Published online Mar 21, 2012. doi: 10.3748/wjg.v18.i11.1191
Revised: July 6, 2011
Accepted: July 13, 2011
Published online: March 21, 2012
Although most cases of acute nonvariceal gastrointestinal hemorrhage either spontaneously resolve or respond to medical management or endoscopic treatment, there are still a significant number of patients who require emergency angiography and transcatheter treatment. Evaluation with noninvasive imaging such as nuclear scintigraphy or computed tomography may localize the bleeding source and/or confirm active hemorrhage prior to angiography. Any angiographic evaluation should begin with selective catheterization of the artery supplying the most likely site of bleeding, as determined by the available clinical, endoscopic and imaging data. If a hemorrhage source is identified, superselective catheterization followed by transcatheter microcoil embolization is usually the most effective means of successfully controlling hemorrhage while minimizing potential complications. This is now well-recognized as a viable and safe alternative to emergency surgery. In selected situations transcatheter intra-arterial infusion of vasopressin may also be useful in controlling acute gastrointestinal bleeding. One must be aware of the various side effects and potential complications associated with this treatment, however, and recognize the high re-bleeding rate. In this article we review the current role of angiography, transcatheter arterial embolization and infusion therapy in the evaluation and management of nonvariceal gastrointestinal hemorrhage.
- Citation: Walker TG, Salazar GM, Waltman AC. Angiographic evaluation and management of acute gastrointestinal hemorrhage. World J Gastroenterol 2012; 18(11): 1191-1201
- URL: https://www.wjgnet.com/1007-9327/full/v18/i11/1191.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i11.1191
Most cases of acute nonvariceal gastrointestinal hemorrhage resolve spontaneously and of those that do not, the majority respond to conservative medical management measures such as fluid resuscitation, correction of any coagulopathy and administration of blood products[1]. In those cases that are refractory to medical management, endoscopy is the mainstay for diagnosis and treatment. However, there is a subset of patients in whom endoscopic management is ineffective, and imaging evaluation with an accompanying alternative intervention is necessary for bleeding control because of recurrence or massive hemorrhage[1]. Localization and characterization of the bleeding source are important in determining the appropriate intervention, as treatment options range from minimally invasive catheter-directed therapy to extensive surgical resection. Although there has generally been a decline in the number of patients who present with acute gastrointestinal hemorrhage requiring angiography and/or transcatheter intervention, there are still patients who are unresponsive to either medical or endoscopic management and thus require emergency angiographic evaluation and possible transcatheter treatment.
The alimentary system is subdivided into the upper and lower gastrointestinal tracts and thus gastrointestinal hemorrhage is subcategorized according to the location of bleeding. The upper gastrointestinal system extends from the esophagus to the ligament of Treitz, while the latter includes the small bowel, colon and rectum. The distinction is important, as there are some characteristics that are relatively unique to each location and these may affect and determine the therapeutic approach to the particular bleeding source. Thus establishing the specific location of the bleeding as well as the etiology is critical to the treatment of patients with massive or recurrent gastrointestinal hemorrhage. Unfortunately the diagnosis can often be challenging because of the intermittent nature of gastrointestinal bleeding.
The initial management of a patient with acute gastrointestinal hemorrhage should be directed at stabilization through administration of fluids and blood products, correction of coagulation abnormalities, placement of appropriate intravenous access lines and insertion of a nasogastric tube if needed. The vital signs should be closely monitored for signs of active bleeding that may manifest as tachycardia, hypotension and potential hypoxemia. Although persistent melena, hematochezia or hematemesis may provide direct clinical evidence of active hemorrhage, hemodynamic instability despite vigorous resuscitation is the best indicator of active bleeding that may be angiographically demonstrable.
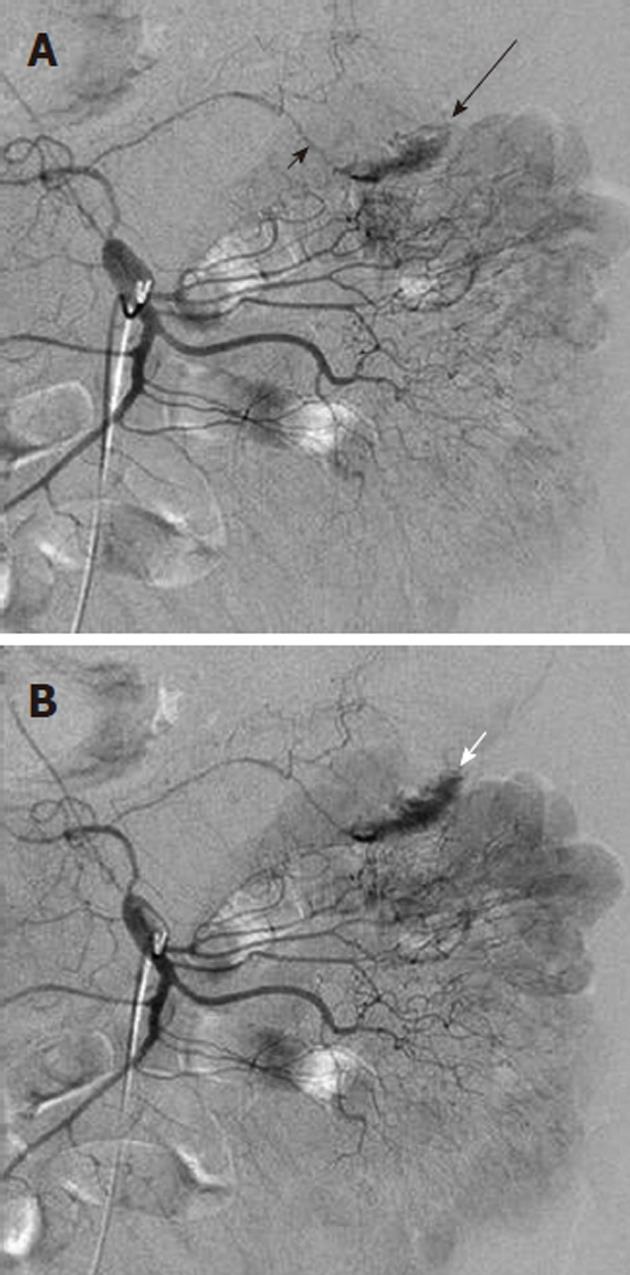
Acute gastrointestinal hemorrhage far more frequently involves an upper than a lower gastrointestinal source, with the former most commonly due to either peptic ulcer disease or gastritis. The most common etiology of lower gastrointestinal hemorrhage, in young adults, is inflammatory bowel disease, while in patients older than 50 years, the cause is diverticulitis (Figure 1) and to a lesser extent, angiodysplasia. The colon is the source of lower gastrointestinal hemorrhage in 80% of individuals, with the bleeding site originating in the ascending colon in one-third, the transverse colon in another one-third, and the remainder in the descending colon and rectosigmoid. A small bowel source of lower gastrointestinal hemorrhage occurs in only 20% of patients.
If an upper gastrointestinal bleeding source seems most likely, endoscopy should be the initial diagnostic study, as the source can usually be identified and treated by the endoscopist. Unfortunately the endoscopic detection and treatment success rates in patients with lower gastrointestinal hemorrhage are generally less than with an upper source, particularly if rapid and significant bleeding is present.
