Published online Mar 21, 2012. doi: 10.3748/wjg.v18.i11.1243
Revised: May 1, 2011
Accepted: May 8, 2011
Published online: March 21, 2012
AIM: To investigate whether, under the influence of polypectomy, the incidence of adenoma decreases with age.
METHODS: Consecutive patients with colonic adenomas identified at index colonoscopy were retrospectively selected if they had undergone three or more complete colonoscopies, at least 24 mo apart. Patients who had any first-degree relative with colorectal cancer were excluded. Data regarding number of adenomas at each colonoscopy, their location, size and histological classification were recorded. The monthly incidence density of adenomas after the index examination was estimated for the study population, by using the person-years method. Baseline adenomas were excluded from incidence calculations but their characteristics were correlated with recurrence at follow-up, using the χ2 test.
RESULTS: One hundred and fifty-six patients were included (109 male, mean age at index colonoscopy 56.8 ± 10.3 years), with follow-up that ranged from 48 to 232 mo. No significant correlations were observed between the number, the presence of villous component, or the size of adenomas at index colonoscopy and the presence of adenomas at subsequent colonoscopies (P = 0.49, 0.12 and 0.78, respectively). The incidence of colonic adenomas was observed to decay from 1.4% person-months at the beginning of the study to values close to 0%, at 12 years after index colonoscopy.
CONCLUSION: Our results suggest the sporadic formation of adenomas occurs within a discrete period and that, when these adenomas are removed, all neoplasia-prone clones may be extinguished.
- Citation: Rosa I, Fidalgo P, Soares J, Vinga S, Oliveira C, Silva JP, Ferro SM, Chaves P, Oliveira AG, Leitão CN. Adenoma incidence decreases under the effect of polypectomy. World J Gastroenterol 2012; 18(11): 1243-1248
- URL: https://www.wjgnet.com/1007-9327/full/v18/i11/1243.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i11.1243
It has long been believed that initiation of sporadic colo-rectal cancer (CRC) increases over time, due to toxicity or loss of fidelity of DNA replication. Therefore, it is believed that the risk of adenoma formation is a function of age[1], which justifies lifelong colonoscopy surveillance. However, current evidence has started to challenge these perceptions.
The data that have suggested that incidence of adenoma increases with age were mostly collected from autopsy studies[2,3]. Although relevant, these studies are prone to bias because the age-specific incidence rates do not adequately represent the time trend for newly formed lesions after removal of index lesions. Moreover, the rather important information of family aggregation of CRC was lacking in all of them.
More recent studies, based on surveillance colonoscopies[4-6], have been mainly limited by short follow-up (3-5 years), which may lead to the inclusion, as new adenomas, of polyps that were missed at a previous examination. These time intervals are also likely to be too short to predict the lifelong dynamics of adenoma formation.
The notion that the formation of colon adenomatous polyps peaks at a certain age and then rapidly declines was first presented in 1975 by Henry et al[7]. He stated that: “some undefined stimulus to continuous polyp formation persists for up to 4 years in about one-third of patients who develop a colonic polyp. Thereafter, either the stimulus to neoplasia is no longer present, or the colonic mucosa adapts so that polyp formation does not persist.” We propose that this undefined stimulus corresponds to the presence of a limited number of mutated clones that are scattered in the colonic epithelium and evolve through cumulative critical gene events selected over several decades. This sequence of events ends at a specific time window, in which most adenomas become endoscopically detectable and after which its incidence declines.
A study in Germany[8] has shown that individuals with negative findings at colonoscopy had a reduced risk of CRC for at least 20 years. Furthermore, when the examination was performed at 55-64 years of age and older, the risk of CRC was even lower. Additionally, according to the latest Guidelines for Colonoscopy Surveillance after Polypectomy issued by the American Gastroenterological Association (AGA)[9], age is not considered to be a reliable predictor of subsequent advanced adenomas.
