hazard regression model analysis (Table 3), tumor stage (P < 0.001), serum AFP (P = 0.036), and the expressions of Wnt5a (P = 0.024) and Ror2 (P = 0.011) were significantly associated with overall survival. Therefore, patients with tumors having a low expression of Wnt5a and Ror2 had a poorer prognosis than those with tumors of high Wnt5a and Ror2 expression.
| Covariate | P-value | Risk ratio | 95% CI |
| Univariate | |||
| Sex (male, female) | 0.130 | 0.482 | 0.187-1.240 |
| Age (< 53 yr, ≥ 53 yr) | 0.166 | 0.640 | 0.341-1.203 |
| Serum AFP level (< 30 μg/L, ≥ 30 μg/L) | 0.036a | 2.162 | 1.051-4.449 |
| HBsAg (positive, negative) | 0.506 | 1.621 | 0.390-6.732 |
| Tumor size ( ≤ 2 cm, > 2 cm) | 0.467 | 2.089 | 0.286-15.239 |
| Histological grade (well, moderately, poorly differentiated) | 0.268 | 1.388 | 0.777-2.482 |
| Liver cirrhosis (present, absent) | 0.738 | 1.123 | 0.568-6.732 |
| T classification (T1-T4) | < 0.001a | 2.339 | 1.487-3.679 |
| Wnt5a (low, high) | 0.024a | 3.288 | 1.167-9.263 |
| Ror2 (low, high) | 0.011a | 0.323 | 0.134-0.774 |
| β-catenin ( normal, abnormal) | 0.052a | 1.966 | 0.995-3.885 |
| Ki-67 (mitosis ≤ 10%, > 10%) | 0.273 | 1.479 | 0.734-2.981 |
| Multivariate | |||
| Sex (male, female) | 0.017a | 0.240 | 0.074-0.776 |
| Age (< 53 yr, ≥ 53 yr) | 0.075 | 0.538 | 0.272-1.065 |
| Serum AFP level (< 30 μg/L, ≥ 30 μg/L) | 0.343 | 1.476 | 0.661-3.296 |
| HBsAg (positive, negative) | 0.515 | 1.731 | 0.332-9.026 |
| Tumor size ( ≤ 2 cm, > 2 cm) | 0.711 | 1.535 | 0.159-14.827 |
| Histological grade (well, moderately, poorly differentiated) | 0.298 | 1.462 | 0.715-2.993 |
| Liver cirrhosis (present, absent) | 0.858 | 0.928 | 0.408-2.111 |
| T classification (T1-T4) | 0.001a | 2.119 | 1.347-3.336 |
| Wnt5a (low, high) | 0.020a | 0.288 | 0.101-0.824 |
| Ror2 (low, high) | 0.144 | 0.509 | 0.205-1.259 |
| β-catenin ( normal, abnormal) | 0.013a | 3.233 | 1.286-8.130 |
| Ki-67 (mitosis ≤ 10%, > 10%) | 0.494 | 0.839 | 0.507-1.387 |
Multivariate Cox regression analysis (Table 3), the expression levels of Wnt5a (P = 0.020), and β-catenin (P = 0.013) showed a significant association with overall survival. However, a significant correlation between the expression levels of Ror2 and overall survival (P = 0.144), serum AFP and overall survival (P = 0.343) were not demonstrated.
Consistent with previous reports[23,24], in this study immunohistochemical analysis showed that the loss of Wnt5a protein expression in HCC tumors frequently occurred in patients with HCC (71%-81%), and this also correlated with increased AFP and poor histologic grade. Wnt5a may act as a tumor suppressor gene in the development of HCC. Similar results were obtained in colon carcinoma, breast cancer and thyroid carcinoma[17,18,35,36]. We also performed a survival analysis for 82 patients with HCC. Our results demonstrated that HCC patients with low expression of Wnt5a had a poorer prognosis than those with high Wnt5a expression, and Wnt5a was an independent prognostic factor for HCC.