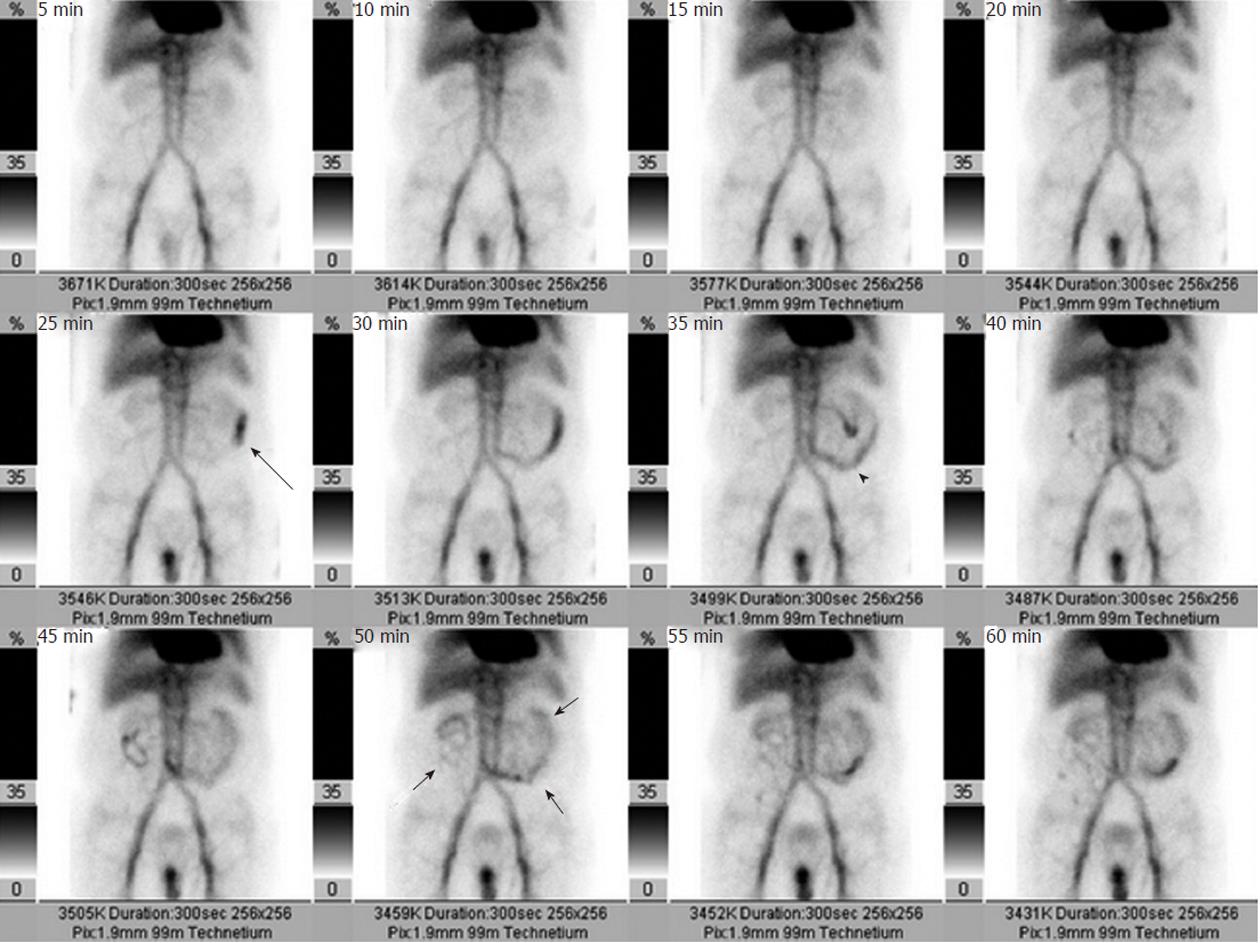
If a bleeding site cannot be identified endoscopically, or if a catheter-based intervention is being considered as a treatment option, obtaining imaging studies may be helpful. In order to successfully demonstrate bleeding by angiography, there must be active ongoing hemorrhage at the time of the examination. Bleeding rates of 0.5-1.0 mL/min have traditionally been considered necessary in order to angiographically demonstrate contrast extravasation[2], but digital subtraction angiography (DSA) may be far more sensitive in detecting active extravasation than was previously thought[3]. Given the well-recognized intermittent nature of gastrointestinal bleeding, many advocate obtaining other imaging before proceeding directly to angiography unless there is massive ongoing hemorrhage or the patient is hemodynamically unstable. Thus a patient may be first evaluated with a radionuclide technetium99m-tagged red blood cell scan, as the nuclear scintigraphy study can demonstrate active bleeding at rates as low as 0.1 mL/min[4-6]. An arteriogram can be obtained following a positive bleeding scan (Figure 2), as a positive scintigram increases the likelihood of a positive angiogram from 22% to 53%[7]. The potential disadvantage of first obtaining a radionuclide study while there is clinical evidence of active bleeding is that prior to angiography the bleeding may cease during the delay necessitated by the nuclear medicine scan. A potentially useful algorithm to consider is to immediately perform angiography in hemodynamically unstable patients, and to first obtain nuclear medicine imaging in hemodynamically stable patients. This approach may potentially decrease the negative angiography examination rate.
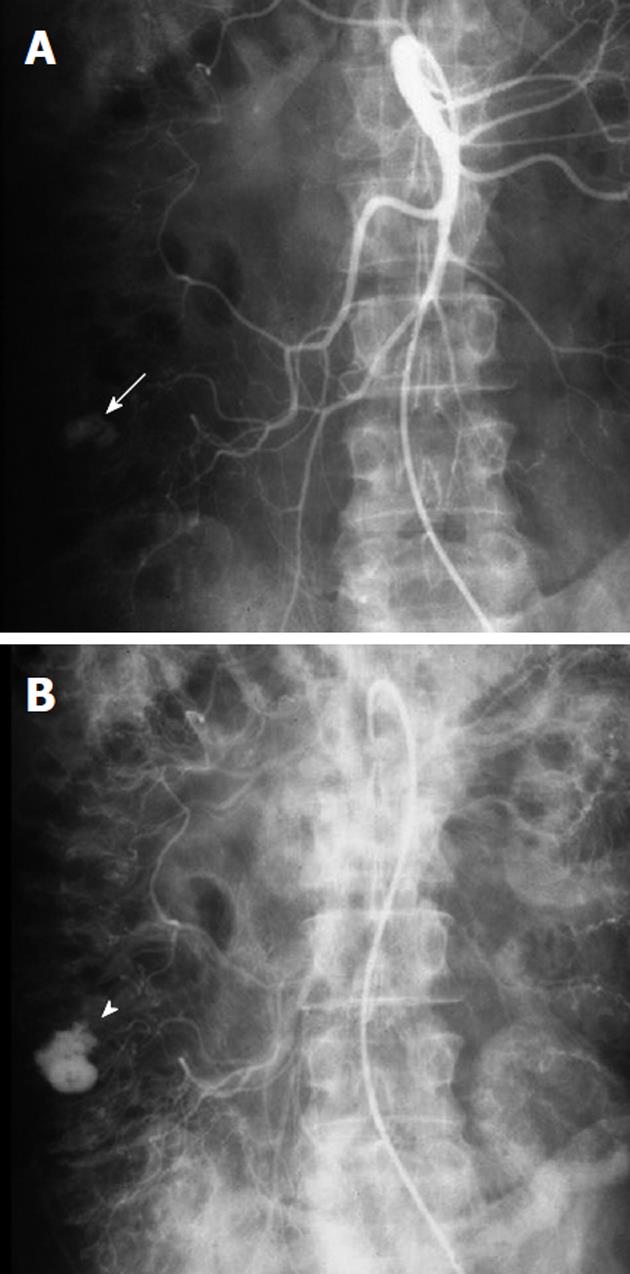
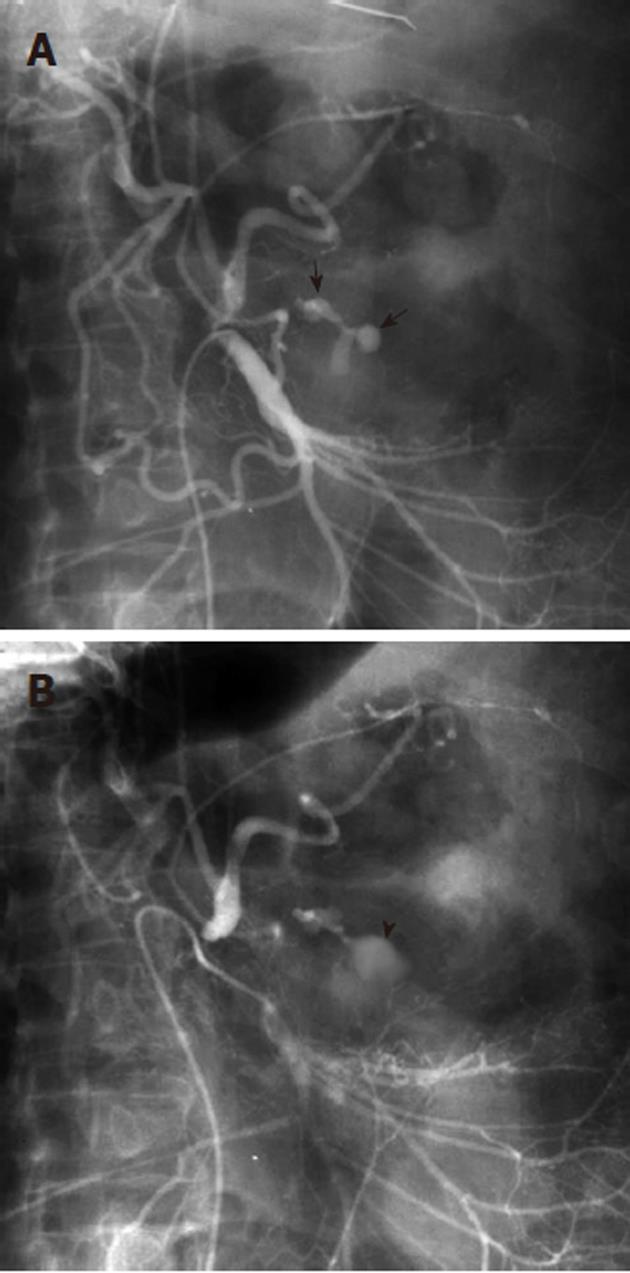
Any angiographic evaluation of a patient with acute gastrointestinal hemorrhage should begin with the selective catheterization of the artery supplying the most likely site of bleeding, as determined by the available clinical, endoscopic and imaging data. Thus for suspected upper gastrointestinal hemorrhage, the celiac artery should first be evaluated (Figure 3), followed by the superior mesenteric artery (SMA), as the latter may contribute to a site of upper gastrointestinal hemorrhage through the pancreaticoduodenal arcade. The lower gastrointestinal tract, however, is the primary territory within the distribution of the SMA. It supplies the small bowel and the ascending and