Taking these data into account and considering the possibility of only a limited time window for sporadic adenoma expression, we aimed to study the temporal trend for adenoma formation, in a standard-risk population under colonoscopic surveillance. According to our hypothesis, which stated that a discrete number of mutated clones were scattered in the colon, we expected the adenoma incidence to decrease under the effect of polypectomy.
Consecutive patients from three Portuguese hospitals (a tertiary oncology center, a tertiary general hospital and a regional hospital) with colonic adenomas who were identified at an index colonoscopy were retrospectively reviewed. These patients were included if they had undergone three or more complete colonoscopies that were at least 24 mo apart. The index colonoscopies were performed between 1978 and 2000, for screening or diagnostic purposes. The subsequent colonoscopies were performed according to the assistant physicians’ choice, based on surveillance guidelines that changed during the time of the study (data were collected until 2007). Only colonoscopies reaching the cecum, with adequate bowel preparation and complete removal of all of the identified polyps were considered for inclusion in the present study.
The patient files were reviewed, and the patients were excluded if they had a previous history of CRC or adenomas, inflammatory bowel disease, hereditary non-polyposis CRC, familial adenomatous polyposis syndrome, a family history of CRC in any first-degree relative, or CRC at the index colonoscopy.
The location and size of all of the polyps had been recorded and the specimens had been sent for pathological evaluation and were classified according to the criteria of the World Health Organization[10]. All of the colonoscopies were performed by certified gastroenterologists, and sedation using intravenous midazolam (with or without pethidine association) or intravenous propofol (performed by an anesthesiologist) was administered on a case-to-case basis. The bowel preparation methods varied among the centers and over time, but were based on oral solutions that contained polyethylene glycol or senna.
The endoscopic and pathological reports were reviewed by the authors, for the results of the index colonoscopy, and of each colonoscopy reported thereafter. The left colon was defined as the splenic flexure and the segment distal to it.
The baseline adenoma characteristics were recorded and correlated with recurrence of adenomas at follow-up. However, these adenomas were specifically excluded from incidence ratio evaluation, because the time frame for their formation was unknown.
The data regarding number of adenomas observed at each colonoscopy, along with their location, size and histological classification were recorded. The analysis of adenoma incidence over time was based on the person-years method. We assumed that the incidence rate of adenomas was constant between two consecutive colonoscopies in each individual and, in that time interval, the monthly incidence of adenomas in each patient was estimated by dividing the number of adenomas found at colonoscopy by the number of months that had elapsed since the previous examination. The incidence of adenomas in a given month after the index colonoscopy was determined for the entire sample by summing the incidence for that month across all of the patients that had been observed up to that time. The monthly incidence density of adenomas was then obtained by dividing the estimated incidence in that month by the population that was still at risk.
The statistical analysis was performed using Excel XP (Microsoft Inc) and Stata 10.0 (Stata Corporation, College Station, TX, United States). The baseline adenoma characteristics were correlated with recurrence at follow-up using the χ2 test.
The present study was a retrospective observational study, in which no experimental intervention was used, and all of the data were kept anonymous. Therefore according to the local regulations, no approval by the Ethics Committee was necessary. All of the patients signed an informed consent document before each endoscopic examination.
The present study included a total of 156 patients (109 male and 47 female). The mean age at the time of the index colonoscopy was 56.8 ± 10.3 years. No procedural complications were recorded. All 156 patients underwent three colonoscopies and 44 of them underwent a fourth examination. The outcomes of these colonoscopies are summarized in Table 1. The index colonoscopy was performed for screening in 31 patients and for diagnostic purposes in the remaining patients (and the symptoms were considered to be unrelated to the adenomas in the majority of cases).
