Published online Mar 21, 2012. doi: 10.3748/wjg.v18.i11.1249
Revised: June 23, 2011
Accepted: June 30, 2011
Published online: March 21, 2012
AIM: To investigate how a complex network of CC chemokine ligands (CCLs) and their receptors influence the progression of tumor and metastasis.
METHODS: In the present study, we used immunohistochemistry to examine the expression of CCL7, CCL8 and CCL21 in 194 gastric cancer samples and adjacent normal tissues. We analyzed their correlation with tumor metastasis, clinicopathologic parameters and clinical outcome.
RESULTS: We found that the higher expression of CCL7 and CCL21 in cancer tissues than in normal tissues was significantly correlated with advanced depth of wall invasion, lymph node metastasis and higher tumor node metastasis stage. Moreover, Kaplan-Meier survival analysis revealed that CCL7 and CCL21 overexpression in cancer tissues was correlated with poor prognosis.
CONCLUSION: These results suggest that overexpression of these two CC chemokine ligands is associated with tumor metastasis and serves as a prognostic factor in patients with gastric cancer.
- Citation: Hwang TL, Lee LY, Wang CC, Liang Y, Huang SF, Wu CM. CCL7 and CCL21 overexpression in gastric cancer is associated with lymph node metastasis and poor prognosis. World J Gastroenterol 2012; 18(11): 1249-1256
- URL: https://www.wjgnet.com/1007-9327/full/v18/i11/1249.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i11.1249
Chemokine ligands (CCLs) belong to the small molecule chemoattractive cytokine family and are grouped into CC and CXC chemokines ligands on the basis of the characteristic presence of four conserved cysteine residues[1-3]. Chemokines mediate their chemical effect on target cells through G-protein-coupled receptors, which are characterized structurally by seven transmembrane spanning domains and are involved in the attraction and activation of mononuclear and polymorphonuclear leukocytes. CCLs and their receptors play an important role in angiogenesis and tumor growth, however the role of CCLs in metastasis has only recently been explored[4,5]. CCL7 promoted the invasion and migration of oral squamous cell carcinoma[4]. CCL21 was significantly highly expressed in breast tumor cells with lymph node metastasis and prognosis[5].
Gastric cancer is one of the commonest malignant tumors of the alimentary tract and is characterized by late clinical presentation, rapid progression, and poor survival[6]. The reason for this poor prognosis is that, at the time of diagnosis, gastric cancer usually shows extensive local tumor invasion and frequent spread to metastatic sites, particularly lymph nodes. Spread of malignant tumors is a multistep process and many of the stages of tumor invasion require degradation or breakdown of the extracellular matrix and connective tissue surrounding tumor cells[7,8]. The matrix metalloproteinases (MMPs) are a family of zinc containing enzymes which are involved in the degradation of different components of the extracellular matrix, and there is considerable evidence to indicate that individual MMPs have important roles in tumor invasion and tumor spread[9-11]. A recent study showed that increased levels of CCL recruit immature myeloid cells that carry the chemokine ligand receptor (CCR) from the blood to the tumor invasion front. These immature myeloid cells produced MMP9 and MMP2 and help the tumor cells to migrate and invade[12].
In the present study, we used immunohistochemistry to examine the expression of CCL7, CCL8 and CCL21 in 194 gastric cancer samples and adjacent normal tissues. We analyzed their correlation with tumor metastasis, clinicopathologic parameters and clinical outcome.
A consecutive series of 194 tissue specimens were collected from patients with gastric cancer who received subtotal or total gastroectomy resection in Chang Gung Memorial Hospital (CGMH) in Taiwan. All operations were performed between January 2001 and December 2002. Written informed consent was obtained before sample collection and this study was approved by the Institutional Review Board of CGMH. There were 114 males and 80 females with a mean age of 62 years (range, 24-90 years). The age and gender of patients, tumor location, tumor size, cell differentiation, depth of wall invasion, status of lymph node metastasis, vascular invasion, lymphatic invasion and desmoplastic reaction were obtained from histopathology records. Stage of gastric cancer was described according to the 1997 tumor node metastasis (TNM) classification of malignant tumors by the American Joint Committee on Cancer. All patients were followed until December 2007 with a minimum 5 years of follow-up. All tissue specimens were formalin-fixed and paraffin-embedded. Formalin fixed tissue sections were stained with haematoxylin and eosin and classified by a pathologist. These results were compared with the histopathology records from CGMH. Final pathology was determined by consensus and review if necessary.
The tissue blocks were constructed according to the me-thod of Schraml et al[13] and the best representative morphological areas of tumors were used in this study. The specimen sections were deparaffinized, treated with 3% hydrogen peroxide and microwaved after pretreatment in 10 mmol/L citric acid to retrieve antigenicity. The sections were incubated with blocking solution containing phosphate buffered saline and 1% bovine serum albumin for 20 min at room temperature, and then incubated overnight at 4 °C with an anti-CCL7 antibody (1:100, R and D), an anti-CCL8 monoclonal antibody (1:50, R and D), or an anti-CCL21 monoclonal antibody (1:50, R and D), respectively. After washing 4 times with Tris Buffered Saline, the sections were incubated with biotinylated secondary antibody (Santa Cruz Biotechnology). The immuno-complex was visualized by the immonoglobulin enzyme bridge technique using the DAKO LSAB 2 System, HRP kit (DAKO corp. Carpinteria, CA) with 3,3’ diaminobenzidine tetrachloride as a substrate. The sections were counterstained with hematoxylin, dehydrated with graded alcohols, cleared with xylene and mounted with a coverslip.
