Published online Mar 28, 2012. doi: 10.3748/wjg.v18.i12.1319
Revised: February 1, 2012
Accepted: February 16, 2012
Published online: March 28, 2012
AIM: To investigate whether hepatitis B virus (HBV) could induce a hepatitis B virus core antigen (HBcAg)-specific cytotoxic T lymphocyte (CTL) response in vitro by dendritic cells (DCs) transduced with lentiviral vector-encoding ubiquitinated hepatitis B virus core antigen (LV-Ub-HBcAg).
METHODS: Recombinant LV-Ub-HBcAg were transfected into highly susceptible 293 T cells to obtain high virus titres. Bone marrow-derived DCs isolated from BALB/c mice were cultured with recombinant granulocyte-macrophage colony-stimulating factor and recombinant interleukin (IL)-4. LV-Ub-HBcAg, lentiviral vector-encoding hepatitis B virus core antigen (LV-HBcAg), lentiviral vector (LV) or lipopolysaccharide were added to induce DC maturation, and the DC phenotypes were analyzed by flow cytometry. The level of IL-12 in the supernatant was detected by enzyme-linked immunosorbent assay (ELISA). T lymphocytes were proliferated using Cell Counting Kit-8. DCs were cultured and induced to mature using different LVs, and co-cultured with allogeneic T cells to detect the secretion levels of IL-2, IL-4, IL-10 and interferon-γ in the supernatants of T cells by ELISA. Intracellular cytokines of proliferative T cells were analyzed by flow cytometry, and specific CTL activity was measured by a lactate dehydrogenase release assay.
RESULTS: LV-Ub-HBcAg-induced DCs secreted more IL-12 and upregulated the expression of CD80, CD86 and major histocompatibility class II. DCs sensitised by different LVs effectively promoted cytokine secretion; the levels of IL-2 and interferon-γ induced by LV-Ub-HBcAg were higher than those induced by LV-HBcAg. Compared with LV-HBcAg-transduced DCs, LV-Ub-HBcAg-transduced DCs more efficiently stimulated the proliferation of T lymphocytes and generated HBcAg-specific cytotoxic T lymphocytes.
CONCLUSION: LV-Ub-HBcAg effectively induced DC maturation. The mature DCs efficiently induced T cell polarisation to Th1 and generated HBcAg-specific CTLs.
- Citation: Chen JH, Yu YS, Chen XH, Liu HH, Zang GQ, Tang ZH. Enhancement of CTLs induced by DCs loaded with ubiquitinated hepatitis B virus core antigen. World J Gastroenterol 2012; 18(12): 1319-1327
- URL: https://www.wjgnet.com/1007-9327/full/v18/i12/1319.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i12.1319
Hepatitis B virus (HBV) infection is a serious public health problem, particularly in Asia and South Africa[1]. Notably, an effective T cell response is critical for virus clearance, and defective cytotoxic T lymphocytes (CTLs) may lead to persistent HBV infection[2]. Moreover, the defective CTL response was ascribed to the impaired dendritic cell (DC) function[3]. Promoting and improving DC function is a promising approach to combating persistent HBV infection.
Various methods have been attempted to modify DC function, including the use of protein antigens, cytokines, costimulatory molecules, and signalling pathway ligands known to activate the immune response[4-7]. Nevertheless, these methods may be insufficient to induce a strong antigen-specific immune response. Further enhancement of the immune response to HBV-specific CTL may be more conducive to clear the HBV. Thus, a novel therapeutic approach is needed to activate T cell expansion and induce a strong antigen-specific T cell response.
Ubiquitin (Ub) is a highly conserved small regulatory protein, ubiquitous in eukaryotes, that usually serves as a signal for the target protein that is recognized and degraded in proteasomes[8]. The Ub-mediated processing of antigens is rapid and efficient and stimulates cell-mediated immune responses. Accordingly, Ub-mediated processing of antigens has been widely used in chronic infection and cancer studies to improve immune response. Wang et al[9] found that an Ub-fused Mycobacterium tuberculosis antigen ESAT-6 DNA vaccine significantly increased the antigen-specific cellular immune response in BALB/c mice. That study confirmed that the Th1-type immune response and CTL activity were enhanced by changing the antigen processing. Zhang et al[10] reported that Ub-fused melanoma antigens induced antigen proteins to execute proteasome-dependent degradation and created epitopes of major histocompatibility complex (MHC) class I, resulting in the preferential activation of antigen-specific CD8+ T cells.
Retroviral and adenoviral vectors have been the focus of many studies because of their high efficiency. Lentivirus vectors (LVs) transfect both dividing and relatively quiescent cells and have been widely used to modify DCs[11,12]. The aim of this study was to investigate the capacity of DCs transfected with LVs encoding the ubiquitinated hepatitis B virus core antigen (LV-Ub-HBcAg-DC) to stimulate lymphocyte proliferation and to generate antigen-specific CTLs. The results may provide effective approaches to the control of persistent HBV infection.
BALB/c mice (H-2d), 6-8 wk old, were purchased from the Shanghai Experimental Animal Centre of the Chinese Academy of Sciences and maintained under pathogen-free conditions. Mice were cared for and treated in accordance with the guidelines established by the Shanghai Public Health Service Policy on the Humane Care and Use of Laboratory Animals.
HEK293T cells were cultured in Dulbecco’s modified Eagle’s medium (Invitrogen, Gaithersburg, MD, United States) supplemented with 10% foetal bovine serum (Gibco, Grand Island, NY, United States), penicillin (100 U/mL), and streptomycin (100 mg/mL) at 37 °C in 5% CO2. The H-2d mastocytoma cell line P815/c (expressing the HBV core antigen) was maintained in our lab.