| Outcomes | Mean | SD |
| Index colonoscopy: 156 patients studied | ||
| Adenomas | 1.68 | 1.01 |
| Tubular adenomas | 1.49 | 1.13 |
| Tubulo-villous/villous adenomas | 0.14/0.05 | 0.35/0.22 |
| Size of the larger adenoma (mm) | 15.51 | 10.36 |
| Adenomas in the left colon/remaining colon | 1.34/0.35 | 0.93/0.69 |
| Second colonoscopy: 156 patients studied (41.6 ± 21.0 mo since the first) | ||
| Adenomas | 0.54 | 0.86 |
| Tubular adenomas | 0.51 | 0.85 |
| Tubulo-villous/villous adenomas | 0.02/0.01 | 0.14/0.11 |
| Size of the larger adenoma (mm) | 8.02 | 7.39 |
| Adenomas in the left colon/remaining colon | 0.36/0.17 | 0.67/0.49 |
| Third colonoscopy: 156 patients studied ( 83.7 ± 27.7 mo since the first) | ||
| Adenomas | 0.47 | 0.92 |
| Tubular adenomas | 0.46 | 0.92 |
| Tubulo-villous/villous adenomas | 0.02/0.0 | 0.18/0.0 |
| Size of the larger adenoma (mm) | 6.19 | 4.22 |
| Adenomas in the left colon/remaining colon | 0.21/0.26 | 0.47/0.71 |
| Fourth colonoscopy: 44 patients studied (116.9 ± 34.1 mo since the first) | ||
| Adenomas | 0.32 | 0.74 |
| Tubular adenomas | 0.32 | 0.74 |
| Tubulo-villous/villous adenomas | 0.0/0.0 | 0.0/0.0 |
| Size of the larger adenoma (mm) | 5.78 | 1.92 |
| Adenomas in the left colon/remaining colon | 0.16/0.16 | 0.43/0.48 |
The total number of adenomas and the numbers of each adenoma subtype (according to the histology or location) declined over the course of the four colonoscopies. Additionally, 12 patients underwent a fifth examination (162.3 ± 32.7 mo after the first), and two of these had a sixth examination (211.5 ± 20.5 mo after the first). No adenomas were found in any of these last examinations.
Of the initial 156 patients, who underwent three colonoscopies, 107 presently have scheduled colonoscopies, in agreement with the latest guidelines for surveillance after polypectomy; 27 patients have been released from follow-up due to advanced age or significant comorbidity; two patients have died of unrelated causes; and 20 have been lost from follow-up.
There was no significant correlation between the number of adenomas at the index colonoscopy and the presence or absence of adenomas at the second or all subsequent colonoscopies (P = 0.68 for the second colonoscopy, P = 0.49 for all subsequent colonoscopies). The presence of three or more adenomas at the index colonoscopy did not correlate with the presence of adenomas at the subsequent colonoscopies (P = 0.57 for the second, P = 0.21 for all subsequent colonoscopies), nor did the presence of adenomas > 1 cm at the index colonoscopy (P = 0.43 for the second colonoscopy, P = 0.78 for all subsequent colonoscopies). The presence of adenomas with a villous component at the index colonoscopy correlated with the presence of adenomas at the second colonoscopy (P = 0.01), but there was no significant correlation when all of the subsequent colonoscopies were considered together (P = 0.12) (Tables 2 and 3).
| Index colonoscopy | Second colonoscopy | χ2test | |
| 0 adenomas (n) | ≥1 adenoma (n) | ||
| > 1 adenoma | 39 | 28 | P = 0.24 |
| ≥ 3 adenomas | 14 | 10 | P = 0.57 |
| ≥ 1 TV adenoma | 8 | 14 | P = 0.004 |
| ≥ 1 villous adenoma | 5 | 3 | P = 0.95 |
| ≥ 1 TV or V adenoma | 13 | 17 | P = 0.01 |
| ≥ 1 adenoma > 1 cm | 67 | 42 | P = 0.43 |
| Index colonoscopy | Subsequent colonoscopies | χ2test | |
| 0 adenomas (n) | ≥1 adenoma (n) | ||
| > 1 adenoma | 31 | 36 | P = 0.5 |
| ≥ 3 adenomas | 9 | 15 | P = 0.2 |
| ≥ 1 TV adenoma | 7 | 15 | P = 0.08 |
| ≥ 1 villous adenoma | 4 | 4 | P = 0.97 |
| ≥ 1 TV or V adenoma | 11 | 19 | P = 0.12 |
| ≥ 1 adenoma > 1 cm | 53 | 56 | P = 0.78 |
The presence of adenomas of the left colon at the index colonoscopy did not predict recurrence in the same segment in the second or all subsequent colonoscopies, and the same was true for the right colon (data not shown).
