INTRODUCTION
Inflammatory pseudotumors (IPT) are unusual lesions from a histological standpoint as they are characterized by nonspecific inflammatory cell infiltration and fibrosis. This tumor type was first described in the lung by Brunn[1] in 1939, and has been reported subsequently in various organs including the liver[2] and spleen[3].
No radiological findings have been found that are characteristics of IPT. This may be because the proportion or the distribution of inflammatory cells and fibrosis differs according to the cause and the period of inflammation[4]. Hence, it is difficult to establish a definite diagnosis by radiological imaging. Moreover, a proper differential diagnosis of IPT from a malignant tumor could delay vital treatment. As a result of these issues, surgical removal is chosen only when the possibility of malignancy cannot be ruled out and when the treatment can be expected to be effective, particularly if growth of the lesion is observed during the evaluation period. There are some reports emphasizing the importance of undertaking a percutaneous needle biopsy in cases of suspected hepatic IPT to avoid unnecessary surgery, although misdiagnoses of adenocarcinoma[5] or an uncertain type of sarcoma[6] have been reported for some microscopic examinations. However, only one case of a needle biopsy for splenic IPT has been reported to date[7], and it is extremely rare to find IPT of the liver and spleen at the same time[8-10].
We report a rare case of IPT involving the liver and spleen which was difficult to diagnose by hematological examination or radiological study, but was confirmed following a percutaneous liver and spleen biopsy.
CASE REPORT
A woman aged over 70 with a past history of early-stage gastric cancer that had been treated by endoscopic mucosal resection at another hospital four years previously, was being followed annually by upper esophagogastroduodenoscopy. In one examination, compression from outside of the stomach at the fundus was observed, and she was referred to our hospital for further investigation.
At the time of the referral, this patient had been suffering from a general malaise lasting more than two months but did not have any other clinical symptoms such as fever, appetite loss, weight loss, or abdominal pain. The initial physical examination revealed nothing of note. Blood tests further showed a reduction in hemoglobin [8.0 g/dL (normal range 12.0-15.0 g/dL)] and an elevation of the white blood cell count [10 300/mm3 (normal range 3300-9400/mm3)], platelet count [549 000/mm3 (normal range 130 000-320 000/mm3)], and C-reactive protein level [10.98 mg/dL (normal range < 0.2 mg/dL)]. There was a slight increase in the total protein content [8.2 g/dL (normal range 6.4-8.1 g/dL)], whereas the albumin concentration was 1.9 g/dL (normal range 3.6-4.7 g/dL), indicating a substantial increase in globulin levels, which was consistent with an elevated IgG [3774 mg/dL (normal range 870-1700 mg/dL)]. The serum aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels were within the normal range, whereas the serum levels of alkaline phosphatase and γ-glutamyl transpeptidase were elevated [507 U/L (normal range 134-359 U/L) and 94 U/L (normal range 8-51 U/L), respectively]. The patient was negative for hepatitis B surface antigen (HBsAg) and hepatitis C virus antibody (anti-HCV). Antinuclear antibodies (ANA) were positive at a titer of 1:40 (normal range < 1:40) whereas other autoantibodies were negative, including antimitochondrial antibody (AMA), cytoplasmic anti-neutrophil cytoplasmic antibody (C-ANCA), and perinuclear anti-neutrophil cytoplasmic antibody (P-ANCA). Carcinoembryonic antigen (CEA), carbohydrate antigen 19-9 (CA19-9), α-fetoprotein (AFP), and des-γ-carboxy prothrombin (DCP) were all found to be within normal limits.