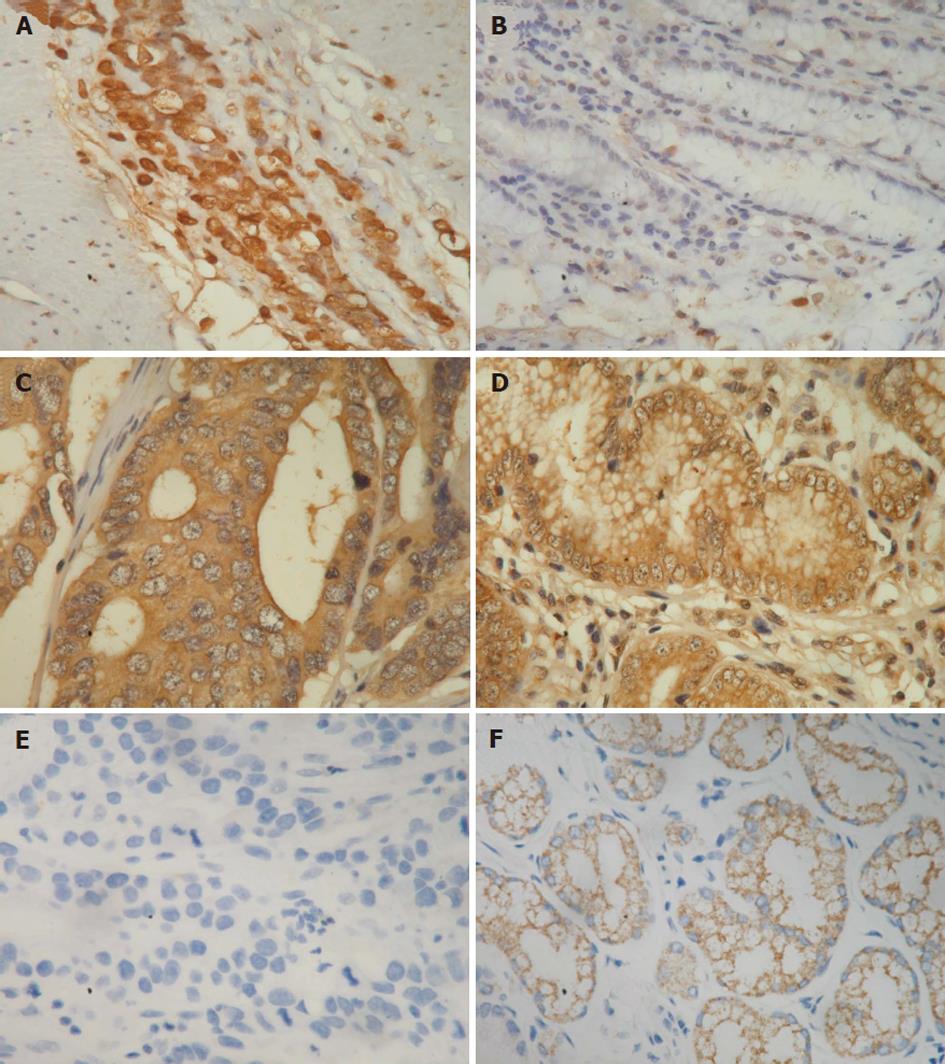
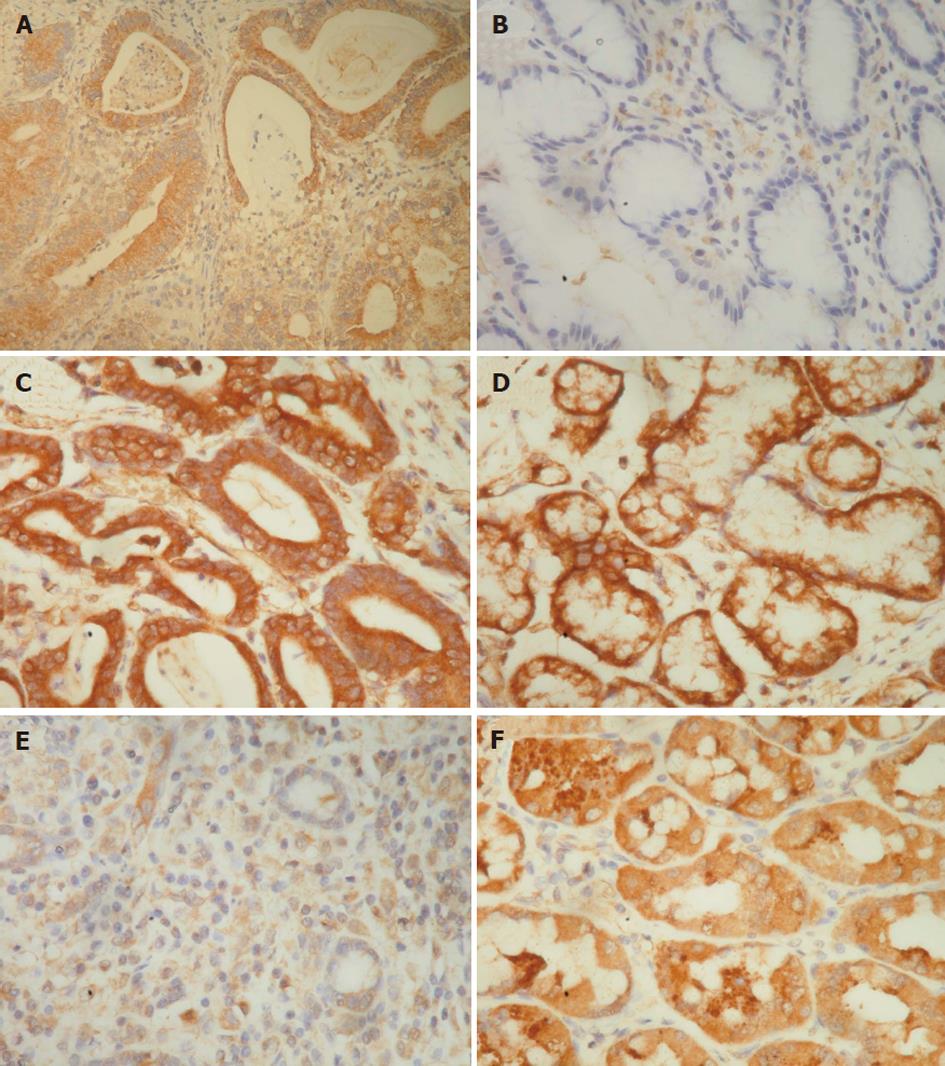
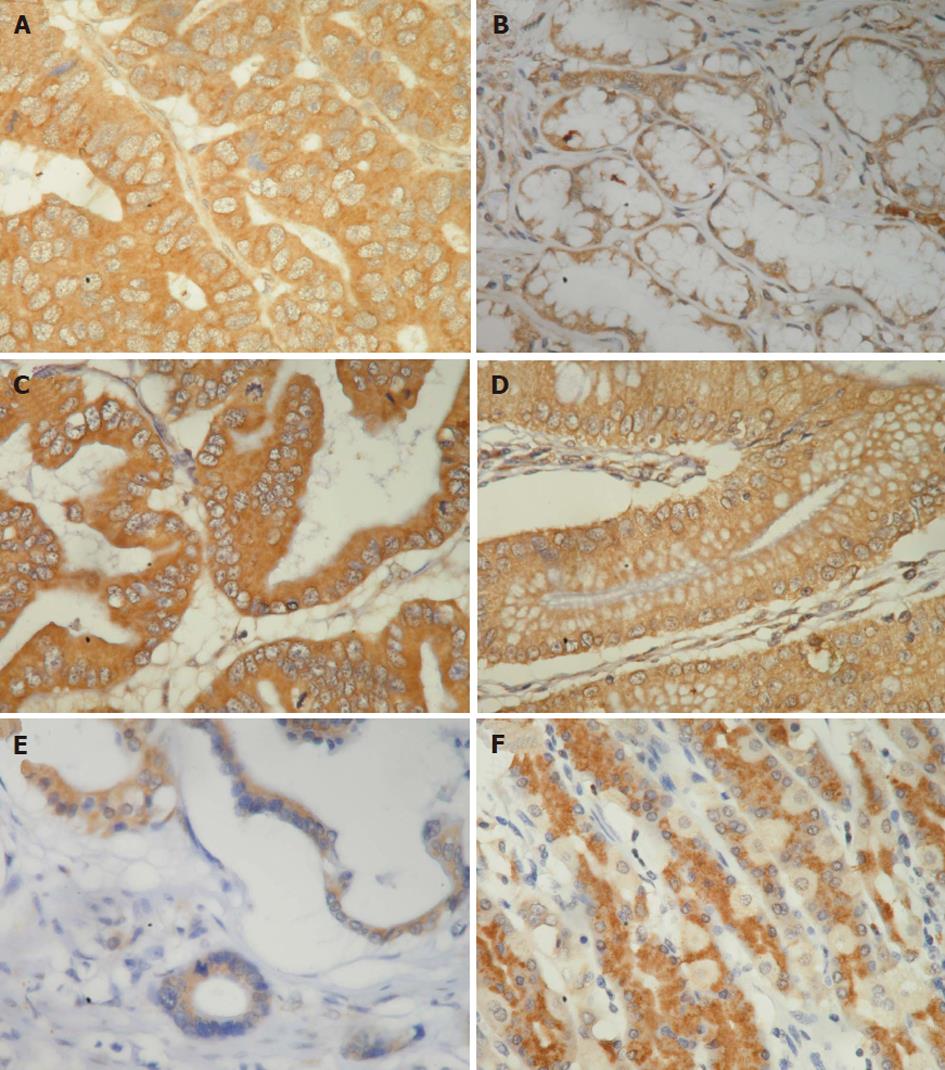
The immunostaining results were scored as follows, according to a previous report[14]. The immunostaining reaction was evaluated by subjective assessments of the median staining intensity (0, no stain; 1, weak; 2, moderate; and 3, strong stain) and by the fraction of stained cells in percentage categories (0, 0%-9%; 1, 10%-49%; 2, 50%-89%; and 3, ≥ 90%). This scoring system was previously shown to be reproducible[15]. The scores of 0 to 3 were obtained as follows: percentage categories and staining were each ranked as indicated above. The ranks for percentage and staining intensity were multiplied by each other, divided by 3, and rounded up to the nearest whole number[15]. The results of immunostaining in tumor and normal tissues were divided into three groups, higher (rank of tumor tissue > rank of normal tissue), equal (rank of tumor tissue = rank of normal tissue), and lower (rank of tumor tissue < rank of normal tissue) (Figures 1-3).
χ2 or Fisher’s exact test was used to test for an association between CCL7, CCL8 and CCL21 expression and patient clinicopathologic parameters. Disease-free survival was defined as the time from surgery to the first relapse of cancer, occurrence of a second primary tumor, or death from any cause. Univariate survival analysis was assessed by the Kaplan-Meier method and significance of difference between groups was analysed using log rank test or log rank test for trend. Stepwise multivariate survival analysis was performed by the Cox proportional hazards model. All reported P values were two-sided and a P value < 0.05 was considered significant.