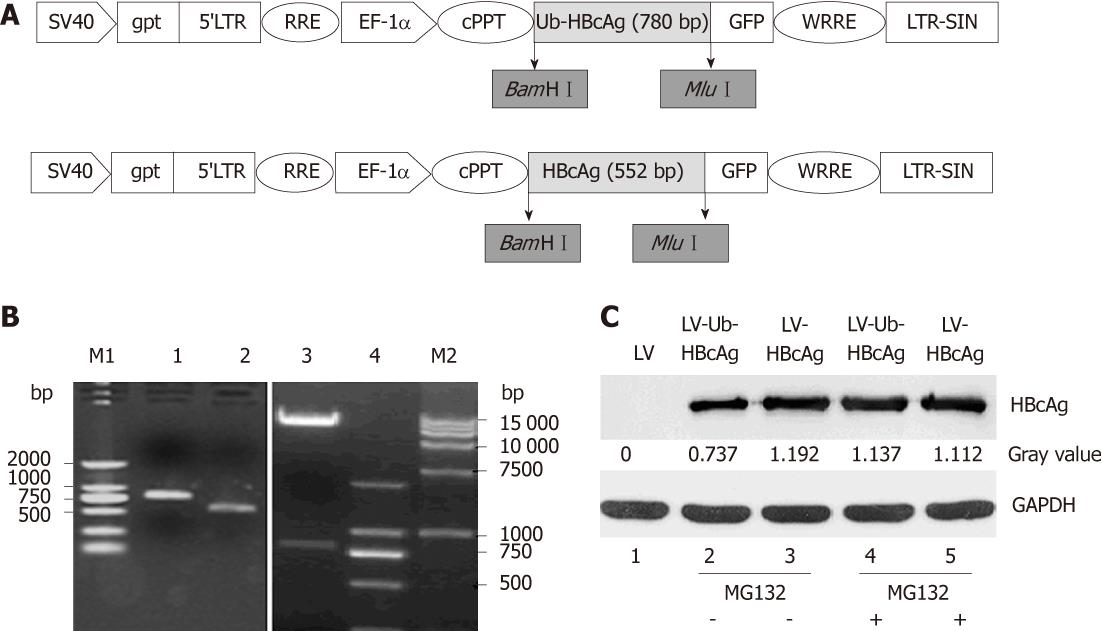
The plasmid pcDNA3.1(-)-Ub-HBcAg was constructed and maintained in our lab. The Ub-HBcAg gene was amplified by polymerase chain reaction (PCR). The primers used were: Ub-HBcAg: forward: CGTGGGATCCATGCA GATCTTCGTGAAG, reverse: CGCACGCGTCTAACATTGAGATTCCCGAG from plasmid pcDNA3.1(-)-Ub-HBcAg. The purified Ub-HBcAg fragment was cloned into the pWPLXd vector (provided by Prof. Jianming Li, Nanjing, China) using BamH I and Mlu I restriction sites. The recombinant pWPLXd- Ub-HBcAg plasmid was confirmed by restriction enzyme digestion and DNA sequencing. LV-Ub-HBcAg was derived by a combined transfection of three elements: 10 μg pWPLXd-Ub-HBcAg backbone plasmid, 5 μg psPAX2 packaging plasmid, and 5 μg PMD2.G envelope plasmid. We transiently transfected 293T cells with plasmids using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, United States). Two days after the transfection, the viral supernatant was collected and filtered through a 0.45-μm filter. Concentrated vectors for the in vitro studies were prepared by ultracentrifugation at 25 000 rpm and 4 °C for 90 min. Viral pellets were resuspended in 2 mL sterile phosphate-buffered saline (PBS) and stored at -80 °C.
The control plasmid was constructed by inserting the HBcAg fragment into the BamH I and Mlu I site of the pWPLXd plasmid and named pWPLXd-HBcAg. LV particles (LV-HBcAg) were produced by Lipofectamine transfection into 293T cells.
To determine the titre of the green fluorescent protein (GFP)-expressing vector, 293T cells (1 × 106 cells/well) were infected with serially diluted viral supernatant. On day 2, the infected cells expressing GFP were counted by flow cytometry. The titre was calculated as: transduction units per mL (TU) = the number of infected cells/volume of virus supernatant.
The 293T cells were seeded in six-well plates at 1 × 106 cells/well. LV-Ub-HBcAg, LV-HBcAg or LV was added at an multiplicity of infection (MOI) of 1. In some experiments, a specific inhibitor of proteasomes, MG132, was used at 10 μmol. The cells were harvested 48 h after infection, washed twice with PBS, gently dispersed into a single-cell suspension and homogenised using RIPA lysis buffer. Protein concentrations were determined using the Pierce BCA Protein Assay Reagent kit (Rockford, IL, United States). Homogenates were diluted to the desired protein concentration with 2 × SDS-PAGE loading buffer (Invitrogen). Samples were boiled and loaded onto polyacrylamide mini-gels (Invitrogen) for electrophoresis. Proteins from the gels were transferred to Immobilon-PVDF membranes (Millipore Corp., Bedford, MA, United States) using a semi-dry apparatus (Bio-Rad, Hercules, CA, United States). A mouse anti-human HBcAg monoclonal antibody (Santa Cruz Biotechnology, Santa Cruz, CA, United States) was used as the primary antibody, and horseradish peroxidase-conjugated goat anti-mouse immunoglobulin-G antibody was used as the secondary antibody.
Femurs and tibiae of Babl/c mice were removed and purified from the surrounding muscle tissues. Thereafter, intact bones were left in 70% ethanol for 5 min for disinfection and then washed with PBS. Both ends were cut with scissors and the marrow was flushed with PBS using a syringe with a 0.45-mm diameter needle. Clusters within the marrow suspension were disintegrated by vigorous pipetting. Bone marrow cells were cultured at 2 × 106 cells/mL in complete RPMI 1640 culture medium (containing 10% FBS, 100 U/mL penicillin, and 100 mg/mL streptomycin) in the presence of 20 ng/mL murine granulocyte-macrophage colony-stimulating factor (GM-CSF) (PeproTech, Rocky Hill, United states) and 10 ng/mL murine IL-4 (mIL-4; PeproTech). Nonadherent single cells were gently removed, and fresh medium containing murine GM-CSF and mIL-4 was added on day 3 after beginning culture.