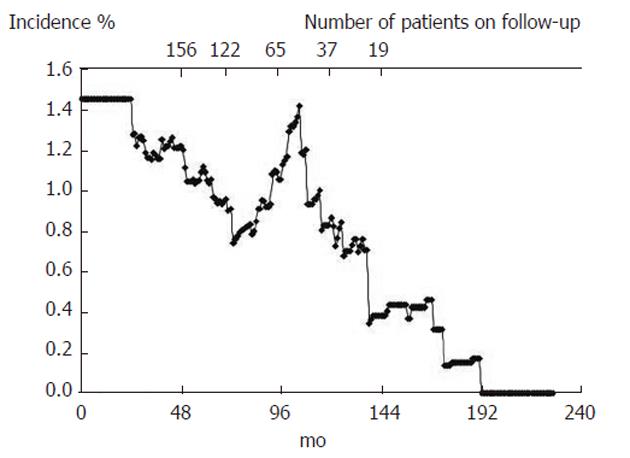
The incidence of colonic adenomas was found to decline from 1.4% person-months, at the beginning of the study, to values close to 0%, 12 years after the index examination (Figure 1).
Eight years after the index colonoscopy (with 65 patients evaluated), a peak in the incidence of adenomas was observed, that approached the baseline values, which was then followed by a steady decline until the end of follow-up (with 37 patients evaluated at 10 years and 19 patients evaluated at 12 years).
There were no reports of flat lesions of the colon in any of these examinations, and there were no colorectal adenocarcinomas reported in these patients during the study period.
To explain the predominant CRC expression in the sixth and seventh decades of life, a necessary sequence of 4-7 known mutations fits a model of a stable mutation clock that ends in full-blown neoplasia at consistent time-intervals. The predominant molecular pathway responsible for CRC begins with the selection of cells with an APC gene loss or a β-catenin mutation, which is followed by the cumulative selection of subsequent critical events in other genes. Estimations of the time taken to acquire such a sequence of mutations suggest that full-blown neoplasia can take several decades to occur. According to recent estimations, to obtain the necessary sequence of mutations, the first event may need to occur at an early age[11-13]; most likely during the exponential phase of embryonic development, when APC is well known to play a key role[14].
In 2007, human colon cancer stem cells were identified by two separate research groups[15,16]. More recently, the location of normal colon stem cells, at the crypt base, has also been demonstrated[17], and these cells seem to be the origin of colon cancer stem cells[18]. It has been proposed that mutations in stem cells are much more likely to occur during the exponential phase of early growth, as opposed to later in life[19].
Accordingly, the results of the present paper strongly suggest that the sporadic formation of adenomas occurs during a limited time period and, when these adenomas are removed, virtually all of the neoplasia-prone clones may be extinguished. If this model is shown to be true, then the concept of a field carcinogenic defect, which progresses with continuous adverse environmental exposure and/or failure of the tight controls that assure DNA replication fidelity, should be replaced by a concept of limited colonic mosaicism. When the endoscopically visible neoplastic expression of this limited mosaicism is removed, the putative carcinogenic impulse no longer compromises colonic epithelia homeostasis.
Our study was limited mainly by its retrospective nature and by the small number of patients reaching longer follow-up. However, this sample was probably representative of the population at risk for sporadic CRC, because it included both symptomatic and asymptomatic individuals who had no relevant family histories, who underwent initial colonoscopy mostly during the sixth decade of life, and who were followed for at least 4 years.