Recent studies have indicated that the upregulation of Wnt5a was associated with tumor invasiveness and metastasis in metastatic melanoma, gastric cancer, and non-small-cell lung carcinoma[17-19]. Wnt5a was expressed predominantly in the metastatic but not primary lesions of metastatic melanoma[34]. Therefore, three cases with lung metastasis of HCC were recruited in the present study. Immuno- histochemical analysis with anti-Wnt5a antibody showed that Wnt5a was not expressed in either primary or metastatic lesions, which confirmed our hypothesis that Wnt5a acts as a tumor suppressor gene in HCC. These observations suggested that the complex Wnt5a-regulated signal pathways and the functional role of Wnt5a depends on cell type as well as stimulus factors during the development of HCC tumor.
Previous reports showed that Ror2 shared a similar structure with the Wnt receptor[25]. Mikels et al[25,37] revealed that Wnt5a suppressed Wnt/β-catenin activity via the Ror2-mediated signal pathway, and confirmed that the Ror2 receptor required tyrosine kinase activity to mediate Wnt5A signaling. He et al[38] demon- strated that Wnt5a levels correlated with those of Ror2 during mammalian palate development. Similar to Wnt5a, Ror2 plays different roles in different human tumor tissues. There is evidence that the enhanced expression of Ror2 is associated with tumor invasiveness and metastasis in metastatic melanoma, renal cell carcinoma, and squamous cell carcinoma[29-31]. In contrast, the mRNA and protein expression of Ror2 was reduced in colon cancer tissues compared with adjacent nontumorous liver tissue, which might be due to the hypermethylated Ror2 promotor[32].
Our current study showed that Ror2 gene transcription and protein translation were both suppressed in tumor tissues of HCC as compared with tissue adjacent to the tumor. This reduced expression of Ror2 in tumor tissues was correlated with decreased Wnt5a expression (P < 0.001), a high Ki-67 LI, increased AFP, high differentiation, and poor prognosis. The consistency of Wnt5a and Ror2 expression in tumor tissues as well as in lung metastasis of HCC implies that Ror2 may be active downstream of Wnt5a and participate in the regulation of the noncanonical Wnt signal pathway. Moreover, the mRNA and protein expression of Ror2 is increased in chronic hepatitis livers and is greatly enhanced in cirrhotic livers as compared with normal liver tissues, suggesting Ror2 may play important roles in regulation of cell repair. The expression of Ror2 is reduced in tumor tissues and is associated with poor prognosis, indicating the impaired regulatory effect of Ror2 in cells, and Ror2 may also serve as an anti-tumor gene. In addition, in this present study, the mRNA expression of Ror2 was decreased in highly differentiated HCC as compared with moderately or poorly differentiated HCC (P < 0.05), whereas similar results were not obtained in the protein expression of Ror2. The underlying mechanism needs to be further elucidated. However, due to the limited sample size in highly differentiated HCC (3 cases), future study will be continued by enlarging the sample size.
β-catenin is recognized as the key mediator in the canonical Wnt signal pathway. Evidence indicates that Wnt5a inhibits the abnormal expression of β-catenin through the Ror2-mediated pathway[25,39,40]. The involvement of β-catenin in tumorigenesis has been intensively researched. In colon carcinoma, the nuclear localization of β-catenin induced by gene mutation contributes to tumorigenesis. However, in HCC associated with HBV infection, β-catenin mutations are rarely seen, and β-catenin mainly accumulates in the cytoplasm[41,42]. Consistent with these observations, statistically reduced membrane expression and elevated cytoplasmic expression of β-catenin were detected in HCC tumor tissue, compared with the β-catenin expression in cell membranes in the adjacent liver tissue. Among 85 HCC cases, 6 exhibited condensed nuclear staining of β-catenin, suggesting that this protein is involved in the development of HCC. Nevertheless, we could not rule out the possibility that the loss of Wnt5a and Ror2 protein expression may decrease β-catenin degradation, which contributes to disease progression. Additionally, the lining shape of β-catenin expression in cell membranes was observed in lung metastasis of HCC, which was different from their cytoplasmic expression in the primary lesion. Since β-catenin not only acts as the key mediator of the canonical Wnt signal pathway, but also binds to E-cadherin and together they contribute to the cell adhesion and migration process[43], we hypothesize that the lung metastasis expression of β-catenin benefits the accumulation and adhesion of tumor cells in metastatic lesions.