The percentages of the higher expression of CCL7, CCL8 and CCL21 in cancer tissues than in normal tissues were 42.3% (82 of 194), 29.9% (58 of 194) and 44.8% (87 of 194), respectively (Figures 1-3 and Table 1). The percentages of the equal expression of CCL7, CCL8 and CCL21 in cancer tissues and in normal tissues were 35.6% (69 of 194), 33% (64 of 194) and 32.5% (63 of 194), respectively (Figures 1-3 and Table 1). The percentages of the lower expression of CCL7, CCL8 and CCL21 in cancer tissues than in normal tissues were 22.2% (43 of 194), 37.1% (72 of 194) and 22.7% (44 of 194), respectively (Figures 1-3 and Table 1).
| Factors | Cases | CCL-7 expression | CCL-8 expression | CCL-21 expression | |||||||||
| Higher | Equal | Lower | P value | Higher | Equal | Lower | P value | Higher | Equal | Lower | P value | ||
| n = 82 | n = 69 | n = 43 | n = 58 | n = 64 | n = 72 | n = 87 | n = 63 | n = 44 | |||||
| Age (yr) | |||||||||||||
| ≤ 60 | 83 | 31 | 31 | 21 | 0.449 | 17 | 28 | 38 | 0.026 | 34 | 24 | 25 | 0.101 |
| > 60 | 111 | 51 | 38 | 22 | 41 | 36 | 34 | 53 | 39 | 19 | |||
| Gender | |||||||||||||
| Male | 114 | 52 | 40 | 22 | 0.412 | 36 | 40 | 38 | 0.429 | 47 | 45 | 22 | 0.041 |
| Female | 80 | 30 | 29 | 21 | 22 | 24 | 34 | 40 | 18 | 22 | |||
| Tumor location | |||||||||||||
| Upper | 22 | 10 | 6 | 6 | 0.025 | 4 | 6 | 12 | 0.004 | 8 | 9 | 5 | 0.026 |
| Middle | 40 | 10 | 17 | 13 | 10 | 6 | 24 | 13 | 11 | 16 | |||
| Lower | 124 | 58 | 46 | 20 | 42 | 48 | 34 | 60 | 43 | 21 | |||
| Whole | 8 | 4 | 0 | 4 | 2 | 4 | 2 | 6 | 0 | 2 | |||
| Tumor size (cm) | |||||||||||||
| ≤ 3 | 95 | 29 | 36 | 30 | 0.001 | 24 | 28 | 43 | 0.068 | 34 | 35 | 26 | 0.043 |
| > 3 | 99 | 53 | 33 | 13 | 34 | 36 | 29 | 53 | 28 | 18 | |||
| Differentiation | |||||||||||||
| Well | 18 | 6 | 8 | 4 | 0.177 | 5 | 6 | 7 | 0.422 | 7 | 7 | 4 | 0.130 |
| Moderate | 55 | 27 | 22 | 6 | 17 | 23 | 15 | 30 | 18 | 7 | |||
| Poor | 52 | 25 | 15 | 12 | 19 | 13 | 20 | 27 | 12 | 13 | |||
| Signet ring cell | 69 | 24 | 24 | 21 | 17 | 22 | 30 | 23 | 26 | 20 | |||
| Depth of wall invasion | |||||||||||||
| T1 | 47 | 12 | 19 | 16 | 0.001 | 8 | 15 | 24 | 0.070 | 9 | 18 | 20 | < 0.0001 |
| T2 | 37 | 8 | 18 | 11 | 13 | 12 | 12 | 9 | 18 | 10 | |||
| T3 | 101 | 58 | 29 | 14 | 35 | 31 | 35 | 64 | 24 | 13 | |||
| T4 | 9 | 4 | 3 | 2 | 2 | 6 | 1 | 5 | 3 | 1 | |||
| Lymph node metastasis | |||||||||||||
| N0 | 88 | 30 | 36 | 22 | 0.020 | 21 | 29 | 38 | 0.488 | 28 | 33 | 27 | 0.003 |
| N1 | 47 | 16 | 16 | 15 | 16 | 17 | 14 | 20 | 17 | 10 | |||
| N2 | 23 | 13 | 7 | 3 | 7 | 6 | 10 | 13 | 5 | 5 | |||
| N3 | 36 | 23 | 10 | 3 | 14 | 12 | 10 | 26 | 8 | 2 | |||
| Vascular invasion | |||||||||||||
| No | 168 | 70 | 60 | 38 | 0.800 | 50 | 58 | 60 | 0.561 | 71 | 57 | 40 | 0.163 |
| Yes | 26 | 12 | 9 | 5 | 8 | 6 | 12 | 16 | 6 | 4 | |||
| Lymphatic invasion | |||||||||||||
| No | 104 | 37 | 39 | 28 | 0.086 | 27 | 34 | 43 | 0.325 | 36 | 38 | 30 | 0.006 |
| Yes | 90 | 45 | 30 | 15 | 31 | 30 | 29 | 51 | 25 | 14 | |||
| Desmoplastic reaction | |||||||||||||
| None | 28 | 6 | 14 | 8 | 0.006 | 5 | 6 | 17 | 0.071 | 7 | 9 | 12 | < 0.0001 |
| Mild | 64 | 22 | 20 | 22 | 18 | 21 | 25 | 17 | 26 | 21 | |||
| Moderate | 75 | 43 | 25 | 7 | 23 | 30 | 22 | 44 | 24 | 7 | |||
| Marked | 27 | 11 | 10 | 6 | 12 | 7 | 8 | 19 | 4 | 43 | |||
| TNM stage | |||||||||||||
| I | 64 | 16 | 27 | 21 | 0.008 | 14 | 20 | 30 | 0.427 | 13 | 26 | 25 | < 0.0001 |
| II | 44 | 17 | 17 | 10 | 13 | 14 | 17 | 18 | 18 | 8 | |||
| III | 38 | 20 | 12 | 6 | 13 | 14 | 11 | 24 | 8 | 6 | |||
| IV | 48 | 29 | 13 | 6 | 18 | 16 | 14 | 32 | 11 | 5 | |||
The overexpression of CCL7 in cancer tissues compared with normal tissues was significantly correlated with tumor location (P = 0.025) and tumor size (P = 0.001). The overexpression of CCL7 was significantly higher in gastric cancer with advanced depth of wall invasion (P = 0.001), lymph node metastasis (P = 0.020), desmoplastic reaction (P = 0.006) and higher TNM stage (P = 0.008), but was not correlated with age, gender, differentiation, vascular invasion or lymphatic invasion (Table 1).