On day 5, immature DCs were cultured for an additional 96 h in the presence of LV-Ub-HBcAg, LV-HBcAg or LV (MOI = 20), and lipopolysaccharide (LPS, 0.5 mg/mL; Sigma-Aldrich, St. Louis, MO, United States) was used as a control group. On day 9, non-adherent and loosely adherent cells were harvested as DCs. The expression of DC surface molecules was analyzed by incubation with allophycocyanin-labelled anti-mouse CD11c, CD80, CD86 and MHC class II (eBioscience, San Diego, CA, United States). The stained cells were analyzed by flow cytometry.
On day 5, immature DCs were infected with LV-Ub-HBcAg, LV-HBcAg, or LV (MOI = 20) for 72 h. On day 8, the IL-12 levels in harvested supernatants of mature DCs were measured using a standard sandwich enzyme-linked immunosorbent assay (ELISA) kit (R and D Systems, Minneapolis, MN, United States) according to the manufacturer’s instructions.
On day 9, harvested mature DCs were pre-treated with 25 μg/mL mitomycin C and 5% CO2 for 30 min at 37 °C. Mouse spleens were dissociated on 200-gauge nylon mesh. Splenocytes were collected and treated with lysis buffer to eliminate red cells, washed, and resuspended in RPMI-1640 with 10% FBS. Lymphocytes were derived from splenocytes using nylon wool columns. Single-cell suspensions of lymphocytes (5 × 105 cells/well) were grown in 96-well plates. Lymphocytes were co-cultured with mature DCs at different responder/stimulator (T cell/DC) ratios (5:1, 10:1 or 20:1) for 72 h. The cells were incubated in a final volume of 200-μL complete RPMI 1640 for 72 h, and 10-μL Cell Counting Kit-8 solution (Beyotime Institute of Biotechnology, Haimen, China) was added to the plates for 4 h at 37 °C. The absorbance was finally read at 450 nm.
Splenocytes from mice were cultured in 96-well culture plates in the presence of mature DCs for 4 d at a T-cell to DC ratio of 10:1, and the supernatants were collected. The levels of different cytokines [interferon (IFN)-γ, IL-2, IL-4 and IL-10] in the supernatants of proliferating T cells were measured using commercial ELISA kits according to the manufacturer’s protocol (R and D Systems). Data were expressed as pg/mL.
IFN-γ production was detected by intracellular staining and flow cytometry. The above proliferative T cells were suspended in complete RPMI 1640 and stimulated for 6 h in the presence of 25 μg/mL phorbol 12-myristate 13-acetate, 1 μg/mL ionomycin and 1.7 μg/mL monensin (Sigma). After washed with PBS, the cells were stained with FITC-conjugated anti-CD8α mAb (eBioscience) for 30 min at 4 °C, washed with PBS, fixed with 4% paraformaldehyde, and permeabilised with PBS containing 0.5% saponin (both from BD, Shanghai, China). Cells were incubated with PE-labelled anti-INF-γ McAb (eBioscience) for 30 min at 4 °C, washed with PBS, and analyzed by flow cytometry.
The former stimulated splenocytes (5 × 106/mL) were used as effectors, and the P815/c cell line was used as target cells. P815/c cells were seeded at a density of 5 × 104 cells/well in 96-well plates. Effector cells were incubated with P815/c at different effector and target (E/T) ratios (12.5:1, 25:1 or 50:1) at 37 °C under 5% CO2 for 4 h. The HBcAg-specific CTL activity was measured using a CytoTox 96® Non-Radioactive Cytotoxicity Assay (Promega, Madison WI, United States) for lactate dehydrogenase (LDH) release according to the manufacturer’s instructions. The absorbance values of the supernatants were recorded at optical density 450 nm. Percent cytotoxicity was calculated as follows: [(Experimental release - Effector spontaneous release - Target spontaneous release)/(Target maximum release - Target spontaneous release)] × 100%.
Results were expressed as mean ± SD. Differences between groups was determined using Student’s t test, and the differences between two or more groups were determined using a one-factor analysis of variance. Data were considered statistically significant at P < 0.05.
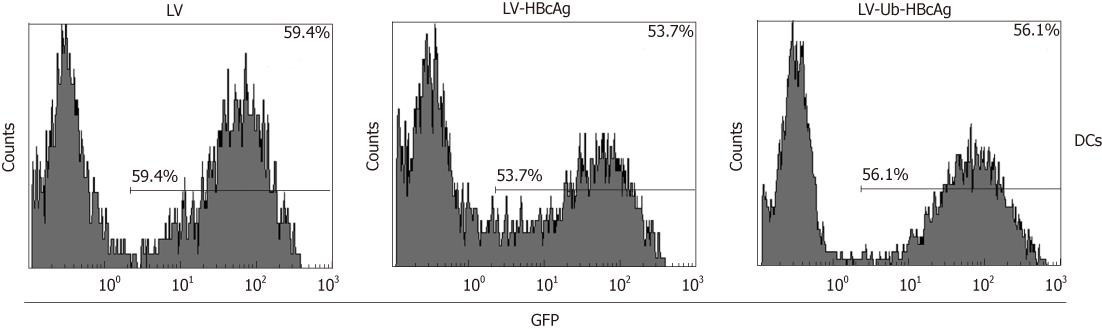
A 780 bp fragment of the Ub-HBcAg gene was cloned into pWPXLd (Figure 1B) and packed into LVs. The HBcAg gene was similarly assembled as a control. After concentration, all vectors in the study achieved a titration of approximately 7.5 × 108 transducing units/mL. The construction procedure is shown in Figure 1A. As expected, Ub-HBcAg expression was lower than that of HBcAg and recovered to the same level as that of HBcAg when MG-132 was added to the culture (Figure 1C). The transduction efficiency of LVs into DCs was evaluated using flow cytometry by detecting GFP expression (Figure 2). On day 4 after infection, 56.1% of GFP-expressing DCs were detected.