In contrast to traditional beliefs, we did not observe a correlation between baseline adenoma characteristics and the risk of recurrence at follow-up. Although the AGA still takes baseline predictors of future adenomas or cancer into consideration, in their latest Guidelines for Colonoscopy Surveillance After Polypectomy[9], several limitations of the available evidence have been raised. van Stolk et al[20] have reported a study in which the number of adenomas at first colonoscopy was a significant predictor of having recurrent adenomas; however, these authors noted that missed polyps were a possible explanation for this relationship. Furthermore, that previous study only included 4 years of follow-up, and the presence of a family history of CRC was not taken into account at all. In another study, by Martínez et al[21], multiple adenomas at baseline, large adenomas (> 1 cm) or adenomas in the proximal colon were predictors of recurrence. However, the maximum follow-up of that study was 2 years, and the baseline colonoscopy was not the first examination for several of the patients. In addition, and although this relationship was not statistically significant, a family history of CRC in first-degree relatives was associated with a higher risk of recurrence in the study population. The initial National Polyp Study also included a follow-up of only 3 years, and investigated the incidence of colorectal cancer but not adenoma[2,22]. The authors included patients regardless of their family history (other than established genetic syndromes)[2] and they admitted that, as a result of the short follow-up, three of the diagnosed cancers may have been missed polyps[23]. A more recent study, by Martinez et al[24], has revealed that the age of the patient and the number and size of prior adenomas were associated with the risk of advanced colorectal neoplasia after polypectomy. However, that study was also limited by a median follow-up of only 4 years and a maximum follow-up of < 6 years[24]. Our study had the advantages of a minimum follow-up of 4 years and exclusion of patients with any family history of CRC in first-degree relatives. This might explain why we did not find that baseline adenoma characteristics were predictive of recurrence, and why we were able to show a decreasing incidence of adenomas with time, after an age peak and under the influence of polypectomy.
At Digestive Disease Week 2007, Zauber et al presented new data regarding the National Polyp Study population indicating that, after a mean follow-up of 14 years, CRC mortality was markedly reduced in all of the patients with adenomas at baseline, when compared to the general population. This was observed even when patients who refused follow-up were considered in the analysis. This finding supports the hypothesis that the major benefit is derived from the first colonoscopy and also brings into question the notion of increasing recurrence of adenomas over time.
With regard to the unexpected second peak that was observed for the incidence of adenomas in our study, after 8 years, we may speculate whether this peak is related to the type of the second hit in the APC gene. It has been reported that, when the first hit happens close to codon 1300, the second hit is most likely loss of heterozygosis, a faster and more efficient mutation process. In all other cases, point mutation is the most common mechanism for the second hit, thus driving a more sluggish development[25]. This confers different selective advantages to each colonic adenoma, according to the specific first hit-second hit combination and may explain why some adenomas appear earlier and why others are only apparent towards the end of the adenoma time window. We acknowledge that, during a limited period of embryonic development, a small number of stem cells may acquire different first mutations, and that these mutations are expressed along incidence waves during a restricted time window.
The results of the present study reinforce the importance of colonoscopy with polypectomy during the fifth to sixth decades of life, and the feasibility of increasing the time for re-examination after a normal examination. Furthermore, our study indirectly supports the concept of a relatively stable mutation clock, which is possibly initiated during the developmental phase of embryogenesis.
We thank Drs. Sandra Faias, Jaime Midões Correia and Anabela Pinto, from Serviço de Gastrenterologia, Instituto Português de Oncologia de Lisboa Francisco Gentil, EPE and Dr. Celeste Fátima Viveiros, from Serviço de Gastrenterologia, Hospital do Espírito Santo - Évora, EPE for assisting with the acquisition of data.
Colorectal cancer (CRC) and adenomas are thought to have an increasing incidence with age, and this has led to almost lifelong surveillance colonoscopy. This view has recently started to be challenged.
APC is a crucial gene in colorectal carcinogenesis. If the first hit on this gene occurs in a colonic stem cell precursor early in life, it could lead to only a few colon crypts that are prone to originate adenoma and cancer, in a restricted time window. If these clones were removed, by polypectomy, the potential for colon cancer could be erradicated in that individual.