The overexpression of CCL8 was significantly correlated with age (P = 0.026) and tumor location (P = 0.004), but not with gender, tumor size, differentiation, depth of wall invasion, lymph node metastasis, vascular invasion, lymphatic invasion, desmoplastic reaction or TNM stage.
The overexpression of CCL21 was significantly higher in females than in males (P = 0.041) and was correlated with tumor location (P = 0.026), tumor size (P = 0.043) and lymphatic invasion (P = 0.006). As with CCL7, the overexpression of CCL21 was significantly higher in gastric cancer with an advanced depth of wall invasion (P < 0.0001), lymph node metastasis (P = 0.003), desmoplastic reaction (P < 0.0001) and higher TNM stage (P < 0.0001), but was not correlated with age, differentiation or vascular invasion (Table 1).
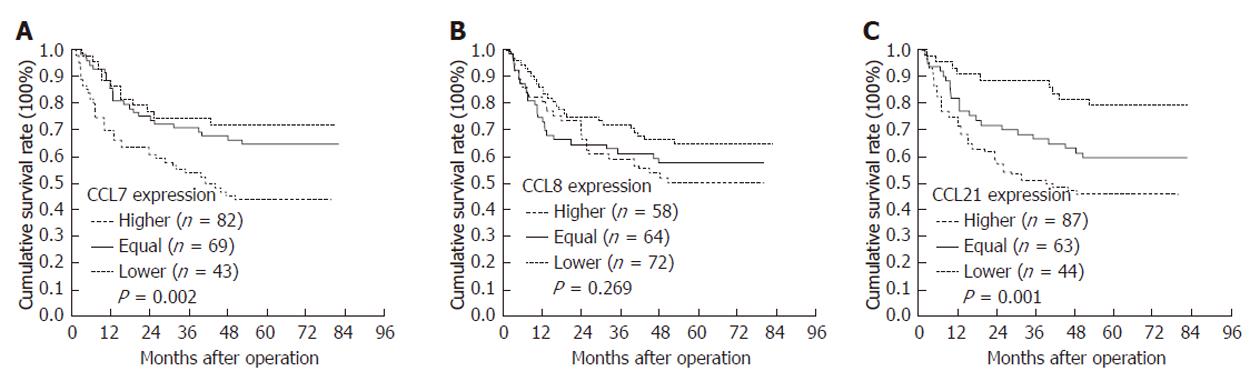
CCL7 and CCL21 overexpression was correlated with a poor prognosis (P = 0.002 and 0.001, Table 2 and Figure 4A and C). CCL8 overexpression was not correlated with survival (Table 2, Figure 4B). Other significant prognostic factors were tumor location, tumor size, differentiation, depth of invasion, lymph node metastases, vascular invasion, lymphatic invasion, marked desmoplastic reaction and higher TNM stage. In multivariate analysis, depth of invasion, lymph node metastasis and desmoplastic reaction were independent prognostic factors (Table 3).
| Factors | Cases | Mean survival (mo) | 95% CI of mean | 5-year survival (%) | P value |
| Age (yr) | |||||
| ≤ 60 | 83 | 57.33 | 50.22-64.44 | 60.2 | 0.516 |
| > 60 | 111 | 53.34 | 46.93-59.76 | 56.1 | |
| Gender | |||||
| Male | 114 | 57.90 | 51.74-64.05 | 62.2 | 0.174 |
| Female | 80 | 51.09 | 43.61-58.56 | 51.7 | |
| Type of gastrectomy | |||||
| Total | 41 | 31.85 | 23.28-40.43 | 33.5 | < 0.0001 |
| Subtotal | 153 | 60.21 | 55.14-65.48 | 64.5 | |
| Tumor location | |||||
| Upper | 22 | 26.01 | 15.81-36.21 | 27.3 | < 0.0001 |
| Middle | 40 | 69.22 | 61.15-71.29 | 77.0 | |
| Lower | 124 | 56.56 | 50.70-62.43 | 59.5 | |
| Diffuse | 8 | 23.41 | 7.46-39.36 | 25.0 | |
| Margin | |||||
| Negative | 173 | 57.71 | 52.73-62.68 | 62.3 | 0.0001 |
| Positive | 21 | 27.41 | 17.26-37.56 | 12.6 | |
| Tumor size (cm) | |||||
| ≤ 3 | 95 | 70.94 | 65.97-75.92 | 80.7 | < 0.0001 |
| > 3 | 99 | 39.02 | 32.23-45.81 | 34.6 | |
| Differentiation | |||||
| Well | 18 | 65.58 | 59.43-71.73 | 94.4 | < 0.0001 |
| Moderate | 55 | 43.11 | 35.16-51.06 | 54.4 | |
| Poor | 52 | 37.91 | 30.28-45.54 | 33.6 | |
| Signet ring cell | 69 | 62.16 | 54.89-69.43 | 67.9 | |
| Depth of invasion | |||||
| T1 | 47 | 79.53 | 75.46-83.60 | 95.7 | < 0.0001 |
| T2 | 37 | 71.92 | 64.56-79.28 | 80.6 | |
| T3 | 101 | 40.04 | 33.53-46.54 | 35.4 | |
| T4 | 9 | 10.57 | 5.94-15.21 | 0.0 | |
| Lymph node metastasis | |||||
| N0 | 88 | 73.64 | 69.17-78.11 | 83.8 | < 0.0001 |
| N1 | 47 | 57.67 | 47.75-67.59 | 64.9 | |
| N2 | 23 | 31.61 | 21.57-41.65 | 23.9 | |
| N3 | 36 | 15.39 | 10.83-19.95 | 0.0 | |
| Vascular invasion | |||||
| No | 168 | 60.36 | 55.48-65.23 | 65.4 | < 0.0001 |
| Yes | 26 | 17.74 | 11.39-24.08 | 4.4 | |
| Lymphatic invasion | |||||
| No | 104 | 70.58 | 65.84-75.33 | 79.2 | < 0.0001 |
| Yes | 90 | 36.48 | 29.43-43.53 | 32.1 | |
| Perineural invasion | |||||