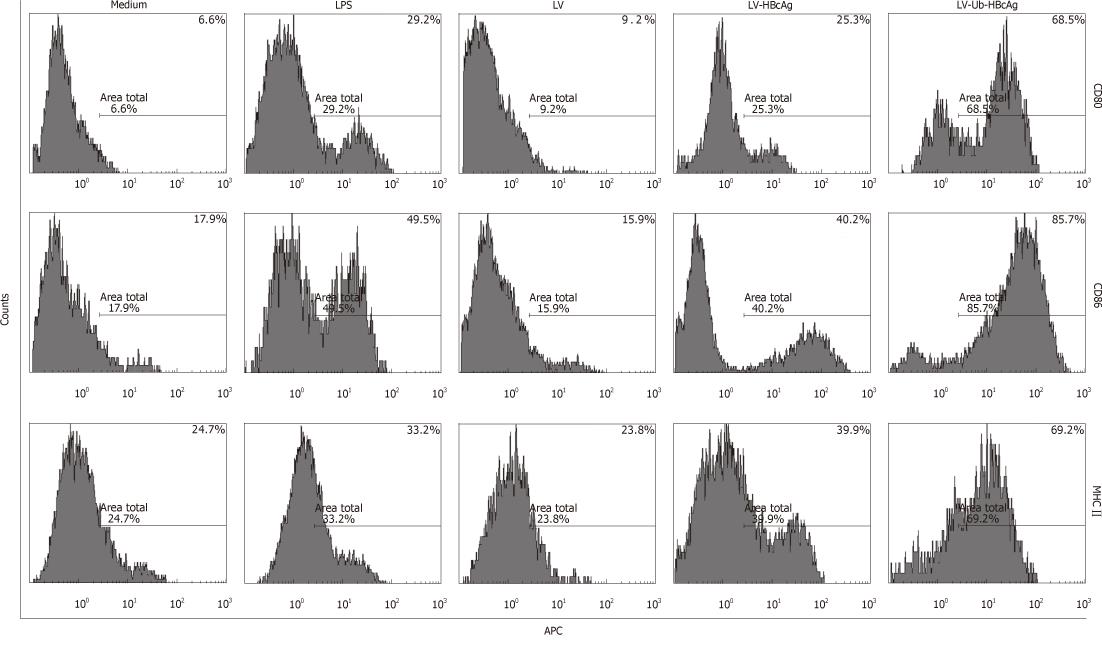
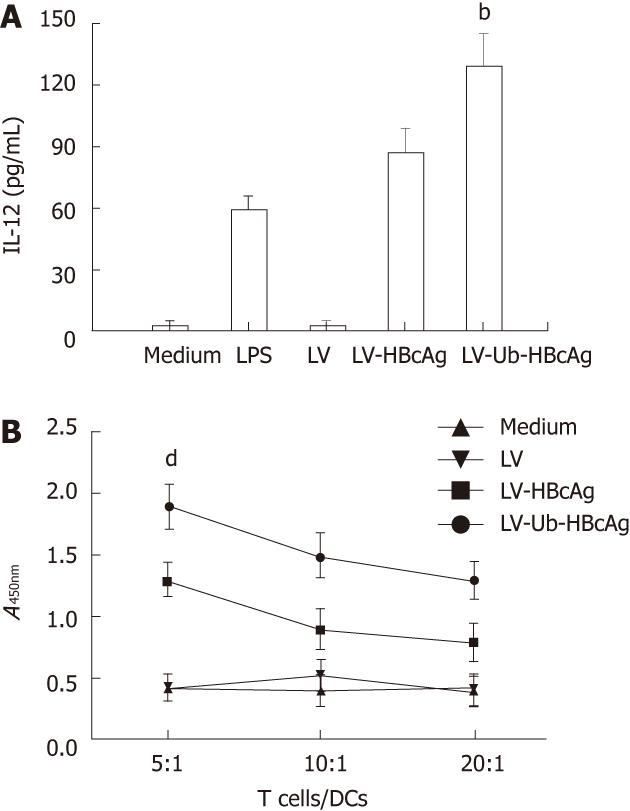
At the end of the treatment, the amount of DCs (CD11c+) was 75% by fluorescence-activated cell sorting analysis. MHC II, CD80 and CD86 molecules, which are characteristic of DCs, were used to evaluate DC differentiation and maturation. These molecules were highly expressed in DCs transduced with LV-Ub-HBcAg compared with those transduced with the alternatives (Figure 3). DC function was evaluated by IL-12 secretion and promotion of lymphocyte proliferation. DCs transduced with LV-Ub-HBcAg showed significantly higher levels of IL-12 production and lymphocyte proliferation than did the others (P < 0.01) (Figure 4A and B). Lymphocyte proliferation capacity was enhanced by the T cell/DC ratio.