transverse portions of the colon, while the inferior mesenteric artery (IMA) supplies the splenic flexure, descending and sigmoid colonic segments as well as the rectum and anus. An additional arterial supply to the rectosigmoid and anus arises from the internal iliac arteries and this may become a dominant vascular pathway if there is IMA occlusion. The SMA becomes another collateral pathway in the presence of IMA occlusion and may provide the entire arterial supply to the descending and sigmoid colon via either the Arc or Riolan or the marginal artery of Drummond. One should be aware of the numerous mesenteric circulatory variations that may occur in the presence of occlusive disease. Furthermore, congenital variant vascular anatomy must be considered during the angiographic evaluation of gastrointestinal bleeding. For example, the entire middle colic artery may originate from the dorsal pancreatic artery in up to 2% of patients (Figure 4). If this situation exists, a celiac arteriogram may be required when investigating lower gastrointestinal bleeding, in order to fully evaluate the colonic arterial supply, particularly in the presence of negative SMA and IMA arteriograms. If SMA, IMA, celiac, and internal iliac arteriography fail to either localize active hemorrhage or to demonstrate all the mesenteric vascular segments, then variant anatomy must be considered. There are known anomalous vessels that may arise directly from the abdominal aorta, such as an anomalous ileocolic artery or a middle mesenteric artery.
The classic angiographic finding denoting active gastrointestinal bleeding is extravasation of contrast material (Figure 5). Extravasation signifies a breach in the integrity of the arterial wall that permits the angiographic contrast to freely exit from the vessel. Extravasated contrast, therefore, does not have the typical tubular appearance of a vascular structure but instead distributes irregularly and often without a pattern. It may sometimes pool within the gastric rugae or within bowel folds or haustra so that the contrast assumes the appearance of a vein, the “pseudo-vein sign” (Figure 6). This may be differentiated from a true venous structure by the unusual location and appearance, as well as by the persistence beyond the venous phase of the contrast injection. Extravasation must also be differentiated from entities that mimic its appearance, such as a hypervascular bowel mucosa, adrenal gland vascular blush and DSA misregistration artifacts from bowel peristalsis or