Colon cancer stem cells have recently been identified, apparently originating from colonic stem cells, at the crypt base. Stem cells seem more prone to mutation during embryogenesis than later in life. Reports have shown that a single colonoscopy with polypectomy reduces CRC incidence. The study shows that, under the influence of polypectomy, adenoma incidence decreases with age.
If the study results are confirmed, surveillance colonoscopy intervals may safely be lengthened, and some people may even be released from surveillance after a few examinations.
APC acts as a tumor suppressor gene by regulating the intranuclear concentration of β-catenin, a protein involved in the transcription of genes that promote proliferation. These genes and proteins are part of the Wnt pathway, which is involved in > 80% of sporadic CRC.
The authors do a nice job to expand on a current line of questioning the need for continuing surveillance and indirectly questioning the concept of age appropriateness for surveillance as well as cost-advantageous practice. This is a very relevant study and well written.
Peer reviewer: Scott Steele, MD, FACS, FASCRS, Chief, Colon and Rectal Surgery, Department of Surgery, Madigan Army Medical Center, Fort Lewis, WA 98431, United States
S- Editor Tian L L- Editor Kerr C E- Editor Li JY
| 1. | Rutter CM, Yu O, Miglioretti DL. A hierarchical non-homogenous Poisson model for meta-analysis of adenoma counts. Stat Med. 2007;26:98-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 35] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 2. | Rickert RR, Auerbach O, Garfinkel L, Hammond EC, Frasca JM. Adenomatous lesions of the large bowel: an autopsy survey. Cancer. 1979;43:1847-1857. [PubMed] |
| 3. | Williams AR, Balasooriya BA, Day DW. Polyps and cancer of the large bowel: a necropsy study in Liverpool. Gut. 1982;23:835-842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 334] [Cited by in RCA: 340] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 4. | Neugut AI, Jacobson JS, Ahsan H, Santos J, Garbowski GC, Forde KA, Treat MR, Waye J. Incidence and recurrence rates of colorectal adenomas: a prospective study. Gastroenterology. 1995;108:402-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 80] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 5. | Avidan B, Sonnenberg A, Schnell TG, Leya J, Metz A, Sontag SJ. New occurrence and recurrence of neoplasms within 5 years of a screening colonoscopy. Am J Gastroenterol. 2002;97:1524-1529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 57] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 6. | Yamaji Y, Mitsushima T, Ikuma H, Watabe H, Okamoto M, Kawabe T, Wada R, Doi H, Omata M. Incidence and recurrence rates of colorectal adenomas estimated by annually repeated colonoscopies on asymptomatic Japanese. Gut. 2004;53:568-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 85] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 7. | Henry LG, Condon RE, Schulte WJ, Aprahamian C, DeCosse JJ. Risk of recurrence of colon polyps. Ann Surg. 1975;182:511-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 53] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 8. | Brenner H, Chang-Claude J, Seiler CM, Stürmer T, Hoffmeister M. Does a negative screening colonoscopy ever need to be repeated? Gut. 2006;55:1145-1150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 120] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 9. | Winawer SJ, Zauber AG, Fletcher RH, Stillman JS, O'Brien MJ, Levin B, Smith RA, Lieberman DA, Burt RW, Levin TR. Guidelines for colonoscopy surveillance after polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer and the American Cancer Society. Gastroenterology. 2006;130:1872-1885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 516] [Cited by in RCA: 493] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 10. | Hamilton SR, Aaltonen LA. WHO Classification of Tumours. Pathology and Genetics of Tumours of the Digestive System. Lyon: IARC Press 2000; . |