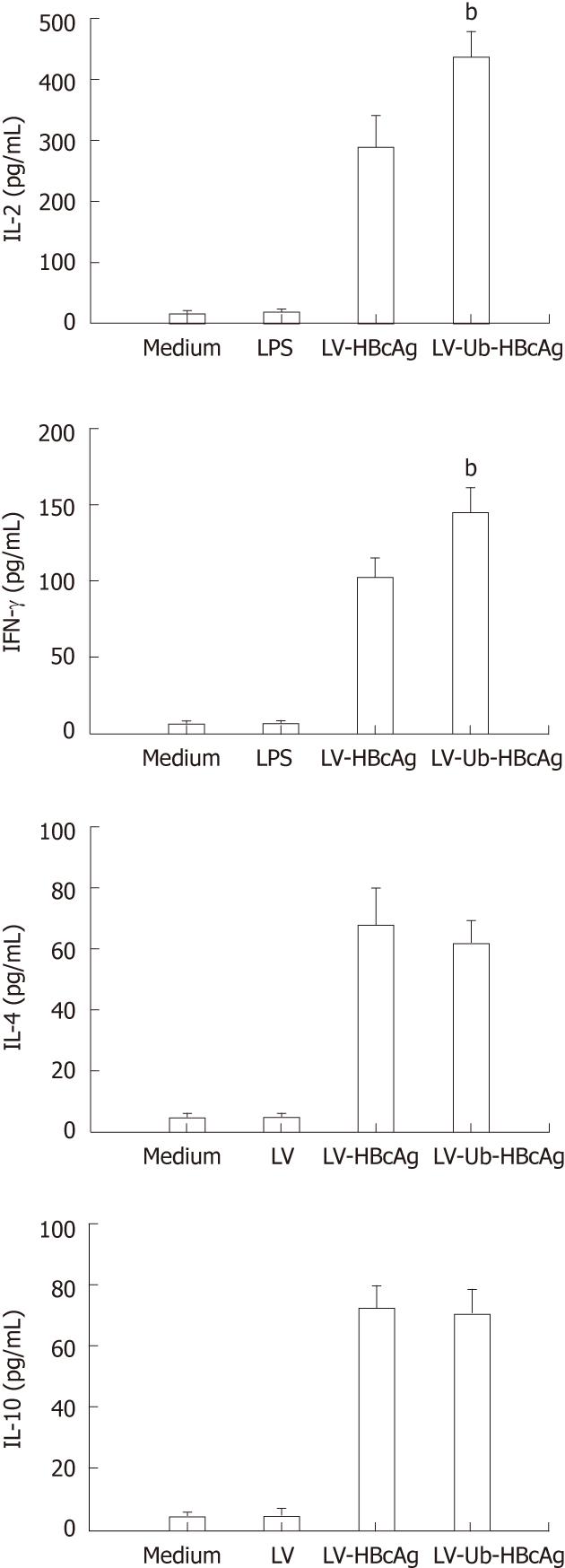
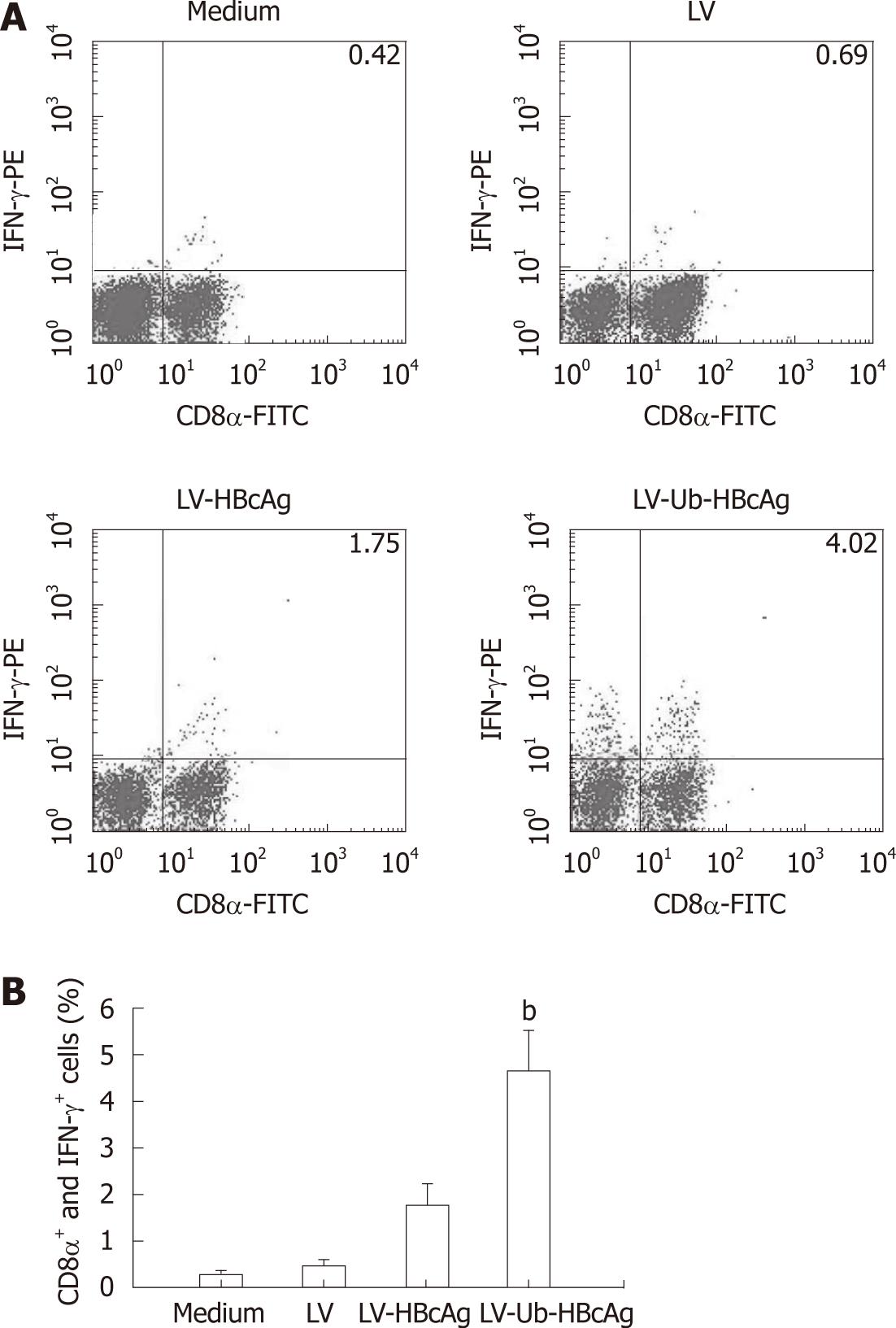
T cells stimulated by DCs transduced with LV-Ub-HBcAg showed increased IFN-γ and IL-2 secretion compared with DCs transduced with LV-HBcAg (Figure 5). No significant difference between the two groups was observed for IL-4 and IL-10 production. CTLs were analyzed by intercellular IFN-γ and CD8α+ levels. The levels and intensities of IFN-γ expression were higher in the LV-Ub-HBcAg than in the LV-HBcAg samples (Figure 6A and B), suggesting that DCs transduced with LV-Ub-HBcAg were effective for inducing CTLs.