| No | 109 | 67.57 | 62.27-72.88 | 76.3 | < 0.0001 |
| Yes | 84 | 37.36 | 30.70-44.02 | 34.1 | |
| Desmoplastic reaction | |||||
| None | 28 | 67.55 | 56.87-78.24 | 78.6 | < 0.0001 |
| Mild | 64 | 70.20 | 64.03-76.37 | 78.7 | |
| Moderate | 75 | 41.99 | 34.35-49.62 | 38.8 | |
| Marked | 27 | 36.67 | 24.49-48.85 | 36.7 | |
| TNM stage | |||||
| I | 64 | 78.86 | 75.66-82.07 | 92.1 | < 0.0001 |
| II | 44 | 66.55 | 58.17-74.92 | 74.2 | |
| III | 38 | 46.52 | 36.06-56.98 | 45.7 | |
| IV | 48 | 14.71 | 10.94-18.48 | 0.0 | |
| CCL-7 | |||||
| Higher | 82 | 45.62 | 37.97-53.28 | 43.6 | 0.002 |
| Equal | 69 | 60.09 | 52.62-67.57 | 64.7 | |
| Lower | 43 | 63.28 | 54.24-72.32 | 71.8 | |
| CCL-8 | |||||
| Higher | 58 | 51.05 | 42.41-59.70 | 50.0 | 0.269 |
| Equal | 64 | 51.89 | 43.39-60.39 | 57.4 | |
| Lower | 72 | 59.97 | 52.60-67.34 | 64.6 | |
| CCL-21 | |||||
| Higher | 87 | 44.96 | 37.83-52.10 | 45.8 | 0.001 |
| Equal | 63 | 56.60 | 48.30-64.90 | 59.5 | |
| Lower | 44 | 70.58 | 63.24-77.93 | 79.2 | |
| Factors | Hazard ratio | 95% CI | P value |
| upper-lower | |||
| Depth of invasion | |||
| T2/T1 | 7.850 | 1.454-42.390 | 0.017 |
| T3/T1 | 23.200 | 4.733-113.716 | 0.000 |
| T4/T1 | 65.052 | 10.830-390.730 | < 0.0001 |
| Lymph node metastasis | |||
| N1/N0 | 1.856 | 0.865-3.982 | 0.112 |
| N2/N0 | 3.520 | 1.597-7.758 | 0.002 |
| N3/N0 | 7.227 | 3.349-15.596 | < 0.0001 |
| Desmoplastic reaction | |||
| Mild/none | 3.663 | 1.272-10.638 | 0.016 |
| Moderate/none | 3.623 | 1.304-10.101 | 0.014 |
| Marked/none | 4.926 | 1.590-15.152 | 0.006 |
| CCL-7 | 0.801 | ||
| CCL-8 | 0.620 | ||
| CCL-21 | 0.084 | ||
In this study, CCL7, CCL8 and CCL21 expression levels were examined in 194 cases of gastric cancer for correlation with patient clinicopathologic factors. We found that the higher expression of CCL7 and CCL21 in cancer tissues than in normal tissues was significantly correlated with advanced depth of wall invasion, lymph node metastasis and higher TNM stage. The mechanism for chemokine ligand promotion of tumor invasion and metastasis is not clear. Using a model of colorectal tumor progression, Kitamura et al[12] showed that tumor-stromal interaction could promote tumor invasion. The colonic tumor can promote the production of CCL9. Increased levels of CCL9 recruited immature myeloid cells that carry the CCL9 receptor CCR1 from the blood to the tumor invasion front. The immature myeloid cells produce MMP2 and MMP9 and help the tumor epithelium to migrate and invade into the stroma. Jung et al[4] also showed the importance of tumor-stromal crosstalk in invasion of oral squamous cell carcinoma (OSCC) via CCL7[4]. To identify key molecular regulators expressed by carcinoma-associated fibroblasts (CAF) that promote cancer cell invasion, Jung et al[4] used microarrays to compare cocultured OSCC and CAF with monoculture controls. Microarray and real-time polymerase chain reaction analysis identified marked upregulation of CCL7 in cocultured CAF. Enzyme-linked immunosorbent assay showed an elevated level of CCL7 secretion from CAF stimulated by coculture with OSCC cells. CCL7 promoted the invasion and migration of OSCC cells, and the invasiveness was inhibited by treatment with CCL7 neutralizing antibody.