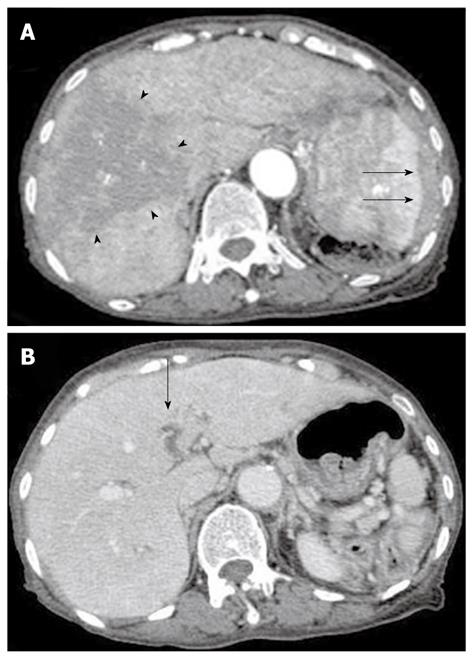
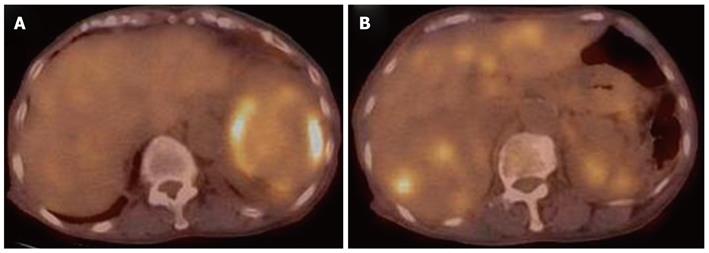
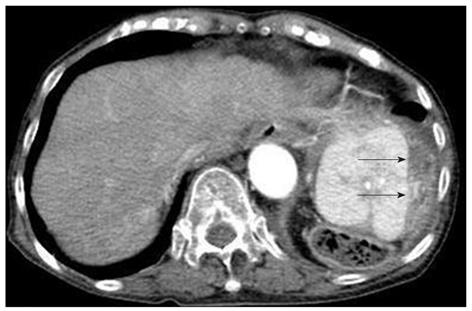
Contrast computed tomography (CT) during the arterial phase showed a diffusely and non-homogeneously enhanced liver, with a less enhanced anterior compartment (Figure 1A). The left branch of the portal vein was occluded in the portal phase (Figure 1B). The spleen was protruding inward, with diffuse and inhomogeneous enhancement of the arterial phase, as observed for the liver. CT also revealed a soft density layer around the spleen (Figure 1A). The results of fluorodeoxyglucose positron emission tomography/computed tomography (FDG-PET/CT) exhibited abnormal metabolic activity with a high standardized uptake value (SUV) of 7.1 around the spleen (Figure 2A). This was consistent with previous CT findings, and with a multiple abnormal uptake in segment six of the liver (Figure 2B). There was no abnormal uptake in other body sites. These radiological findings indicated the presence of a malignancy of the area surrounding the spleen and liver, with the possibility of a differential diagnosis of angiosarcoma, malignant lymphoma, hepatocellular carcinoma or metastatic cancer of the liver, with tumor thrombosis of the portal vein and the spleen.
Figure 1 Contrast computed tomography scanning of the arterial phase revealed a diffusely and non-homogeneously enhanced liver, with the anterior compartment showing less enhancement (arrow-head).
The spleen was found to be protruding inward, and also showed a diffuse and inhomogeneous enhancement. A: Computed tomography (CT) scans also revealed a soft density layer around the spleen (arrow). B: A CT of the portal phase showed an occlusion (arrow) of the left branch of the portal vein.
Figure 2 Fluorodeoxyglucose positron emission tomography/computed tomography analysis showing abnormal metabolic activity with a high standardized uptake value of 7.
1 surrounding the spleen (A), and multiple abnormal uptakes in segment six of the liver (B).