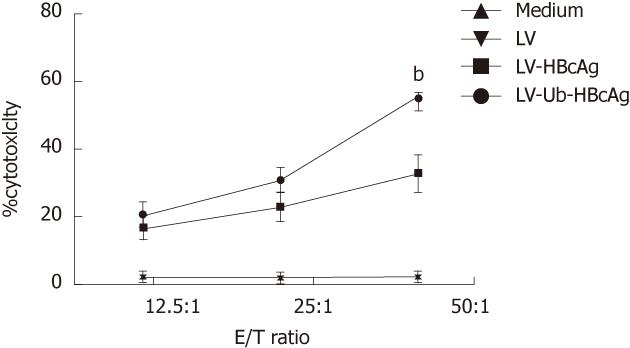
The LDH relaxation index was determined to evaluate the specific cytotoxicity of T lymphocytes in response to different LV-transduced DCs. HBcAg-specific CTL activities with different effector/target ratios are shown in Figure 7. T lymphocytes from the LV-Ub-HBcAg-transduced DCs killed 55.0% ± 4.3% target cells at an effector and target ratio of 50:1, which was significantly higher than that of the LV-HBcAg-transduced DC group (32.4% ± 5.2%) (P < 0.01). Accordingly, these results indicated that LV-Ub-HBcAg-transduced DCs induced strong specific CTL responses.
The development of novel immunotherapies has been highly anticipated because HBV infection is one of the leading causes of cancer or hepatocellular carcinoma-related death. Several studies have demonstrated that the main cause of viral persistence during HBV infection is an inadequate antiviral immune response to the viral antigens[13,14]. The viral-specific CD8+ T cell response plays an important role in the process of viral clearance. Patients with chronic hepatitis B (CHB) or therapeutic failure show deficient Th1 immunity associated with inefficient CD8+ T cell cytotoxicity[15]. Therefore, induction of CTL responses specific to HBV represents a promising strategy to protect against HBV infection.
DCs are key antigen-presenting cells that induce primary and memory immune responses. Impaired DC function is found in chronic HBV infection, in which patients are generally in an immunocompromised state of immune tolerance[16-18]. Considerable effort has been made to introduce antigens into DCs in the forms of peptides, proteins, or transgenic protein antigens using viral vectors[19,20]. Various viral vectors, including poxvirus and adenovirus, have been used to genetically modify DCs, but low transduction efficiencies have limited their application. We chose LVs as a gene transfer vector because they can transduce non-dividing, monocyte-derived DCs and bone marrow-derived DCs with very high transduction efficiencies[21,22]. Several reports have demonstrated that immunizing mice with LVs by delivering viral or tumor model antigens mice elicited broad and long-lasting specific immune responses. For example, lentiviral transduction of DCs expressing ovalbumin effectively processed and presented the ovalbumin antigens and induced ovalbumin-specific T cell responses[23]. Our results confirmed that lentivirus-mediated gene transfer could offer the unique opportunity to investigate the biologic activity of DCs.
The ubiquitin-proteasome system (UPS) is a highly selective adenosine-5’-triphosphate-dependent proteolytic system present in all eukaryotic cells and plays a key role in antigen presentation[8]. It is well established that short antigenic peptides must be presented on MHC class I molecules of target cells to be recognized by specific CTLs. Proteasomes are responsible for the proteolysis of intracellular proteins, including viral antigens, to generate MHC class I ligands.
Attachment of Ub to a protein is the initial signal for targeted protein degradation. To prevent fusion gene (Ub-HBcAg) cleavage by deubiquitination enzymes, we constructed a pWPXLd vector encoding HBcAg fused with Ub, in which the Ub C-terminal glycine was replaced with alanine[24]. Additionally, HBcAg with a modified N-terminal Met residue was replaced by Arg. By this method, the fusion protein can be quickly recognized by the UPS, resulting in a promotion of HBcAg degradation[25-27]. In our study, Western blotting analyses identified efficient expression of HBcAg from the 293T cells transduced with recombinant LVs. Ub-fused HBcAg was converted into an excellent substrate for the UPS. We found that the 293T cells transduced with LV-Ub-HBcAg showed low levels of protein expression in the absence of MG-132.
Immature DCs expressed low levels of surface MHC molecules, producing almost no expression of CD40, CD80 or CD86. Fully matured DCs showed strong surface expression of MHC class II and co-stimulatory molecules (CD80 and CD86). In our study, the surface molecules CD80, CD86, and MHC class II DCs were markedly upregulated by LV-Ub-HBcAg stimulation, whereas no significant change was observed after LPS or LV-HBcAg stimulation. An important sign of mature DCs is IL-12 secretion. Mature DCs secrete high levels of IL-12 that promote activation of effector cells (e.g., natural killer cells, lymphokine-activated killer cells, tumor-infiltrating lymphocytes and macrophages) and induce a variety of cytokines (e.g., IFN-γ, GM-CSF, IL-2 and IL-8)[28]. IL-12 is produced by mature DCs in response to infection by various intracellular pathogens. This response plays a critical role initiating a specific T cell-mediated immune response and drives Th1 cell activation and differentiation[29,30]. We examined IL-12 production after adding different maturation factors to the culture medium. As expected, IL-12-induced LV-Ub-HBcAg production of DCs was markedly elevated compared with that produced by other treatments. In this study, LV-Ub-HBcAg-transduced DCs not only promoted DC surface molecule expression, but also promoted further secretion of IL-12, which helped stimulate the immune response.
Higher rates of intracellular antigen traffic should increase the number and varieties of peptides available for MHC class I binding, which may result in an increase in the cell immune response to the expressed antigen. DCs pulsed with HBV antigens effectively abrogated CTL tolerance in HBV transgenic mice. Chen et al[4] demonstrated that DCs loaded with HBcAg not only induce the production of HBV-specific T cells but also restore the impaired function of such cells. The DCs generated by transfection of LV-Ub-HBcAg were able to stimulate proliferation of naive allogeneic T lymphocytes and to increase the number of antigen-specific CD8+/IFN-γ+ T cells in vitro. Th1 cells primarily secrete IL-2 and IFN-γ, whereas Th2 cells secrete type II cytokines IL-4 and IL-10. Th1/Th2 immune balance plays a key role in the outcome of HBV infection. Dominant Thl cells tend to lead to an acute self-limited HBV infection; dominant Th2 cells tend to occur with a chronic persistent HBV infection. In our study, we observed that the LV-Ub-HBcAg group had higher levels of both IL-2 and IFN-γ in the lymphocyte supernatant compared with those in the LV-HBcAg group. This was further supported by enhanced levels of IFN-γ-producing CD8+ T cells. These results clearly indicate that the immune responses were directed toward a Th1 type rather than a Th2 type. Th1 cells are correlated with the induction of CTL activity, which is beneficial for viral or tumor eradication[31,32]. In this study, the LV-Ub-HBcAg-transfected DCs stimulated T lymphocytes and generated antigen-specific cytotoxic T lymphocytes more efficiently than those of the LV-HBcAg-transfected DCs. Thus, LVs carrying Ub-fused HBcAg effectively activated antigen-specific CD8+ T cells. Inadequate endogenous antigen presentation by MHC class I molecules to CD8+ T cells is one of the reasons for the failure of the immune system to eliminate pathogens. Patients with CHB or therapeutic failure showed deficient Th1 immunity associated with inefficient CD8+ T cell cytotoxicity. In our study, enhanced antigen presentation increased the number of antigen-specific CD8+/IFN-γ+ T cells in the LV-Ub-HBcAg-transfected DC group. Ub-fused HBcAg was rapidly degraded by the proteasome, resulting in efficient production of a variety of peptides, including many CTL epitopes that may be presented by many types of MHC class I molecules.