However, other studies have shown that CC chemokine ligands promote T cells to kill the tumor cells. Wu et al[16] investigated the effect of exogenous CCL21 expressed in breast cancer MCF-7 cells on human monocyte-derived dendritic cells (DCs). Stimulation of CCL21-transfected MCF-7 cells prompted DC function: migration, antigen uptake and presentation. The stimulated DCs facilitated Th 1 type cytokine production, perforin-forming CD8+ T cell transformation and final T cell-associated clearance of MCF-7 cells. Wetzel et al[17] showed that human CCL7 can reduce tumorigenicity and augment infiltration of dendritic cells and neutrophils toward mouse mastocytoma; it also inhibits mouse melanoma growth through activation of T lymphocytes and natural killer cells.
The differences between the expression of CCL7 and CCL21 correlated with clinicopathologic parameters were gender and lymphatic invasion. The overexpression of CCL7 in gastric cancer was not correlated with gender and lymphatic invasion, but that of CCL21 was correlated with these two parameters. The overexpression of CCL21 was significantly higher in females than in males. The reason for the significance is not clear and more studies are necessary to clarify the significance. The overexpression of CCL21 was also correlated with lymphatic invasion. Recently, metastatic gastric carcinoma cells have been shown to express the receptor for chemokine CCL21, chemokine receptors CCR7, a property that may allow them to access the lymphatic system and spread to regional lymph nodes[18]. Thus the “chemoattraction” theory of metastasis may be reflected by malignant cells expressing functional chemokine receptors that can respond to organ-specific chemoattractant molecules and migrate directionally along chemokine gradients to set up site-specific metastases in the target organs. Such chemotactic migration of tumors would mirror the physiologic mechanisms of lymphocyte homing into lymphoid organs.
Kaplan-Meier survival analysis revealed that CCL7 and CCL21 overexpression in cancer tissues was correlated with poor prognosis. If tumor-infiltrating leukocytes are able, in some instances, to promote cancer, then the local production of chemokines that attract leukocytes could be a poor prognostic sign. This is the case in human breast cancer, where levels of CCL5 and CCL2 correlate with tumor progression and there is a positive correlation between the extent of the macrophage infiltrate, lymph-node metastasis and clinical aggressiveness[19-21]. In esophageal squamous cell carcinoma, CCL2 expression has been associated with the extent of macrophage infiltration, tumor cell invasion and tumor vascularity[22].
In conclusion, the higher expression of CCL7 and CCL21 in gastric cancer tissues than in normal tissues was significantly correlated with advanced depth of wall invasion, lymph node metastasis and higher TNM stage. Moreover, Kaplan-Meier survival analysis revealed that CCL7 and CCL21 overexpression in cancer tissues was correlated with poor prognosis. These results suggest that overexpression of these two CC chemokine ligands is associated with tumor metastasis and serves as a prognostic factor in patients with gastric cancer.
Gastric cancer is one of the commonest malignant tumors of the alimentary tract and is characterized by late clinical presentation, rapid progression, and poor survival. The reason for this poor prognosis is that, at the time of diagnosis, gastric cancer usually shows extensive local tumor invasion and frequent spread to metastatic sites, particularly lymph nodes. Spread of malignant tumors is a multistep process and many of the stages of tumor invasion require degradation or breakdown of the extracellular matrix and connective tissue surrounding tumor cells.
The matrix metalloproteinases (MMPs) are a family of zinc containing enzymes which are involved in the degradation of different components of the extracellular matrix, and there is considerable evidence to indicate that individual MMPs have important roles in tumor invasion and tumor spread. A recent study showed that increased levels of chemokine ligand (CCL) recruit immature myeloid cells that carry chemokine ligand receptor from the blood to the tumor invasion front. These immature myeloid cells produced MMP9 and MMP2 and help the tumor cells to migrate and invade.
In the present study, the authors used immunohistochemistry to examine the expression of CCL7, CCL8 and CCL21 in 194 gastric cancer samples and adjacent normal tissues. The authors analyzed their correlation with tumor metastasis, clinicopathologic parameters and clinical outcome. They found that the higher expression of CCL7 and CCL21 in cancer tissues than in normal tissues was significantly correlated with advanced depth of wall invasion, lymph node metastasis and higher tumor node metastasis (TNM) stage. Moreover, Kaplan-Meier survival analysis revealed that CCL7 and CCL21 overexpression in cancer tissues was correlated with poor prognosis.
These results suggest that overexpression of CCL7 and CCL21 is associated with tumor metastasis and serves as a prognostic factor in patients with gastric cancer.
The authors used immunohistochemistry to examine the expression of CCL7, CCL8 and CCL21 in 194 gastric cancer samples and adjacent normal tissues. They found that the higher expression of CCL7 and CCL21 in cancer tissues than in normal tissues was significantly correlated with advanced depth of wall invasion, lymph node metastasis and higher TNM stage. Moreover, Kaplan-Meier survival analysis revealed that CCL7 and CCL21 overexpression in cancer tissues was correlated with poor prognosis.