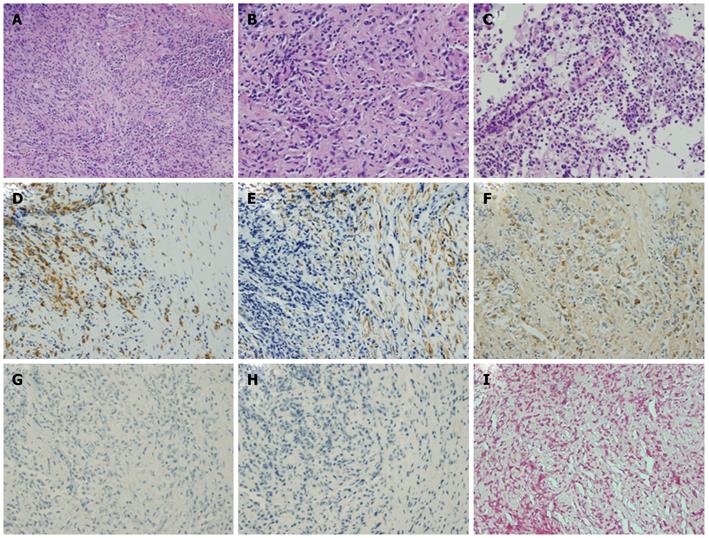
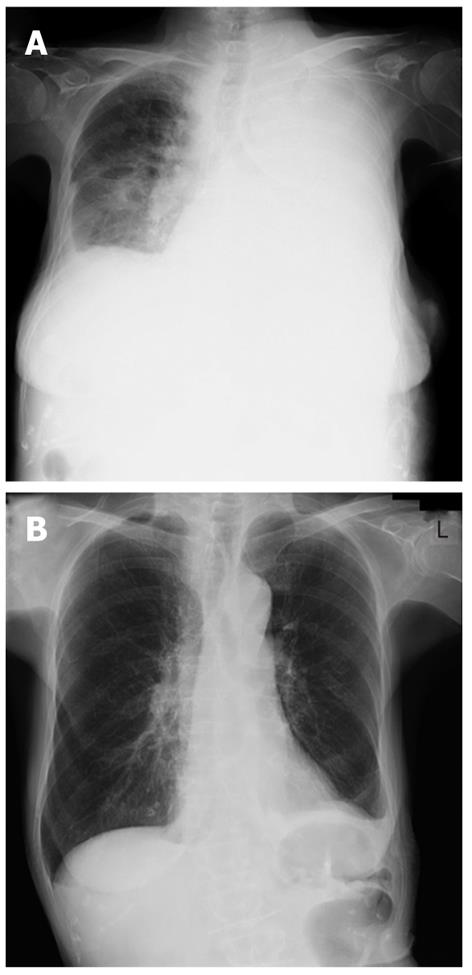
A percutaneous needle liver biopsy was performed under ultrasonic guidance to enable a more definite diagnosis. Sonographic examination revealed rough echogenicity of the right hepatic lobe, and a liver biopsy was subsequently performed but did not show a focal liver mass. The sonographic appearance of the spleen was similar to the liver biopsy region. The liver specimen showed a patchy fibrosis and inflammatory cell infiltration, mainly consisting of lymphocytes and plasma cells, but was free of malignant cells (Figure 3A and B). From this point of view, we considered a possible diagnosis of IPT, but the radiological findings so strongly indicated malignancy that we planned splenectomy for diagnostic confirmation. However, due to the patient’s severe shortness of breath, caused by massive pleural effusion, a splenectomy was considered unsafe at that time (Figure 4A). Subsequent cytologic analysis showed that the pleural effusion was transudative and negative for malignancy. Hence, a percutaneous needle biopsy of the splenic lesion was performed as an alternative examination under ultrasonic guidance in the supine position via an intracostal approach. Microscopic examination of the spleen specimen revealed infiltration by lymphocytes and plasma cells, but no evidence of malignant cells (Figure 3C), as found in the liver specimen. Immunohistochemical examination of the liver specimen showed that the lesion was positive for CD68, α-smooth muscle actin (SMA) and IgG, but was negative for IgG4, anaplastic lymphoma kinase (ALK), and Epstein-Barr virus (EBV) encoded RNA (EBER) (Figure 3D-I). We were thus able to rule out an IgG4-related lesion, inflammatory myofibroblastic tumor (IMT), and an EBV-associated ITP-like follicular dendritic cell (FDC) tumor.