In summary, we have successfully transfected murine bone marrow-derived DCs with LVs encoding the Ub-HBcAg fusion gene. The Ub-HBcAg-transfected DCs proliferated and generated HBcAg-specific CTLs more efficiently than did the HBcAg-transfected DCs. Therefore, this novel strategy may have therapeutic value that can be applied to the treatment of infectious diseases.
Hepatitis B virus (HBV) infection is a serious public health problem. Defective cytotoxic T lymphocytes (CTLs) may lead to persistent HBV infection, and the defective CTL response was ascribed to the impaired dendritic cell (DC) function. Promoting and improving DC function is a promising approach to combating persistent HBV infection. Ubiquitin (Ub) is a highly conserved small regulatory protein, and the Ub-mediated processing of antigens is rapid and efficient and stimulates cell-mediated immune responses.
Ub-fused melanoma antigens could induce antigen proteins to execute proteasome-dependent degradation and created epitopes of major histocompatibility complex (MHC) class I, resulting in the preferential activation of antigen-specific CD8+ T cells.
The study reported for the first time the capacity of DCs transfected with lentivirus encoding the ubiquitinated hepatitis B virus core antigen (Ub-HBcAg) to stimulate lymphocyte proliferation and to generate antigen-specific CTLs.
The authors found that lentivirus encoding Ub-HBcAg effectively could induce DC maturation. The mature DCs efficiently induced T cell polarization to Th1 and generated HBcAg-specific CTLs. These results may be helpful in seeking novel approaches to the control of persistent HBV infection.
Ubiquitin is a highly conserved small regulatory protein, ubiquitous in eukaryotes, that usually serves as a signal for the target protein that is recognized and degraded in proteasomes.
The topic is novel, with very few articles published in this field till now. The manuscript is well organized with objectives, methods, results being adequately described, and the conclusions are based on the results found. Tables and figures are appropriate. Statistical analysis needs better description by providing P values through the text where comparisons between groups are present.
Peer reviewer: Elena Vezali, Dr., Department of Hepatology, Hygeia Diagnostic and Therapeutic Center of Athens, Erythrou Staurou 4, Maroussi, Athens 15123, Greece
S- Editor Gou SX L- Editor Ma JY E- Editor Xiong L
| 1. | Te HS, Jensen DM. Epidemiology of hepatitis B and C viruses: a global overview. Clin Liver Dis. 2010;14:1-21, vii. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 2. | Yang SH, Lee CG, Park SH, Im SJ, Kim YM, Son JM, Wang JS, Yoon SK, Song MK, Ambrozaitis A. Correlation of antiviral T-cell responses with suppression of viral rebound in chronic hepatitis B carriers: a proof-of-concept study. Gene Ther. 2006;13:1110-1117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 94] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 3. | Almand B, Resser JR, Lindman B, Nadaf S, Clark JI, Kwon ED, Carbone DP, Gabrilovich DI. Clinical significance of defective dendritic cell differentiation in cancer. Clin Cancer Res. 2000;6:1755-1766. [PubMed] |
| 4. | Chen W, Shi M, Shi F, Mao Y, Tang Z, Zhang B, Zhang H, Chen L, Chen L, Xin S. HBcAg-pulsed dendritic cell vaccine induces Th1 polarization and production of hepatitis B virus-specific cytotoxic T lymphocytes. Hepatol Res. 2009;39:355-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 5. | Creusot RJ, Yaghoubi SS, Kodama K, Dang DN, Dang VH, Breckpot K, Thielemans K, Gambhir SS, Fathman CG. Tissue-targeted therapy of autoimmune diabetes using dendritic cells transduced to express IL-4 in NOD mice. Clin Immunol. 2008;127:176-187. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 60] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 6. | Wang Q, Liu Y, Wang J, Ding G, Zhang W, Chen G, Zhang M, Zheng S, Cao X. Induction of allospecific tolerance by immature dendritic cells genetically modified to express soluble TNF receptor. J Immunol. 2006;177:2175-2185. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 60] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 7. | Tiao MM, Lu L, Tao R, Harnaha J, Fung JJ, Huang LT, Qian S. Application of recipient-derived dendritic cells to induce donor-specific T-cell hyporesponsiveness. Transplant Proc. 2004;36:1592-1594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 8. | Gao G, Luo H. The ubiquitin-proteasome pathway in viral infections. Can J Physiol Pharmacol. 2006;84:5-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 139] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 9. | Wang QM, Kang L, Wang XH. Improved cellular immune response elicited by a ubiquitin-fused ESAT-6 DNA vaccine against Mycobacterium tuberculosis. Microbiol Immunol. 2009;53:384-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 10. | Zhang M, Obata C, Hisaeda H, Ishii K, Murata S, Chiba T, Tanaka K, Li Y, Furue M, Chou B. A novel DNA vaccine based on ubiquitin-proteasome pathway targeting 'self'-antigens expressed in melanoma/melanocyte. Gene Ther. 2005;12:1049-1057. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 11. | Breckpot K, Escors D, Arce F, Lopes L, Karwacz K, Van Lint S, Keyaerts M, Collins M. HIV-1 lentiviral vector immunogenicity is mediated by Toll-like receptor 3 (TLR3) and TLR7. J Virol. 2010;84:5627-5636. [PubMed] |