respiratory motion. There are also angiographic findings other than contrast extravasation that may be seen in certain pathologic conditions and are suggestive of the cause and/or source of the gastrointestinal bleeding. In peptic ulcer disease, for example, small contrast collections may be seen within an ulcer crater, or may outline the gastric or duodenal mucosa. The angiographic demonstration of a gastric ulcer usually requires subselective catheterization of celiac arterial branches such as the left gastric artery, although the bleeding source may also potentially arise from the right gastric artery, the short gastric artery, or either the left or right gastroepiploic arteries. Additionally, the gastroduodenal artery may supply a bleeding pyloric or duodenal ulcer.
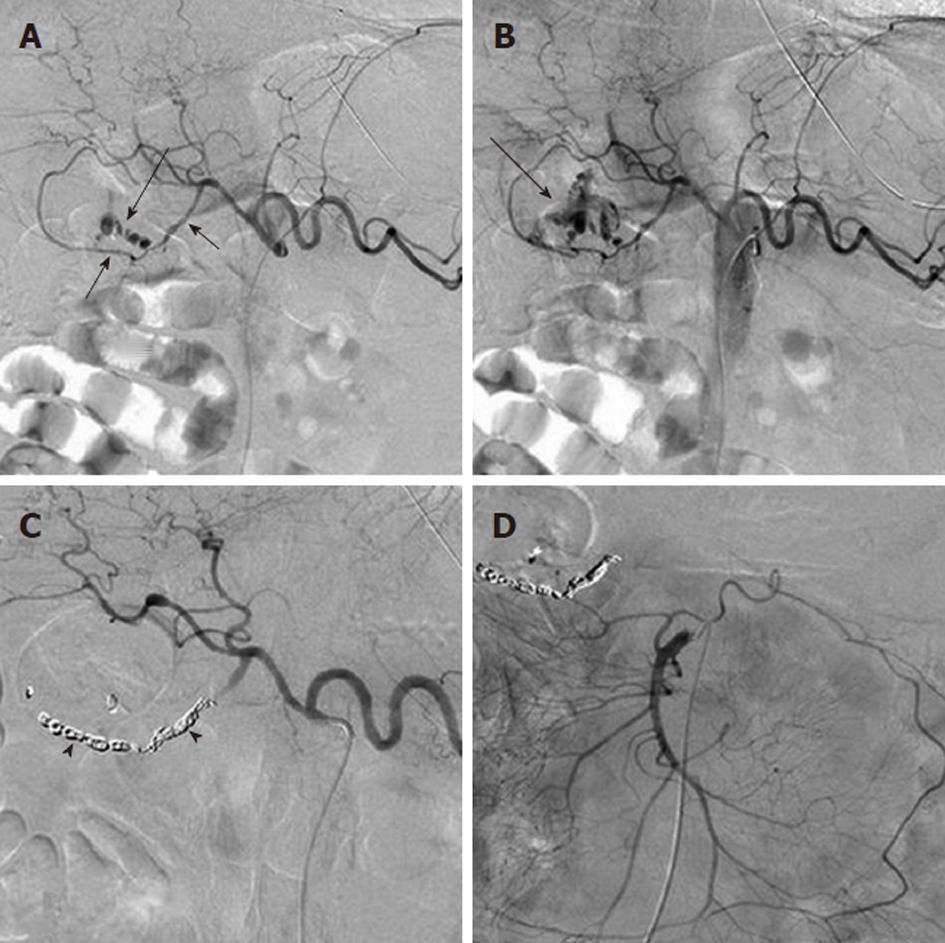
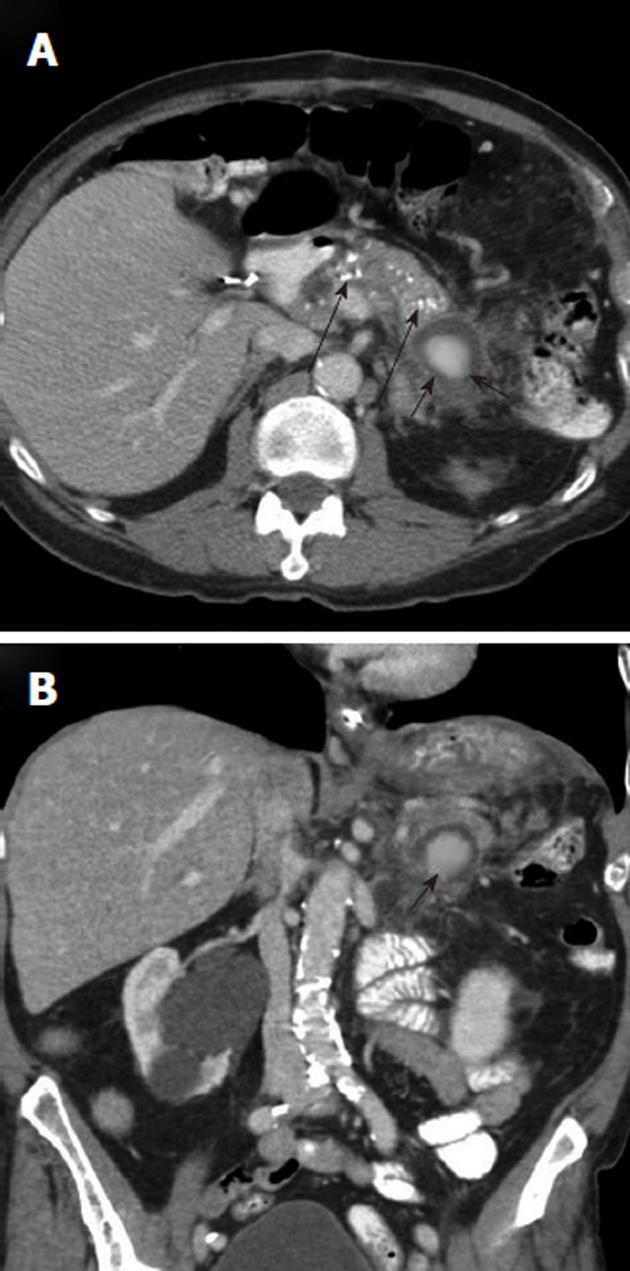
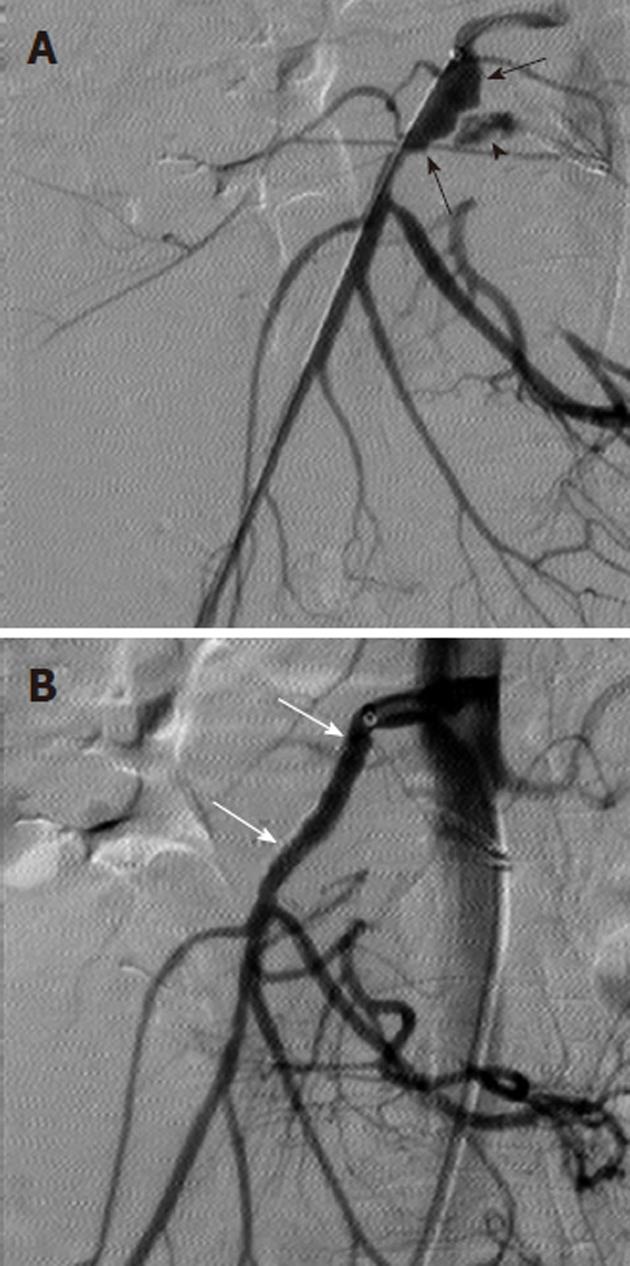
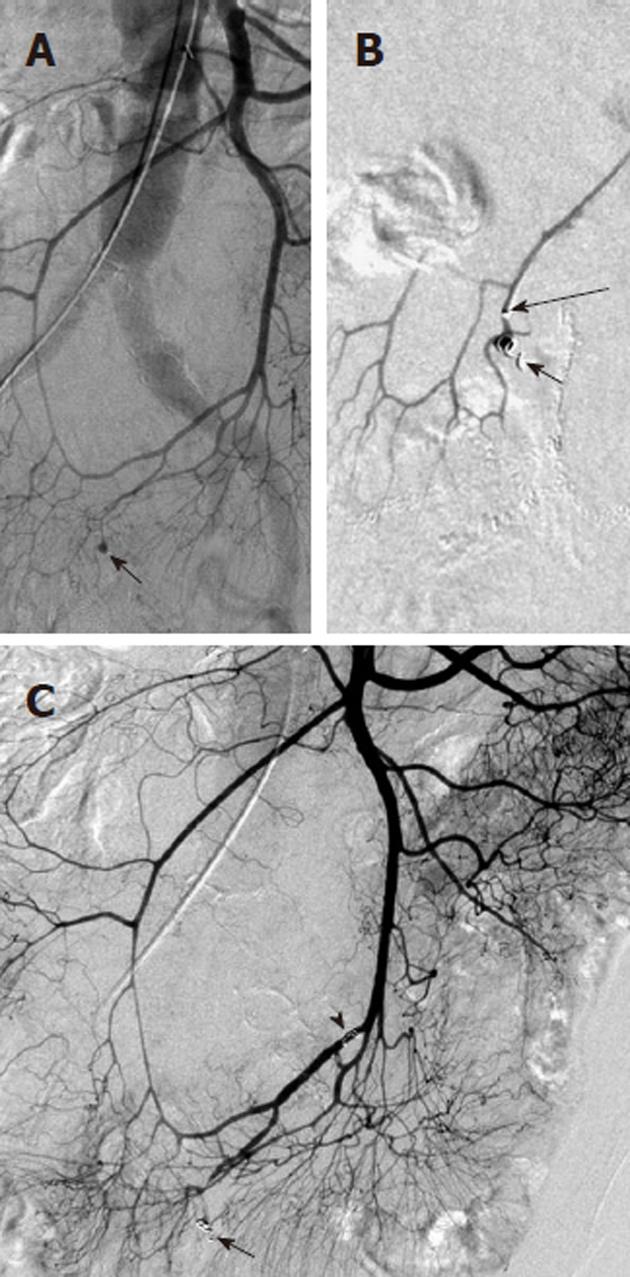
Arterial pseudoaneurysm is another angiographic abnormality that may be identified as a source of gastrointestional hemorrhage. These occur most frequently in patients who have chronic pancreatitis. Hemosuccus pancreaticus refers to bleeding through the pancreatic duct, and may be caused by an arterial pseudoaneurysm that has resulted from chronic exposure of the arterial wall to the inflammatory effects of the pancreatic digestive enzymes[8]. The weakened arterial wall may permit the intermittent bleeding that characterizes hemosuccus pancreaticus, or the pseudoaneurysm may catastrophically rupture and cause acute massive, life-threatening intra-abdominal hemorrhage. Contrast enhanced computed tomographic angiography can be very effective in demonstrating these characteristic pseudoaneurysms (Figure 7) and thus has an increasingly important role in the diagnosis of acute gastrointestinal hemorrhage that is secondary to pancreatitis, while angiographic transcatheter therapy provides the best treatment option in these patients (Figures 8 and 9).
Given both the diffuse nature of the inflammatory process seen in pancreatitis, and the pancreatic arterial supply from both the celiac artery and SMA, pseudoaneurysms may be present involving either or both of these arteries or their branches. The angiographic evaluation must thus include both the celiac and SMA and transcatheter treatment may involve multiple sites. Surgical intervention is very difficult in these patients because of the extensive inflammatory process that characterizes this cause of bleeding (Figure 10).