Figure 3 Histological findings for the liver via hematoxylin and eosin staining showing patchy fibroses and inflammatory cell infiltration (original magnification × 100).
A: Mainly consisting of lymphocytes and plasma cells (original magnification × 200); B and C: Histological findings for the spleen following HE staining showed infiltration by plasma cells (original magnification × 200); D-I: Immunohistochemical analysis of the liver showed that the lesion was positive for CD68 (D), α-smooth muscle actin (SMA) (E), and IgG (F), but not for IgG4 (G), anaplastic lymphoma kinase (ALK) (H), or Epstein-Barr virus (EBV) encoded RNA (EBER) (I). HE: Hematoxylin and eosin.
Figure 4 Chest X-ray showing massive pleural effusion of the left side before treatment (A), and full correction following steroid pulse therapy (B).
Finally, based on the results of our microscopic examination of both the liver and the spleen specimens, we diagnosed the pathological status of this patient as an IPT. However, despite an extensive clinical search for the potential cause of this IPT, mainly for infectious diseases including tuberculosis, its etiology could not be identified. As IgG levels were elevated in this patient and the ANA test was positive, we speculated that an autoimmune process could be possible. The patient did not respond for 19 d to intravenous antibiotics, including ampicillin hydrate (ABPC), sultamicillin tosilate hydrate (SBTPC), ciprofloxacin hydrochloride (CPFX), and meropenem hydrate (MEPN) and no underlying disease that could be a possible cause of IPT was detected. Because of the lack of any evidence for infection and her deteriorating clinical condition, we commenced steroid pulse therapy (three days of intravenous methylprednisolone; 1000 mg). C-reactive protein levels decreased immediately from 9.25 mg/dL to 0.93 mg/dL and there was no subsequent flare-up of inflammation during the gradual tapering of the steroid dose. The pleural effusion also decreased gradually (Figure 4B) accompanied by the restoration of normal breathing. Follow-up CT imaging after one month showed a nearly complete resolution of the hepatic and splenic lesions, except for the soft density layer remaining around the spleen (Figure 5). The occlusion of the portal vein remained unresolved however, and was subsequently found to be due to a thrombus which may have resulted from the severe inflammation. At 15 mo after the initial steroid pulse therapy, the patient was in good health and free of recurrence with continuing treatment of 5 mg of oral prednisone.
Figure 5 Follow-up computed tomography showing nearly complete resolution of the hepatic and splenic lesions other than the remaining soft density layer around the spleen (arrow).
DISCUSSION
The sites of predilection for IPT are the lungs and eyes followed by the liver, but involvement of the spleen is rare. The coexistence of IPT of the liver and spleen is therefore unusual.
It is very difficult for a physician to diagnose IPT because of the absence of specific symptoms, hematological abnormalities or anomalous radiological findings. Patients with IPT can present with fever, abdominal pain, abdominal discomfort, or leukocytosis, but these symptoms are not specific to IPT. Radiological results for IPT are also inconsistent, because fatty depositions, tissue inflammation and necrosis, fibrosis and bleeding can affect the imaging[11]. Moreover, the degree and distribution of proliferating capillaries influences the staining patterns obtained by CT or MRI examination. A further problem is that IPT imaging findings are often similar to those of malignant tumors. For example, delayed enhancement on contrast CT, especially at the periphery of the lesions, is considered to be characteristic of IPT[12,13], but cholangiocellular carcinoma or metastatic tumor shows the same enhancement pattern. IPT sometimes shows an early enhancement pattern followed by a washout in the delayed phase on contrast CT[14], but this is a typical finding also for hepatocellular carcinoma. FDG uptake can be semiquantitatively measured using an SUV, which is generally higher in malignant tumors than in inflammatory disease. However, this method of differentiation is of limited value for IPT as the FDG uptake varies with the proportion of fibrosis and inflammatory cell infiltration. In fact, there have been some FDG-PET studies of IPT that have reported a high SUV[15,16] that is comparable to malignancy.