| 12. | Rossetti M, Gregori S, Hauben E, Brown BD, Sergi LS, Naldini L, Roncarolo MG. HIV-1-derived lentiviral vectors directly activate plasmacytoid dendritic cells, which in turn induce the maturation of myeloid dendritic cells. Hum Gene Ther. 2011;22:177-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 13. | Stoop JN, van der Molen RG, Baan CC, van der Laan LJ, Kuipers EJ, Kusters JG, Janssen HL. Regulatory T cells contribute to the impaired immune response in patients with chronic hepatitis B virus infection. Hepatology. 2005;41:771-778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 390] [Cited by in RCA: 407] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 14. | Woltman AM, Op den Brouw ML, Biesta PJ, Shi CC, Janssen HL. Hepatitis B virus lacks immune activating capacity, but actively inhibits plasmacytoid dendritic cell function. PLoS One. 2011;6:e15324. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 105] [Cited by in RCA: 120] [Article Influence: 8.6] [Reference Citation Analysis (2)] |
| 15. | Tsai SL, Sheen IS, Chien RN, Chu CM, Huang HC, Chuang YL, Lee TH, Liao SK, Lin CL, Kuo GC. Activation of Th1 immunity is a common immune mechanism for the successful treatment of hepatitis B and C: tetramer assay and therapeutic implications. J Biomed Sci. 2003;10:120-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 51] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 16. | Tavakoli S, Mederacke I, Herzog-Hauff S, Glebe D, Grün S, Strand D, Urban S, Gehring A, Galle PR, Böcher WO. Peripheral blood dendritic cells are phenotypically and functionally intact in chronic hepatitis B virus (HBV) infection. Clin Exp Immunol. 2008;151:61-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 50] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 17. | Akbar SM, Horiike N, Onji M. Immune therapy including dendritic cell based therapy in chronic hepatitis B virus infection. World J Gastroenterol. 2006;12:2876-2883. [PubMed] |
| 18. | Op den Brouw ML, Binda RS, van Roosmalen MH, Protzer U, Janssen HL, van der Molen RG, Woltman AM. Hepatitis B virus surface antigen impairs myeloid dendritic cell function: a possible immune escape mechanism of hepatitis B virus. Immunology. 2009;126:280-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 184] [Article Influence: 10.8] [Reference Citation Analysis (2)] |
| 19. | Jirmo AC, Koya RC, Sundarasetty BS, Pincha M, Yu GY, Lai M, Bakshi R, Schlaphoff V, Grabowski J, Behrens G. Monocytes transduced with lentiviral vectors expressing hepatitis C virus non-structural proteins and differentiated into dendritic cells stimulate multi-antigenic CD8(+) T cell responses. Vaccine. 2010;28:922-933. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 20. | Dyall J, Latouche JB, Schnell S, Sadelain M. Lentivirus-transduced human monocyte-derived dendritic cells efficiently stimulate antigen-specific cytotoxic T lymphocytes. Blood. 2001;97:114-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 104] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 21. | Zhang L, Procuik M, Fang T, Kung SK. Functional analysis of the quantitative expression of a costimulatory molecule on dendritic cells using lentiviral vector-mediated RNA interference. J Immunol Methods. 2009;344:87-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 22. | Veron P, Boutin S, Martin S, Chaperot L, Plumas J, Davoust J, Masurier C. Highly efficient transduction of human plasmacytoid dendritic cells without phenotypic and functional maturation. J Transl Med. 2009;7:10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 23. | He Y, Zhang J, Mi Z, Robbins P, Falo LD. Immunization with lentiviral vector-transduced dendritic cells induces strong and long-lasting T cell responses and therapeutic immunity. J Immunol. 2005;174:3808-3817. [PubMed] |
| 24. | Imai T, Duan X, Hisaeda H, Himeno K. Antigen-specific CD8+ T cells induced by the ubiquitin fusion degradation pathway. Biochem Biophys Res Commun. 2008;365:758-763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 25. | Mogk A, Schmidt R, Bukau B. The N-end rule pathway for regulated proteolysis: prokaryotic and eukaryotic strategies. Trends Cell Biol. 2007;17:165-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 202] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 26. | Delogu G, Howard A, Collins FM, Morris SL. DNA vaccination against tuberculosis: expression of a ubiquitin-conjugated tuberculosis protein enhances antimycobacterial immunity. Infect Immun. 2000;68:3097-3102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 80] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 27. | Rodriguez F, Zhang J, Whitton JL. DNA immunization: ubiquitination of a viral protein enhances cytotoxic T-lymphocyte induction and antiviral protection but abrogates antibody induction. J Virol. 1997;71:8497-8503. [PubMed] |
| 28. | Tatsumi T, Takehara T, Yamaguchi S, Sasakawa A, Miyagi T, Jinushi M, Sakamori R, Kohga K, Uemura A, Ohkawa K. Injection of IL-12 gene-transduced dendritic cells into mouse liver tumor lesions activates both innate and acquired immunity. Gene Ther. 2007;14:863-871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 29. | Giermasz AS, Urban JA, Nakamura Y, Watchmaker P, Cumberland RL, Gooding W, Kalinski P. Type-1 polarized dendritic cells primed for high IL-12 production show enhanced activity as cancer vaccines. Cancer Immunol Immunother. 2009;58:1329-1336. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 60] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 30. | Jan RH, Lin YL, Chen LK, Huang MT, Wang LC, Chiang BL. Hepatitis B virus surface antigen can activate dendritic cells and modulate T helper type immune response. Microbiol Immunol. 2011;55:51-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 31. | Chamoto K, Kosaka A, Tsuji T, Matsuzaki J, Sato T, Takeshima T, Iwakabe K, Togashi Y, Koda T, Nishimura T. Critical role of the Th1/Tc1 circuit for the generation of tumor-specific CTL during tumor eradication in vivo by Th1-cell therapy. Cancer Sci. 2003;94:924-928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 46] [Article Influence: 2.1] [Reference Citation Analysis (0)] |