In summary, in patients with chronic hepatitis or cirrhosis, loss of Wnt5a and Ror2 protein expression in HCC tumor tissue frequently occurs during the progression of HCC and is associated with patient prognosis. We hypothesize that Wnt5a acts upstream of Ror2. Wnt5a and Ror2 synergistically execute an anti-tumor effect during the development of HCC. The decreased expression of Wnt5a and Ror2 in HCC tissues may be directly or indirectly correlated with the abnormal activity of β-catenin. It is possible that the Wnt5a-mediated noncanonical Wnt signal pathway and the β-catenin-mediated canonical signal pathway contribute to the pathogenesis and progression of HCC. These critical mediators may be novel promising targets for gene therapy. Our study showed that HCC patients with reduced Wnt5a and Ror2 expression had poorer prognosis, indicating that protein expression of Wnt5a and Ror2 might be used as clinicopathological biomarkers for prognosis of HCC.
Hepatocellular carcinoma (HCC) is one of the most common cancers in the world. Understanding the molecular biological features of HCC is necessary for early diagnosis and better prognosis. The potential role of Wnt member 5a (Wnt5a) and receptor tyrosine kinase-like orphan receptor 2 (Ror2) in human HCC is receiving increasing attention.
Recent work in a wide of human tumors has indicated that Wnt5a and Ror2 have a critical role in malignant progression. However, little is known about the association of Wnt5a expression with Ror2 and canonical Wnt in HCC. In this study, the authors demonstrate that Wnt5a, in conjunction with Ror2 and β-catenin, may take part in the progression of HCC.
The loss of Wnt5a and Ror2 protein expression in HCC tumor tissue frequently occurs during the progression of HCC and is associated with patient poor prognosis. Wnt5a and Ror2 synergistically execute an anti-tumor effect during the development of HCC. The loss of Wnt5a and Ror2 protein expression was shown to be associated with abnormal β-catenin expression. This is the first study to report an association of Wnt5a expression with Ror2 and β-catenin in HCC.
The study results suggest that protein expression of Wnt5a and Ror2 may be used as clinicopathological biomarkers for prognosis of HCC.
Wnt5a is a non-canonical member of the Wnt family of secreted glycoproteins that acts through the family of frizzled G-protein-coupled receptor, Ror2, to mediate important events during development and cancer.
This paper reported that the loss of Wnt5a and Ror2 protein expression in HCC was associated with poor patient prognosis. Based on reduction in tumors, the authors conclude these markers could be tumor suppressor genes and good prognostic markers for HCC patients. The work is purely descriptive and relevance to clinical practice is significant.