| 11. | Luebeck EG, Moolgavkar SH. Multistage carcinogenesis and the incidence of colorectal cancer. Proc Natl Acad Sci USA. 2002;99:15095-15100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 239] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 12. | Calabrese P, Mecklin JP, Järvinen HJ, Aaltonen LA, Tavaré S, Shibata D. Numbers of mutations to different types of colorectal cancer. BMC Cancer. 2005;5:126. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 13. | Meza R, Luebeck EG, Moolgavkar SH. Gestational mutations and carcinogenesis. Math Biosci. 2005;197:188-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 14. | Jordan CT, Guzman ML, Noble M. Cancer stem cells. N Engl J Med. 2006;355:1253-1261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1172] [Cited by in RCA: 1189] [Article Influence: 62.6] [Reference Citation Analysis (0)] |
| 15. | O'Brien CA, Pollett A, Gallinger S, Dick JE. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature. 2007;445:106-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2977] [Cited by in RCA: 3048] [Article Influence: 160.4] [Reference Citation Analysis (0)] |
| 16. | Ricci-Vitiani L, Lombardi DG, Pilozzi E, Biffoni M, Todaro M, Peschle C, De Maria R. Identification and expansion of human colon-cancer-initiating cells. Nature. 2007;445:111-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2952] [Cited by in RCA: 3035] [Article Influence: 159.7] [Reference Citation Analysis (0)] |
| 17. | Barker N, Clevers H. Leucine-rich repeat-containing G-protein-coupled receptors as markers of adult stem cells. Gastroenterology. 2010;138:1681-1696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 241] [Cited by in RCA: 274] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 18. | Barker N, Ridgway RA, van Es JH, van de Wetering M, Begthel H, van den Born M, Danenberg E, Clarke AR, Sansom OJ, Clevers H. Crypt stem cells as the cells-of-origin of intestinal cancer. Nature. 2009;457:608-611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1797] [Cited by in RCA: 1667] [Article Influence: 104.2] [Reference Citation Analysis (0)] |
| 19. | Frank SA, Nowak MA. Cell biology: Developmental predisposition to cancer. Nature. 2003;422:494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 70] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 20. | van Stolk RU, Beck GJ, Baron JA, Haile R, Summers R. Adenoma characteristics at first colonoscopy as predictors of adenoma recurrence and characteristics at follow-up. The Polyp Prevention Study Group. Gastroenterology. 1998;115:13-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 118] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 21. | Martínez ME, Sampliner R, Marshall JR, Bhattacharyya AK, Reid ME, Alberts DS. Adenoma characteristics as risk factors for recurrence of advanced adenomas. Gastroenterology. 2001;120:1077-1083. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 174] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 22. | Winawer SJ, Zauber AG, O'Brien MJ, Gottlieb LS, Sternberg SS, Stewart ET, Bond JH, Schapiro M, Panish JF, Waye JD. The National Polyp Study. Design, methods, and characteristics of patients with newly diagnosed polyps. The National Polyp Study Workgroup. Cancer. 1992;70:1236-1245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 23. | Winawer SJ, Zauber AG, Ho MN, O'Brien MJ, Gottlieb LS, Sternberg SS, Waye JD, Schapiro M, Bond JH, Panish JF. Prevention of colorectal cancer by colonoscopic polypectomy. The National Polyp Study Workgroup. N Engl J Med. 1993;329:1977-1981. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3107] [Cited by in RCA: 3126] [Article Influence: 97.7] [Reference Citation Analysis (1)] |
| 24. | Martínez ME, Baron JA, Lieberman DA, Schatzkin A, Lanza E, Winawer SJ, Zauber AG, Jiang R, Ahnen DJ, Bond JH. A pooled analysis of advanced colorectal neoplasia diagnoses after colonoscopic polypectomy. Gastroenterology. 2009;136:832-841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 396] [Cited by in RCA: 427] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 25. | Albuquerque C, Breukel C, van der Luijt R, Fidalgo P, Lage P, Slors FJ, Leitão CN, Fodde R, Smits R. The 'just-right' signaling model: APC somatic mutations are selected based on a specific level of activation of the beta-catenin signaling cascade. Hum Mol Genet. 2002;11:1549-1560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 260] [Cited by in RCA: 282] [Article Influence: 12.3] [Reference Citation Analysis (0)] |