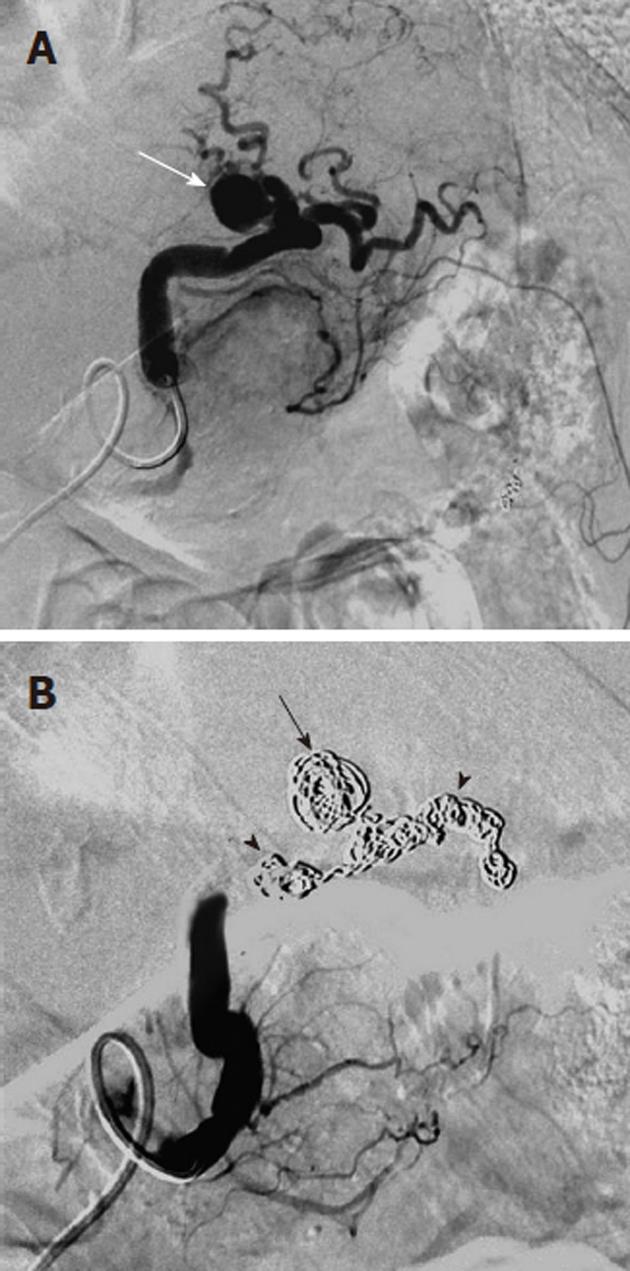
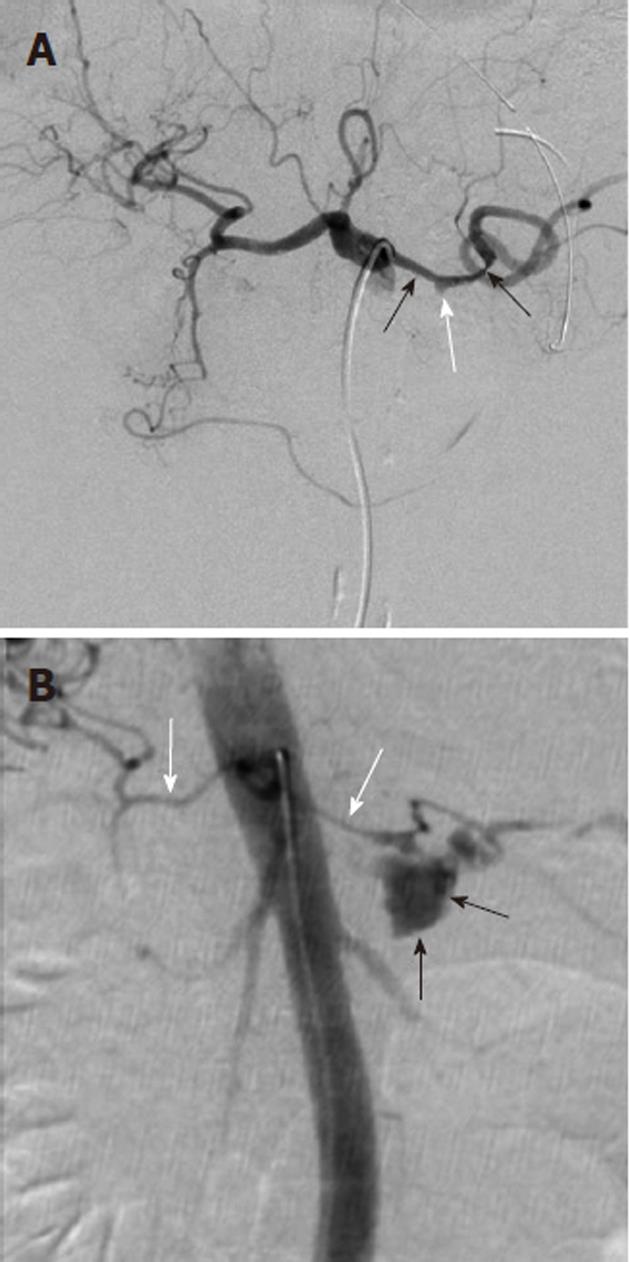
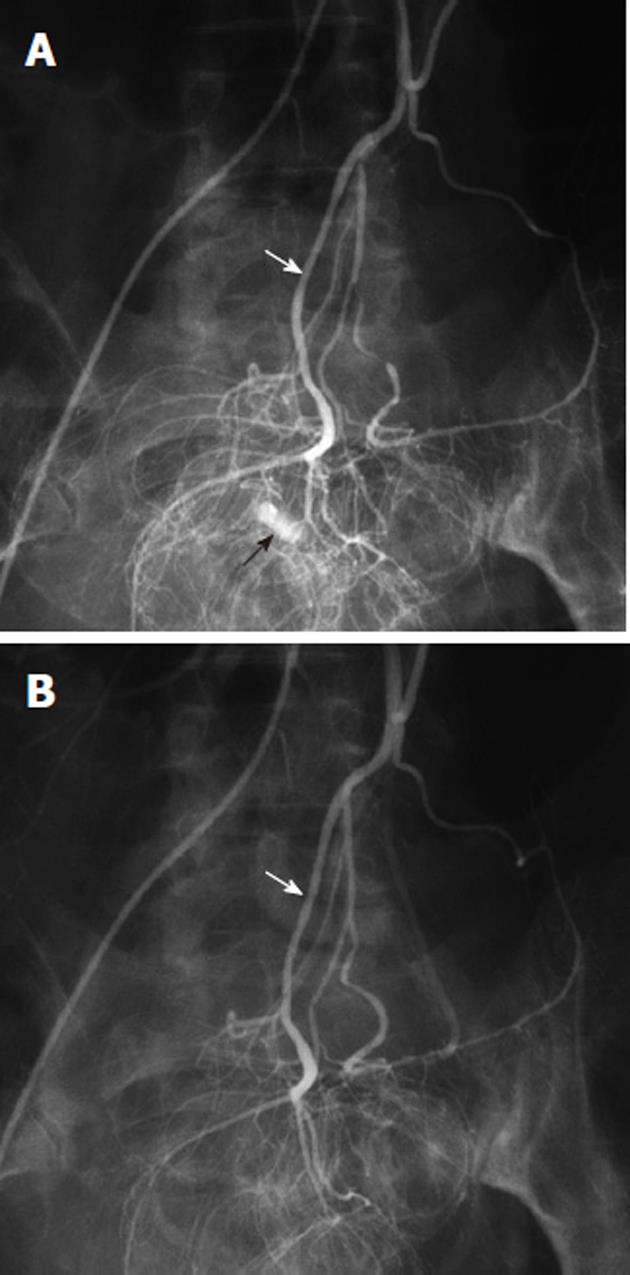
Arterial pseudoaneurysms may also be seen in patients with chronic occlusive disease of the celiac artery, and involve the pancreaticoduodenal arteries (Figure 11). These pseudoaneurysms are rupture-prone and may cause massive acute upper gastrointestinal hemorrhage. Because of the occlusion of the origin of the celiac artery, the angiographic evaluation involves the selective catheterization of the SMA, in order to image the pancreaticoduodenal arcade. Any intervention, such as coil embolization, must typically also be performed via an SMA access, although there are cases in which a percutaneous route has been used to treat such an abnormality.