Peer reviewers: Hitoshi Tsuda, MD, PhD, Diagnostic Pathology Section, Clinical Laboratory Division, National Cancer Center Hospital, 5-1-1 Tsukiji, Chuo-ku, Tokyo 104-0045, Japan; Haruhiko Sugimura, Professor, Hamamatsu University School of Medicine, 1-20-1 Handayama, Higashi-ku, Hamamatsu 431-3192, Japan
S- Editor Gou SX L- Editor A E- Editor Zheng XM
| 1. | El-Serag HB. Hepatocellular carcinoma: an epidemiologic view. J Clin Gastroenterol. 2002;35:S72-S78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 451] [Cited by in RCA: 467] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 2. | Bosch FX, Ribes J, Cléries R, Díaz M. Epidemiology of hepatocellular carcinoma. Clin Liver Dis. 2005;9:191-211, v. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 625] [Cited by in RCA: 605] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 3. | Breuhahn K, Vreden S, Haddad R, Beckebaum S, Stippel D, Flemming P, Nussbaum T, Caselmann WH, Haab BB, Schirmacher P. Molecular profiling of human hepatocellular carcinoma defines mutually exclusive interferon regulation and insulin-like growth factor II overexpression. Cancer Res. 2004;64:6058-6064. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 101] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 4. | Zhang J, Wang WL, Li Q, Qiao Q. Expression of transforming growth factor-alpha and hepatitis B surface antigen in human hepatocellular carcinoma tissues and its significance. World J Gastroenterol. 2004;10:830-833. [PubMed] |
| 5. | Breuhahn K, Longerich T, Schirmacher P. Dysregulation of growth factor signaling in human hepatocellular carcinoma. Oncogene. 2006;25:3787-3800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 300] [Cited by in RCA: 307] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 6. | Idobe Y, Murawaki Y, Kitamura Y, Kawasaki H. Expression of transforming growth factor-beta 1 in hepatocellular carcinoma in comparison with the non-tumor tissue. Hepatogastroenterology. 2003;50:54-59. [PubMed] |
| 7. | Reya T, Clevers H. Wnt signalling in stem cells and cancer. Nature. 2005;434:843-850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2659] [Cited by in RCA: 2765] [Article Influence: 138.3] [Reference Citation Analysis (0)] |
| 8. | Rijsewijk F, Schuermann M, Wagenaar E, Parren P, Weigel D, Nusse R. The Drosophila homolog of the mouse mammary oncogene int-1 is identical to the segment polarity gene wingless. Cell. 1987;50:649-657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 646] [Cited by in RCA: 662] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 9. | Veeman MT, Axelrod JD, Moon RT. A second canon. Functions and mechanisms of beta-catenin-independent Wnt signaling. Dev Cell. 2003;5:367-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1007] [Cited by in RCA: 1064] [Article Influence: 48.4] [Reference Citation Analysis (0)] |
| 10. | Yamada T, Takaoka AS, Naishiro Y, Hayashi R, Maruyama K, Maesawa C, Ochiai A, Hirohashi S. Transactivation of the multidrug resistance 1 gene by T-cell factor 4/beta-catenin complex in early colorectal carcinogenesis. Cancer Res. 2000;60:4761-4766. [PubMed] |
| 11. | Korinek V, Barker N, Willert K, Molenaar M, Roose J, Wagenaar G, Markman M, Lamers W, Destree O, Clevers H. Two members of the Tcf family implicated in Wnt/beta-catenin signaling during embryogenesis in the mouse. Mol Cell Biol. 1998;18:1248-1256. [PubMed] |
| 12. | Qian D, Jones C, Rzadzinska A, Mark S, Zhang X, Steel KP, Dai X, Chen P. Wnt5a functions in planar cell polarity regulation in mice. Dev Biol. 2007;306:121-133. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 343] [Cited by in RCA: 325] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 13. | Slusarski DC, Corces VG, Moon RT. Interaction of Wnt and a Frizzled homologue triggers G-protein-linked phosphatidylinositol signalling. Nature. 1997;390:410-413. [PubMed] |