Due to the difficulty in diagnosing IPT through an assessment of clinical symptoms, blood examinations or radiological imaging, a definitive diagnosis often requires histopathological confirmation. Percutaneous needle liver biopsies are usually performed to diagnose IPT of the liver but may sometimes lead to a misdiagnosis[5,6]. Despite the absence of malignant findings by histological examination, there are also some IPT cases in which the possible existence of malignancy cannot be completely excluded even though various clinical factors are considered in tandem. Exploratory laparotomies or hepatectomies are often then performed[17] for confirmation. In cases of splenic IPT, almost all final diagnoses require surgery since a splenectomy is not only diagnostic but can also be curative. Recently, a less invasive laparoscopic splenectomy has been introduced in clinical settings[18], making it easier to perform. On the other hand, only one case of splenic IPT diagnosed by percutaneous spleen biopsy has been reported to date[7].
Only three cases of the coexistence of IPT of the liver and spleen have been previously reported[8-10]. Each was at first clinically or radiologically believed to be another disease such as lymphoma or metastatic cancer, but was eventually histologically diagnosed as IPT. One case was diagnosed by percutaneous liver biopsy without further radical study including spleen biopsy, as the patient was in the remission stage at the time of the biopsy. In the other two cases, a splenectomy was performed to enable the final diagnosis. In our current case, CT and FDG-PET findings strongly suggested the existence of a malignant tumor of the liver and the spleen with peritoneal dissemination, even though the histological appearance of the liver biopsy sample showed inflammatory cell infiltration and fibrosis indicating IPT. We therefore planned to conduct a splenectomy to confirm the diagnosis, but this became too risky as a massive pleural effusion had reduced the patient’s respiratory function and her clinical status was worsening rapidly at that time. We therefore decided to conduct a spleen biopsy after obtaining informed consent from the patient. The results from the earlier liver biopsy were confirmed by the matching evidence obtained from the spleen sample.
IPT is clinically classified into several types according to their etiology, and the treatment options vary. Infections and autoimmune disorders are representative of originally-defined IPT, which is a benign and a reactive lesion. IPT of infectious origin can be caused by Mycobacterium tuberculosis[19], non-tuberculous mycobacteria[20], Escherichia coli[21], gram-positive cocci[5] and Klebsiella pneumonia[22]. Detection of the infecting organism by bacterial cultivation can confirm the existence of such instances of IPT, and the diagnosis can be further validated by the patient’s response to administered antibacterial agents.
In our current patient, IMT[23], an EBV associated IPT-like FDC tumor[24], and IgG4-related diseases[25] were all excluded by immunohistochemical staining. In addition, although we obtained blood cultures and analyzed tissue from the lesions, no microorganisms were detected. Furthermore, the patient did not respond to a serial course of treatment with antibiotics, thus excluding the possibility of active infection. There are some reports of IgG4-related IPT[25] indicating the possibility that an autoimmune response contributes to the disease pathogenesis. Although the etiology of IPT remains unclear, based on the deteriorating clinical condition of our patient, we undertook steroid pulse therapy which proved to be very effective. The indication for steroid therapy has not been firmly established, but seems to be appropriate for IPT considering that this disorder was originally attributed to an inflammatory or a reactive process.
In conclusion, we report a rare case of the coexistence of hepatic and splenic IPT which was diagnosed by percutaneous liver and spleen biopsies. We conclude that a percutaneous biopsy can be an effective choice for suspected cases of IPT, even when a laparotomy or splenectomy is clinically difficult to perform.