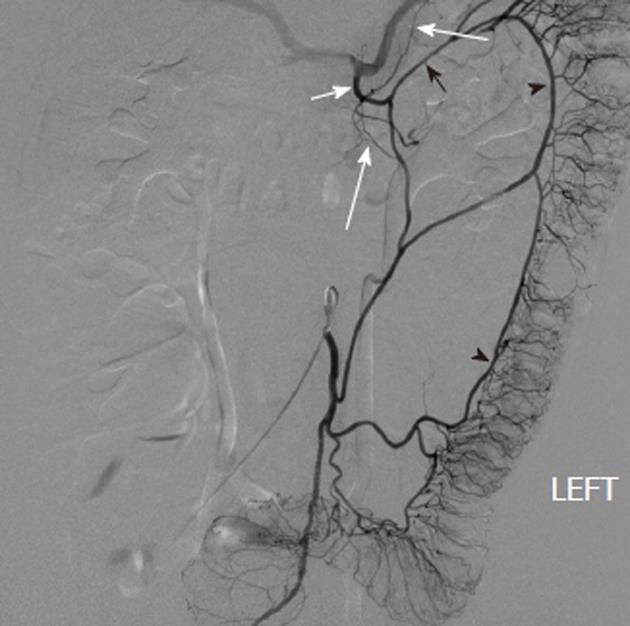
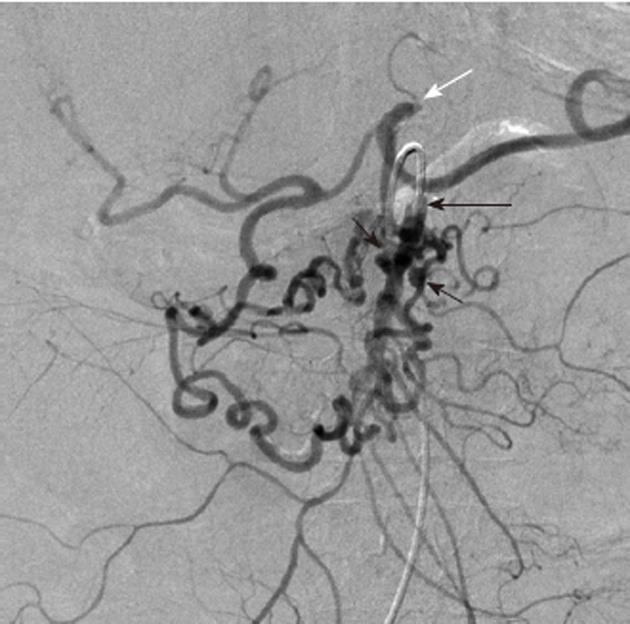
Angiodysplasia and arteriovenous malformations (AVMs) of the bowel are characterized by the early and prolonged opacification of a draining vein, accompanied by an abnormal tangle of vessels that may appear as a blush (Figure 12). The simultaneous filling of the feeding artery and the draining vein may give a characteristic “tram-track” sign. Although up to 80% of angiodysplastic lesions are in the right colon, other parts of the gut, particularly the lower small intestine, may be affected. Angiodysplasia is relatively common in older patients, aged 60 to 80 years, and may be an asymptomatic incidentally noted lesion identified at colonoscopy. Because the dilated vessels are superficial, however, they may bleed spontaneously and patients can either present acutely with overt hemorrhage or insidiously with iron deficiency anemia. Once bleeding has begun, recurrent episodic hemorrhage or persistent iron deficiency anemia requiring repeated transfusion is not uncommon. Symptomatic lesions may be effectively treated endoscopically with laser or heat coagulation or with sclerotherapy. These procedures are not risk-free in the thin cecal wall and have been known to cause serosal irritation and post-treatment bleeding. For patients with repeated bleeding from intestinal vascular malformations, pharmacological treatment using estrogens (e.g., 0.05 mg ethinyl estradiol and 1 mg norethisterone) can be effective in reducing transfusion requirements. Although transcatheter arterial embolization of colonic angiodysplasia can be an effective treatment in emergencies[8-11], it carries a significant risk of inducing colonic ischemia[12]. Definitive and curative treatment usually requires surgical resection.
Transcatheter arterial embolization is an effective means of interrupting blood flow to the bleeding source, while maintaining bowel viability. Although there is a risk of bowel ischemia and/or infarction, the coaxial catheter systems and the variety of available embolic agents that are now used for embolotherapy allow for very selective and precise treatment, and thus have decreased the incidence of these complications. Additionally, the gastrointestinal tract has a rich collateral blood supply, with extensive vascular arcades that permit safe embolization if certain principles are observed. The objective of embolization therapy is to achieve a compromise between selective arterial inflow reduction and maintenance of collateral arterial blood flow. Arterial inflow must be sufficiently decreased to allow for hemostasis, but not to an extent that causes complete devascularization. Achieving a superselective embolization[11] in the presence of an intact coagulation cascade is key to attaining a successful outcome using this form of treatment.
In most of the early literature, embolization was performed proximally in the visceral arteries because microcatheters facilitating superselective embolization were not yet available. The technique that is currently used for transcatheter arterial embolization involves the initial placement of a larger caliber diagnostic catheter (4 or 5 Fr) into the main trunk of the feeding artery, followed by coaxial introduction of a microcatheter. The latter is the crucial step in modern embolization and requires the superselective catheterization of the target artery using a 3-Fr coaxial microcatheter over a 0.018 inch or 0.014 inch guidewire. The guidewire tip must be carefully advanced under fluoroscopic monitoring in a smooth and controlled fashion in order to avoid vasospasm, dissection, or vessel perforation. Vasodilators such as verapamil (100-200 μg) or nitroglycerin (100-300 μg) may be used to treat any vasospasm that may be induced by guidewire and/or microcatheter passage. Using the wire as a guide, the catheter should travel closely behind and eventually engage the target vessel.
The optimal level of embolization varies according to the bleeding site. In general, embolization should not be routinely attempted unless a microcatheter has been advanced close to the bleeding point so that the embolic agent can be deployed as selectively as possible. The risk of infarction is related to both the embolic agent and the proximity of embolization. In the colon, for example, ischemia and infarction may result from embolization of proximal branches supplying a large area of bowel or embolization of multiple distal arteries that do not have sufficient collateral flow. Submucosal collateral blood flow may be preserved only when arteries to a short segment of bowel are embolized, so one should attempt to occlude arteries to as limited a segment of bowel as possible. One must be aware that embolization in the setting of prior gastrointestinal surgery or radiation therapy may impose a greater risk of infarction because of the associated interruption of collaterals.
Various agents may be used for transcatheter embolization. The most commonly used agents include pledgets of absorbable gelatin sponge (Gelfoam®, Pfizer, Inc., NY, United States), particulate agents such as polyvinyl alcohol (e.g., Bead BlockTM, Biocompatibles International, Farnham, United Kingdom) and other spherical agents (e.g., Embospheres® BioSphere Medical, Inc., Rockland, MA, United States; EmbozeneTM microspheres CeloNova BioSciences, Inc., Newnan, GA, United States) and microcoils of various sizes and configurations. Microcoils have the advantage of good radiopacity that allows for a precise deployment permitting reduction of the arterial perfusion pressure to the bleeding site while preserving sufficient collateral flow. The wide range of coil sizes allows one to appropriately match the coil to the target vessel diameter. Each microcoil is delivered sequentially, until hemostasis has been achieved. Intra-arterial microcoil placement is analogous to placement of a surgical ligature. The coil physically occludes the vascular lumen and causes a decreased perfusion pressure, while the attached synthetic fibers maximize thrombogenicity.
Gelfoam pledgets and particulate agents may also be used successfully, but are more difficult to control than microcoils. Gelfoam is a temporary agent and often cannot easily be deployed superselectively. A disadvantage of the particulate agents is that small diameters may reach the intramural circulation distal to the collaterals, thereby risking bowel infarction, or may reflux into non-target arteries.
The liquid embolic agents n-butyl cyanoacrylate, known by the proprietary name Trufill® (Cordis Neurovascular, Miami Lakes, FL, United States), and liquid polyvinyl alcohol copolymer (Onyx®, ev3 Neurovascular, Irvine, CA, United States) have also been used successfully in treating gastrointestinal hemorrhage[13,14]. An advantage of liquid agents is that they may be used effectively in very small caliber vessels. However, the operator must be very familiar with the use of these agents, in order to achieve optimal outcomes and minimize complications.