| 14. | Pandur P, Maurus D, Kühl M. Increasingly complex: new players enter the Wnt signaling network. Bioessays. 2002;24:881-884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 141] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 15. | Topol L, Jiang X, Choi H, Garrett-Beal L, Carolan PJ, Yang Y. Wnt-5a inhibits the canonical Wnt pathway by promoting GSK-3-independent beta-catenin degradation. J Cell Biol. 2003;162:899-908. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 573] [Cited by in RCA: 592] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 16. | Ishitani T, Kishida S, Hyodo-Miura J, Ueno N, Yasuda J, Waterman M, Shibuya H, Moon RT, Ninomiya-Tsuji J, Matsumoto K. The TAK1-NLK mitogen-activated protein kinase cascade functions in the Wnt-5a/Ca(2+) pathway to antagonize Wnt/beta-catenin signaling. Mol Cell Biol. 2003;23:131-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 424] [Cited by in RCA: 440] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 17. | Pukrop T, Binder C. The complex pathways of Wnt 5a in cancer progression. J Mol Med (Berl). 2008;86:259-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 58] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 18. | McDonald SL, Silver A. The opposing roles of Wnt-5a in cancer. Br J Cancer. 2009;101:209-214. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 165] [Cited by in RCA: 193] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 19. | Kurayoshi M, Oue N, Yamamoto H, Kishida M, Inoue A, Asahara T, Yasui W, Kikuchi A. Expression of Wnt-5a is correlated with aggressiveness of gastric cancer by stimulating cell migration and invasion. Cancer Res. 2006;66:10439-10448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 322] [Cited by in RCA: 348] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 20. | Iozzo RV, Eichstetter I, Danielson KG. Aberrant expression of the growth factor Wnt-5A in human malignancy. Cancer Res. 1995;55:3495-3499. [PubMed] |
| 21. | Weeraratna AT, Jiang Y, Hostetter G, Rosenblatt K, Duray P, Bittner M, Trent JM. Wnt5a signaling directly affects cell motility and invasion of metastatic melanoma. Cancer Cell. 2002;1:279-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 713] [Cited by in RCA: 683] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 22. | Pukrop T, Klemm F, Hagemann T, Gradl D, Schulz M, Siemes S, Trümper L, Binder C. Wnt 5a signaling is critical for macrophage-induced invasion of breast cancer cell lines. Proc Natl Acad Sci U S A. 2006;103:5454-5459. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 279] [Cited by in RCA: 277] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 23. | Liu XH, Pan MH, Lu ZF, Wu B, Rao Q, Zhou ZY, Zhou XJ. Expression of Wnt-5a and its clinicopathological significance in hepatocellular carcinoma. Dig Liver Dis. 2008;40:560-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 24. | Liu X, Wang L, Zhang S, Lin J, Zhang S, Feitelson MA, Gao H, Zhu M. Mutations in the C-terminus of the X protein of hepatitis B virus regulate Wnt-5a expression in hepatoma Huh7 cells: cDNA microarray and proteomic analyses. Carcinogenesis. 2008;29:1207-1214. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 46] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 25. | Mikels AJ, Nusse R. Purified Wnt5a protein activates or inhibits beta-catenin-TCF signaling depending on receptor context. PLoS Biol. 2006;4:e115. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 941] [Cited by in RCA: 998] [Article Influence: 52.5] [Reference Citation Analysis (0)] |
| 26. | MacLeod RJ, Hayes M, Pacheco I. Wnt5a secretion stimulated by the extracellular calcium-sensing receptor inhibits defective Wnt signaling in colon cancer cells. Am J Physiol Gastrointest Liver Physiol. 2007;293:G403-G411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 102] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 27. | Forrester WC. The Ror receptor tyrosine kinase family. Cell Mol Life Sci. 2002;59:83-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 84] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 28. | Afzal AR, Rajab A, Fenske CD, Oldridge M, Elanko N, Ternes-Pereira E, Tüysüz B, Murday VA, Patton MA, Wilkie AO. Recessive Robinow syndrome, allelic to dominant brachydactyly type B, is caused by mutation of ROR2. Nat Genet. 2000;25:419-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 214] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 29. | Kobayashi M, Shibuya Y, Takeuchi J, Murata M, Suzuki H, Yokoo S, Umeda M, Minami Y, Komori T. Ror2 expression in squamous cell carcinoma and epithelial dysplasia of the oral cavity. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;107:398-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 30. | Wright TM, Brannon AR, Gordan JD, Mikels AJ, Mitchell C, Chen S, Espinosa I, van de Rijn M, Pruthi R, Wallen E. Ror2, a developmentally regulated kinase, promotes tumor growth potential in renal cell carcinoma. Oncogene. 2009;28:2513-2523. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 93] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 31. | O'Connell MP, Fiori JL, Xu M, Carter AD, Frank BP, Camilli TC, French AD, Dissanayake SK, Indig FE, Bernier M. The orphan tyrosine kinase receptor, ROR2, mediates Wnt5A signaling in metastatic melanoma. Oncogene. 2010;29:34-44. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 140] [Cited by in RCA: 165] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 32. | Lara E, Calvanese V, Huidobro C, Fernández AF, Moncada-Pazos A, Obaya AJ, Aguilera O, González-Sancho JM, Sánchez L, Astudillo A. Epigenetic repression of ROR2 has a Wnt-mediated, pro-tumourigenic role in colon cancer. Mol Cancer. 2010;9:170. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 57] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 33. | Hirohashi S, Blum HE, Ishak KG. Tumours of the liver and intrahepatic bile ducts. World Health Organisation Classification of Tumours: Pathology and genetics of tumours of the digestive system. Lyon: IARC Press 2000; 157-202. |
| 34. | Dissanayake SK, Olkhanud PB, O'Connell MP, Carter A, French AD, Camilli TC, Emeche CD, Hewitt KJ, Rosenthal DT, Leotlela PD. Wnt5A regulates expression of tumor-associated antigens in melanoma via changes in signal transducers and activators of transcription 3 phosphorylation. Cancer Res. 2008;68:10205-10214. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 104] [Cited by in RCA: 94] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 35. | Jönsson M, Dejmek J, Bendahl PO, Andersson T. Loss of Wnt-5a protein is associated with early relapse in invasive ductal breast carcinomas. Cancer Res. 2002;62:409-416. [PubMed] |
| 36. | Kremenevskaja N, von Wasielewski R, Rao AS, Schöfl C, Andersson T, Brabant G. Wnt-5a has tumor suppressor activity in thyroid carcinoma. Oncogene. 2005;24:2144-2154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 205] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 37. | Mikels A, Minami Y, Nusse R. Ror2 receptor requires tyrosine kinase activity to mediate Wnt5A signaling. J Biol Chem. 2009;284:30167-30176. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 124] [Cited by in RCA: 138] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 38. | He F, Xiong W, Yu X, Espinoza-Lewis R, Liu C, Gu S, Nishita M, Suzuki K, Yamada G, Minami Y. Wnt5a regulates directional cell migration and cell proliferation via Ror2-mediated noncanonical pathway in mammalian palate development. Development. 2008;135:3871-3879. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 192] [Cited by in RCA: 181] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 39. | Yamamoto H, Yoo SK, Nishita M, Kikuchi A, Minami Y. Wnt5a modulates glycogen synthase kinase 3 to induce phosphorylation of receptor tyrosine kinase Ror2. Genes Cells. 2007;12:1215-1223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 75] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 40. | Kurayoshi M, Yamamoto H, Izumi S, Kikuchi A. Post-translational palmitoylation and glycosylation of Wnt-5a are necessary for its signalling. Biochem J. 2007;402:515-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 171] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 41. | Huang H, Fujii H, Sankila A, Mahler-Araujo BM, Matsuda M, Cathomas G, Ohgaki H. Beta-catenin mutations are frequent in human hepatocellular carcinomas associated with hepatitis C virus infection. Am J Pathol. 1999;155:1795-1801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 205] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 42. | Joo M, Lee HK, Kang YK. Expression of beta-catenin in hepatocellular carcinoma in relation to tumor cell proliferation and cyclin D1 expression. J Korean Med Sci. 2003;18:211-217. [PubMed] |
| 43. | Jamora C, Fuchs E. Intercellular adhesion, signalling and the cytoskeleton. Nat Cell Biol. 2002;4:E101-E108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 452] [Cited by in RCA: 450] [Article Influence: 19.6] [Reference Citation Analysis (0)] |