Peer reviewer: Richard Hu, MD, MSc, Division of Gastroenterology, Department of Medicine, Olive view-UCLA Medical Center, 14445 Olive View Drive, Los Angeles, CA 91342, United States
S- Editor Tian L L- Editor O’Neill M E- Editor Li JY
| 1. | Baggiolini M, Dewald B, Moser B. Human chemokines: an update. Annu Rev Immunol. 1997;15:675-705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1634] [Cited by in RCA: 1633] [Article Influence: 58.3] [Reference Citation Analysis (0)] |
| 3. | Kelner GS, Kennedy J, Bacon KB, Kleyensteuber S, Largaespada DA, Jenkins NA, Copeland NG, Bazan JF, Moore KW, Schall TJ. Lymphotactin: a cytokine that represents a new class of chemokine. Science. 1994;266:1395-1399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 479] [Cited by in RCA: 458] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 4. | Jung DW, Che ZM, Kim J, Kim K, Kim KY, Williams D, Kim J. Tumor-stromal crosstalk in invasion of oral squamous cell carcinoma: a pivotal role of CCL7. Int J Cancer. 2010;127:332-344. [PubMed] |
| 5. | Liu Y, Ji R, Li J, Gu Q, Zhao X, Sun T, Wang J, Li J, Du Q, Sun B. Correlation effect of EGFR and CXCR4 and CCR7 chemokine receptors in predicting breast cancer metastasis and prognosis. J Exp Clin Cancer Res. 2010;29:16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 105] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 6. | Morson BC, Dawson IMP, Day DW. Morson and Dawson’s gastrointestinal pathology. 3rd ed. Oxford: Blackwell Science 1990; 53-70. |
| 7. | Hart IR, Saini A. Biology of tumour metastasis. Lancet. 1992;339:1453-1457. [RCA] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 235] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 8. | Kohn EC, Liotta LA. Molecular insights into cancer invasion: strategies for prevention and intervention. Cancer Res. 1995;55:1856-1862. [PubMed] |
| 9. | Murphy G, Docherty AJ. The matrix metalloproteinases and their inhibitors. Am J Respir Cell Mol Biol. 1992;7:120-125. [PubMed] |
| 10. | Stetler-Stevenson WG, Liotta LA, Kleiner DE. Extracellular matrix 6: role of matrix metalloproteinases in tumor invasion and metastasis. FASEB J. 1993;7:1434-1441. [PubMed] |
| 11. | Davies B, Waxman J, Wasan H, Abel P, Williams G, Krausz T, Neal D, Thomas D, Hanby A, Balkwill F. Levels of matrix metalloproteases in bladder cancer correlate with tumor grade and invasion. Cancer Res. 1993;53:5365-5369. [PubMed] |
| 12. | Kitamura T, Taketo MM. Keeping out the bad guys: gateway to cellular target therapy. Cancer Res. 2007;67:10099-10102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 13. | Schraml P, Bucher C, Bissig H, Nocito A, Haas P, Wilber K, Seelig S, Kononen J, Mihatsch MJ, Dirnhofer S. Cyclin E overexpression and amplification in human tumours. J Pathol. 2003;200:375-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 113] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 14. | Ravn V, Havsteen H, Thorpe SM. Immunohistochemical evaluation of estrogen and progesterone receptors in paraffin-embedded, formalin-fixed endometrial tissues: comparison with enzyme immunoassay and immunohistochemical analysis of frozen tissue. Mod Pathol. 1998;11:709-715. [PubMed] |
| 15. | Ravn V, Rasmussen BB, Højholt L, Barfoed M, Heiberg I, Thorpe SM. Reproducibility of subjective immunohistochemical estrogen- and progesterone receptor determination in human endometrium. Pathol Res Pract. 1993;189:1015-1022. [PubMed] |
| 16. | Wu S, Xing W, Peng J, Yuan X, Zhao X, Lei P, Li W, Wang M, Zhu H, Huang B. Tumor transfected with CCL21 enhanced reactivity and apoptosis resistance of human monocyte-derived dendritic cells. Immunobiology. 2008;213:417-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 17. | Wetzel K, Struyf S, Van Damme J, Kayser T, Vecchi A, Sozzani S, Rommelaere J, Cornelis JJ, Dinsart C. MCP-3 (CCL7) delivered by parvovirus MVMp reduces tumorigenicity of mouse melanoma cells through activation of T lymphocytes and NK cells. Int J Cancer. 2007;120:1364-1371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 18. | Mashino K, Sadanaga N, Yamaguchi H, Tanaka F, Ohta M, Shibuta K, Inoue H, Mori M. Expression of chemokine receptor CCR7 is associated with lymph node metastasis of gastric carcinoma. Cancer Res. 2002;62:2937-2941. [PubMed] |
| 19. | Azenshtein E, Luboshits G, Shina S, Neumark E, Shahbazian D, Weil M, Wigler N, Keydar I, Ben-Baruch A. The CC chemokine RANTES in breast carcinoma progression: regulation of expression and potential mechanisms of promalignant activity. Cancer Res. 2002;62:1093-1102. [PubMed] |
| 20. | Saji H, Koike M, Yamori T, Saji S, Seiki M, Matsushima K, Toi M. Significant correlation of monocyte chemoattractant protein-1 expression with neovascularization and progression of breast carcinoma. Cancer. 2001;92:1085-1091. [PubMed] |
| 21. | Luboshits G, Shina S, Kaplan O, Engelberg S, Nass D, Lifshitz-Mercer B, Chaitchik S, Keydar I, Ben-Baruch A. Elevated expression of the CC chemokine regulated on activation, normal T cell expressed and secreted (RANTES) in advanced breast carcinoma. Cancer Res. 1999;59:4681-4687. [PubMed] |
| 22. | Ohta M, Kitadai Y, Tanaka S, Yoshihara M, Yasui W, Mukaida N, Haruma K, Chayama K. Monocyte chemoattractant protein-1 expression correlates with macrophage infiltration and tumor vascularity in human esophageal squamous cell carcinomas. Int J Cancer. 2002;102:220-224. [PubMed] |