Because of the intermittent nature of gastrointestinal hemorrhage, arteriography fails to demonstrate a distinct bleeding site in a considerable number of patients, and thus embolization is not possible. Furthermore, a negative arteriogram fails to guide emergency surgery and delays operative decision-making. In such circumstances, some investigators have advocated provocation of bleeding with vasodilators, anticoagulants, and/or thrombolytics in association with tagged red blood cell scans or angiography[15,16]. This may be appropriate for a patient who has undergone multiple blood transfusions and a prior exhaustive work-up that has failed to localize the occult bleeding site. Additionally there must be no contraindications to the administration of a thrombolytic agent. If provocative angiography is undertaken, one should arrange for surgical backup in the event that uncontrollable bleeding occurs.
Different methods of inducing bleeding and different rates of success have been reported. An optimal protocol has yet to be established and the procedure has also yet to become accepted by clinicians as part of the evaluation of gastrointestinal bleeding. The technique continues to evolve as experience and comfort with the use of thrombolytic agents in the setting of nonlocalized bleeding increases. One reported protocol used a combination of intravenous heparin, intra-arterial tolazoline, and intra-arterial tissue-type plasminogen activator (t-PA) to provoke bleeding. Doses used included 3000-10 000 U heparin, 25-100 mg intra-arterial tolazoline, and 10-50 mg intra-arterial t-PA (mean, 20.3 mg). The investigators also noted that more patients had bleeding provoked after smaller rather than larger doses of t-PA[16]. Tolazoline (Priscoline) was formerly used for a vasodilatory effect, but was withdrawn from the United States market in 2002 by Novartis Pharmaceuticals, the sole manufacturer of this drug. Alternative intra-arterial vasodilators that may be used include verapamil (100-200 μg) and nitroglycerin (100-300 μg), with the former showing the greater vasodilatory effects. In the authors’ practice, we have occasionally used a similar transcatheter regimen, in which a vasodilator and a dose of 5000 U intra-arterial heparin and 5-10 mg intra-arterial t-PA were administered, with resultant provocation of bleeding that allowed for subsequent successful transcatheter embolization.
Superselective embolization of the arterial supply to the bleeding source may be technically demanding, particularly in older patients who may have significant atherosclerotic disease. The mesenteric vasculature and the various arterial arcades are often tortuous, and the smaller arteries are prone to vasospasm and care must be taken to avoid dissection.
An arterial bleeding site may receive a dual blood supply as a result of the rich collateral arcades that characterize the mesenteric circulation. One must therefore catheterize and inject both potential sources of perfusion to the lesion and be prepared to embolize two separate vessels if necessary. Although this dual approach will control the hemorrhage, it will also increase the risk of bowel ischemia[17].
While angiodysplasia and AVMs may initially respond to embolization, recurrent hemorrhage is frequent and, as noted, surgical resection of the involved bowel segment is often required. A small bowel AVM is much more easily localized at surgery if an embolization coil has been placed distally in the arterial branch that supplies the lesion, so that it is palpable or visible to the surgeon[18].
The current technique of embolization in the treatment of acute gastrointestinal hemorrhage successfully controls bleeding in about 80%-90% of patients[19-23]. Recurrent hemorrhage is infrequent, with the exception of angiodysplasia, AVMs and inflammatory lesions. Recurrences can usually be angiographically re-evaluated and, if a bleeding source is identified, treated with repeat embolization.
Transcatheter arterial embolization for the treatment of acute gastrointestinal hemorrhage is safe, with major adverse events occurring in less than 2% of patients. A fraction of patients embolized superselectively will develop minor, asymptomatic and self-limited ischemic changes such as small ulcers that can only be detected incidentally via objective follow-up methods such as endoscopy, pathologic surgical specimen or by a radiographic imaging examination. Additionally, superselective microcoil embolization is unlikely to result in delayed infarction. If major bowel ischemia occurs several months to years later, it is more likely to be attributable to a new and acute insult such as thromboembolic disease affecting the mesenteric arterial bed.
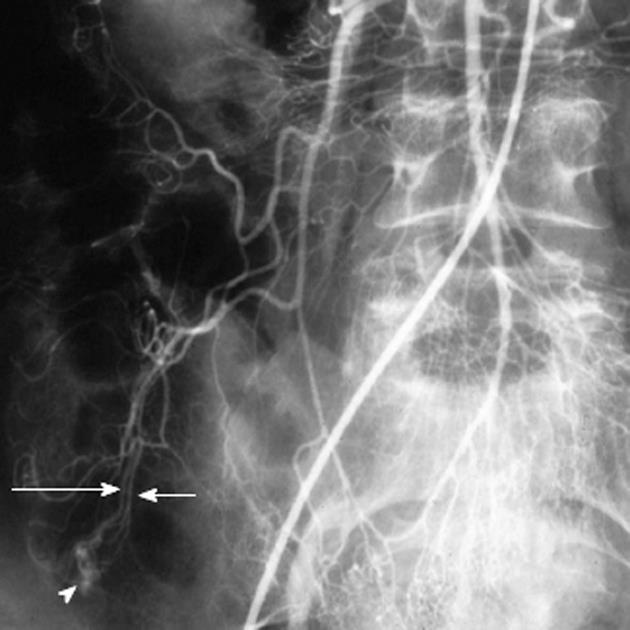
Non-target embolization with microcoils is rare, as the coils are introduced only after a microcatheter has been successfully negotiated into the target vessel. One must carefully choose appropriate sized microcoils however, as a coil that is oversized relative to the target vessel may displace the microcatheter from its superselective position. This could lead to deployment of the microcoil in a non-target location (Figure 13). Similarly, undersized coils may fail to adequately occlude the target vessel or may lodge distal to the lesion that is to be treated.
Vasopressin (Pitressin) is a naturally occurring hormone that causes constriction of both the mesenteric arteries and of the smooth muscle of the bowel wall. The intra-arterial transcatheter infusion of vasopressin proximal to a mesenteric arterial bleeding site will reduce blood flow, thereby lowering the perfusion pressure and permitting clot formation at the lesion (Figure 14). There are several situations in which this form of treatment for gastrointestinal bleeding should not be used. These include bleeding that originates from a large diameter artery such as the gastroduodenal, splenic or proximal SMA or which occurs at a site with a dual blood supply such as the duodenum. It is also contraindicated in patients who have severe coronary artery disease, extreme hypertension, limb ischemia or cardiac arrhythmias. Superselective embolotherapy is now used preferentially over vasopressin infusion for treating gastrointestinal hemorrhage because embolization poses fewer risks and can be completed more rapidly than a vasopressin infusion protocol. Vasopressin may still be useful in certain situations, despite the numerous side effects and the high re-bleeding rates. One may consider using vasopressin for treating lesions that are inaccessible to a microcatheter, for diffuse mucosal oozing, and for controlling multiple sites of hemorrhage in high-risk surgical patients.
Vasopressin is typically infused into the central vessel (e.g., celiac artery, SMA or IMA) that supplies the bleeding site via a catheter that has been placed proximally; to avoid bowel ischemia and potential infarction, a distal infusion should not be attempted. Vasopressin (100 U) is mixed in 500 mL of normal saline and the infusion is started at 0.2 U/min for 20 min using an arterial infusion pump set at 60 mL/min. If there is no cessation of bleeding, the dose is increased by 0.1 U/min up to a maximum of 0.4 U/min; each dosage change is followed by a repeat angiogram 20 to 30 min later to assess the effectiveness[24]. If the bleeding stops, the catheter is left in place for a 24-h infusion at the effective dosage, with monitoring in the intensive care unit. After another repeat angiogram shows control of bleeding, the vasopressin infusion is gradually reduced over 24 to 48 h and then vasopressin is replaced with an infusion of normal saline or 5% dextrose in water for 6-12 h.
This form of therapy is particularly effective in controlling diverticular and gastric mucosal hemorrhage, with initial success rates ranging from 60%-90%[24-27]. As previously noted, however, there is a very high rate of re-bleeding that may be up to 50%.
Mild abdominal pain may occur at the initiation of the infusion and should be closely monitored, as persistence and/or worsening can be an indicator of bowel ischemia. If side effects develop during treatment, the vasopressin can be tapered to a lower dose or may need to be discontinued. Additional side effects and potential complications of vasopressin therapy include angina, myocardial infarction, hypertension, volume overload, abdominal cramps, and mesenteric ischemia. Some of the potential adverse effects may be treated or even pretreated. The simultaneous administration of intravenous, sublingual or transdermal nitroglycerin may prevent or reverse the cardiotoxic side effects of vasopressin infusion[28].
Technical refinements and advances both in diagnostic angiography and in transcatheter arterial embolization have strengthened these options for the evaluation and management of acute gastrointestinal hemorrhage that is refractory to medical and endoscopic therapy. Highly sensitive noninvasive imaging modalities such as nuclear scintigraphy and contrast-enhanced computed tomography are extremely useful adjuncts to angiography as they often can localize and characterize the bleeding source, confirm active hemorrhage and aid in planning an appropriate transcatheter intervention. Superselective catheterization using a coaxial system that allows for microcoil embolization is an effective and safe alternative to emergency surgery. Other embolic agents such as gelatin sponge, spherical particles and liquids also have a role in transcatheter management of gastrointestinal bleeding as do transcatheter therapies such as covered stent placement and vasopressin infusion.
Peer reviewers: Yuji Naito, Professor, Kyoto Prefectural University of Medicine, Kamigyo-ku, Kyoto 602-8566, Japan; Seng-Kee Chuah, MD, Division of Hepatogastroenterology, Chang Kaohsiung Gang Memorial Hospital, 123, Ta-Pei Road, Niaosung Hsiang, Kaohsiung 833, Taiwan, China; Kevin Cheng-Wen Hsiao, MD, Assistant Professor, Colon and Rectal Surgery, Tri-Service General Hospital, No. 325, Sec 2, Cheng-Kung Rd, Nei-Hu District, Taipei 114, Taiwan, China
S- Editor Sun H L- Editor Cant MR E- Editor Zhang DN
| 1. | Welch CE, Hedberg S. Gastrointestinal hemorrhage. I. General considerations of diagnosis and the rapy. Adv Surg. 1973;7:95-148. [PubMed] |
| 2. | Nusbaum M, Baum S. Radiographic demonstration of unknown sites of gastrointestinal bleeding. Surg Forum. 1963;14:374-375. [PubMed] |
| 3. | Krüger K, Heindel W, Dölken W, Landwehr P, Lackner K. Angiographic detection of gastrointestinal bleeding. An experimental comparison of conventional screen-film angiography and digital subtraction angiography. Invest Radiol. 1996;31:451-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 4. | Winzelberg GG, McKusick KA, Froelich JW, Callahan RJ, Strauss HW. Detection of gastrointestinal bleeding with 99mTc-labeled red blood cells. Semin Nucl Med. 1982;12:139-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 55] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 5. | Bentley DE, Richardson JD. The role of tagged red blood cell imaging in the localization of gastrointestinal bleeding. Arch Surg. 1991;126:821-824. [PubMed] |
| 6. | Smith R, Copely DJ, Bolen FH. 99mTc RBC scintigraphy: correlation of gastrointestinal bleeding rates with scintigraphic findings. AJR Am J Roentgenol. 1987;148:869-874. [PubMed] |
| 7. | Gunderman R, Leef J, Ong K, Reba R, Metz C. Scintigraphic screening prior to visceral arteriography in acute lower gastrointestinal bleeding. J Nucl Med. 1998;39:1081-1083. [PubMed] |
| 8. | Bilbao JI, Barettino MD, Longo JM, Aquerreta JD, Larrea JA, Caballero AD. Permanent therapeutic embolization of cecal angiodysplasia. Am J Gastroenterol. 1996;91:1287-1288. [PubMed] |
| 9. | Defreyne L, Vanlangenhove P, De Vos M, Pattyn P, Van Maele G, Decruyenaere J, Troisi R, Kunnen M. Embolization as a first approach with endoscopically unmanageable acute nonvariceal gastrointestinal hemorrhage. Radiology. 2001;218:739-748. [PubMed] |
| 10. | Defreyne L, Vanlangenhove P, Decruyenaere J, Van Maele G, De Vos M, Troisi R, Pattyn P. Outcome of acute nonvariceal gastrointestinal haemorrhage after nontherapeutic arteriography compared with embolization. Eur Radiol. 2003;13:2604-2614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 11. | Funaki B, Kostelic JK, Lorenz J, Ha TV, Yip DL, Rosenblum JD, Leef JA, Straus C, Zaleski GX. Superselective microcoil embolization of colonic hemorrhage. AJR Am J Roentgenol. 2001;177:829-836. [PubMed] |
| 12. | Breton JO, Breton M, Colombier JP, Serres JJ. [A case of iatrogenic ischemic colitis]. J Radiol Electrol Med Nucl. 1978;59:435. [PubMed] |
| 13. | Frodsham A, Berkmen T, Ananian C, Fung A. Initial experience using N-butyl cyanoacrylate for embolization of lower gastrointestinal hemorrhage. J Vasc Interv Radiol. 2009;20:1312-1319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 47] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 14. | Lenhart M, Paetzel C, Sackmann M, Schneider H, Jung EM, Schreyer AG, Feuerbach S, Zorger N. Superselective arterial embolisation with a liquid polyvinyl alcohol copolymer in patients with acute gastrointestinal haemorrhage. Eur Radiol. 2010;20:1994-1999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 40] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 15. | Bloomfeld RS, Smith TP, Schneider AM, Rockey DC. Provocative angiography in patients with gastrointestinal hemorrhage of obscure origin. Am J Gastroenterol. 2000;95:2807-2812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 83] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 16. | Ryan JM, Key SM, Dumbleton SA, Smith TP. Nonlocalized lower gastrointestinal bleeding: provocative bleeding studies with intraarterial tPA, heparin, and tolazoline. J Vasc Interv Radiol. 2001;12:1273-1277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 69] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 17. | Bell SD, Lau KY, Sniderman KW. Synchronous embolization of the gastroduodenal artery and the inferior pancreaticoduodenal artery in patients with massive duodenal hemorrhage. J Vasc Interv Radiol. 1995;6:531-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 18. | Schmidt SP, Boskind JF, Smith DC, Catalano RD. Angiographic localization of small bowel angiodysplasia with use of platinum coils. J Vasc Interv Radiol. 1993;4:737-739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 19. | Patel TH, Cordts PR, Abcarian P, Sawyer MA. Will transcatheter embolotherapy replace surgery in the treatment of gastrointestinal bleeding?(2)(2). Curr Surg. 2001;58:323-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 31] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 20. | Bandi R, Shetty PC, Sharma RP, Burke TH, Burke MW, Kastan D. Superselective arterial embolization for the treatment of lower gastrointestinal hemorrhage. J Vasc Interv Radiol. 2001;12:1399-1405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 122] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 21. | Kuo WT. Transcatheter treatment for lower gastrointestinal hemorrhage. Tech Vasc Interv Radiol. 2004;7:143-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 20] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 22. | Kuo WT, Lee DE, Saad WE, Patel N, Sahler LG, Waldman DL. Superselective microcoil embolization for the treatment of lower gastrointestinal hemorrhage. J Vasc Interv Radiol. 2003;14:1503-1509. [PubMed] |
| 23. | Schenker MP, Duszak R, Soulen MC, Smith KP, Baum RA, Cope C, Freiman DB, Roberts DA, Shlansky-Goldberg RD. Upper gastrointestinal hemorrhage and transcatheter embolotherapy: clinical and technical factors impacting success and survival. J Vasc Interv Radiol. 2001;12:1263-1271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 136] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 24. | Athanasoulis CA, Baum S, Rösch J, Waltman AC, Ring EJ, Smith JC, Sugarbaker E, Wood W. Mesenteric arterial infusions of vasopressin for hemorrhage from colonic diverticulosis. Am J Surg. 1975;129:212-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 85] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 25. | Baum S, Nusbaum M. The control of gastrointestinal hemorrhage by selective mesenteric arterial infusion of vasopressin. Radiology. 1971;98:497-505. [PubMed] |
| 26. | Nusbaum M, Baum S, Blakemore WS, Tumen H. Clinical experience with selective intra-arterial infusion of vasopressin in the control of gastrointestinal bleeding from arterial sources. Am J Surg. 1972;123:165-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 27] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 27. | Clark RA, Colley DP, Eggers FM. Acute arterial gastrointestinal hemorrhage: efficacy of transcatheter control. AJR Am J Roentgenol. 1981;136:1185-1189. [PubMed] |
| 28. | Gimson AE, Westaby D, Hegarty J, Watson A, Williams R. A randomized trial of vasopressin and vasopressin plus nitroglycerin in the control of acute variceal hemorrhage. Hepatology. 1986;6:410-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 123] [Article Influence: 3.2] [Reference Citation Analysis (0)] |