Published online Jan 7, 2012. doi: 10.3748/wjg.v18.i1.1
Revised: June 15, 2011
Accepted: June 22, 2011
Published online: January 7, 2012
Primary sclerosing cholangitis (PSC) is a chronic progressive inflammatory disease affecting the bile ducts, leading to fibrosis and eventually cirrhosis in most patients. Its etiology is unknown and so far no effective medical therapy is available. Liver transplantation (LTX) is the only curative treatment and at present PSC is the main indication for LTX in the Scandinavian countries. Close to half of the PSC patients experience one or more episodes of acute cellular rejection (ACR) following transplantation and approximately 1/5 of the transplanted patients develop recurrent disease in the graft. In addition, some reports indicate that ACR early after LTX for PSC can influence the risk for recurrent disease. For these important post-transplantation entities affecting PSC patients, we have reviewed the current literature on epidemiology, pathogenesis, treatment and the possible influence of rejection on the risk of recurrent disease in the allograft.
- Citation: Fosby B, Karlsen TH, Melum E. Recurrence and rejection in liver transplantation for primary sclerosing cholangitis. World J Gastroenterol 2012; 18(1): 1-15
- URL: https://www.wjgnet.com/1007-9327/full/v18/i1/1.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i1.1
Primary sclerosing cholangitis (PSC) is a chronic cholestatic liver disease of unknown etiology. Histologically, it is characterized by the existence of intrahepatic and/or extrahepatic concentric, obliterative fibrosis of the biliary ducts. It is a progressive disorder that eventually leads to the development of cirrhosis and hepatic decompensation in the majority of the patients[1,2]. PSC is closely associated with inflammatory bowel disease (IBD), especially ulcerative colitis (UC), which is diagnosed in approximately two-thirds of northern European PSC patients[3-5]. Approximately 2/3 of the PSC patients are male[6] and most affected individuals are less than 40 years of age at the time of diagnosis. There is still no medical treatment with proven efficacy[7] and liver transplantation (LTX) is currently considered as the only curative option for end-stage liver disease due to PSC[8]. Although graft and patient survival following LTX has improved dramatically over the last two decades, and is now close to 80% at 5 years[9], it has become increasingly obvious that the disease can recur in the transplanted liver. In the earliest reports, recurrent primary sclerosing cholangitis (rPSC) did not seem to influence graft and patient survival[10,11]. On the contrary, more recent data have indicated that a significant proportion of the affected patients will develop graft loss[12], thus influencing the long-term patient survival[13]. Therefore, rPSC represents an important condition in a steadily growing cohort of patients, for which little is known regarding its epidemiology, pathogenesis and treatment.
PSC patients are also believed to be at an increased risk of rejection, and probably up to 50% of the patients will experience one or more episodes of acute cellular rejection (ACR) during the first few weeks following transplantation. The need for increased immunosuppression represents a problem in the clinical management of these patients[14-16]. In addition, the immunological reactions related to rejection could influence the risk of developing rPSC in the graft as more reactive lymphocytes are likely to be present. To date, a possible effect of rejection on the risk of developing recurrent disease is supported by three published reports. This deserves increased attention and will be addressed in this review.
The incidence of PSC varies considerably among different parts of the world. Population-based studies of disease frequency from Norway, Great Britain and United States, indicate a comparable incidence (0.9-1.3/100 000 per year) and prevalence (8.5-13.6/100 000)[17-19] while the numbers from southern Europe and Asia have been reported to be 10-100 times lower[20-22]. PSC is today the number one indication for liver transplantation in the Scandinavian countries, while it in the United States is the fifth most common indication[23]. The first report of suspected PSC recurrence in a liver graft was published in 1988 by Lerut et al[24] describing a transplanted PSC patient developing intrahepatic biliary strictures in the absence of allograft rejection within the first year after the transplantation. Since then, numerous reports have corroborated rPSC as a clinical entity[25-28]. The incidence of recurrence varies widely in reports from different centers, probably reflecting variation in diagnostic criteria, study design, and length and type of follow-up. We have in this review identified, after exclusion of cohorts duplicated in previous reports[29-32], a total of 22 publications containing data from original studies on the outcome of LTX for PSC, which analyzed the incidence of recurrent disease (Table 1). For studies based on the same clinical material, the most recent and largest study was included. Of the total number of 1399 patients transplanted for PSC in these studies, 259 (18.5%) developed recurrence (range, 5.7%-59.1% in the individual studies). In the largest series reported so far (n = 230), focusing on the risk factors for rPSC, recurrence occurred in 23.5% of the patients at a median of 4.6 years after LTX[13]. This number is in accordance with the overall percentage for the studies we analyzed.
| Ref. | Yr | n | Median follow-up(range) in months | Recurrencen (%) | Median time to recurrence (range) in months | Diagnostic criteriafor recurrence | Risk factor(s)for recurrence |
| Goss et al[11] | 1997 | 127 | 36 (ND) | 11 (8.6) | ND (ND) | Radiographic and histological evidence | ND |
| Jeyarajah et al[70] | 1998 | 100 | ND (12-108) | 18 (18) | 21 (mean) (ND) | Radiographic and/or histological evidence | ACR |
| Graziadei et al[87] | 1999 | 120 | 55 (mean) (4-136) | 24 (20) | 8.5 (3-42) | Radiographic and/or histological evidence | None |
| Kubota et al[169] | 1999 | 53 | ND (12-156) | 3 (5.7) | ND (16-48) | Radiographic and histological evidence | ND |
| Liden et al[170] | 2001 | 61 | ND (ND) | 5 (8.2) | ND (ND) | Radiographic and histological evidence | ND |
| Renz et al[171] | 2002 | 49 | 66 (ND) | 7 (14) | ND (ND) | Radiographic or histological evidence | ND |
| Yusoff et al[172] | 2002 | 12 | 58 (mean) (4-174) | 2 (17) | ND | Radiographic and histological evidence | ND |
| Khettry et al[71] | 2003 | 42 | ND (24-168) | 6 (14.3) | ND (ND) | Radiographic and/or histological evidence | Recipient-donor mismatch |
| Kugelmas et al[76] | 2003 | 71 | ND (14-91) | 15 (21.1) | 53 (mean) (12-110) | Radiographic or histological evidence | ND |
| Brandsaeter et al[75] | 2005 | 49 | 77 (17-182) | 9 (18) | ND | Radiographic evidence | Steroid-resistant ACR |
| Khuroo et al[173] | 2005 | 5 | 90 (1-186) | 2 (40) | ND | Radiographic evidence | ND |
| Oldakowska-Jedynak et al[174] | 2005 | 17 | 32 (mean) (0.9-91) | 2 (12) | 29 (18-51) | Radiographic and/or histological evidence | ND |
| Cholongitas et al[77] | 2007 | 53 | 110 (12-185) | 7 (13.2) | 60 (4-120) | Radiographic and histological evidence | Steroids for UC (> 3 mo post-LTX) |
| Campsen et al[78] | 2007 | 130 | 66 (ND) | 22 (16.9) | ND (ND) | Radiographic evidence | Pre-LTX CCA |
| Yamagiwa et al[175] | 2007 | 44 (all LDLT) | ND (ND) | 11 (25) | ND (ND) | Radiographic and histological evidence | ND |
| Tamura et al[176] | 2007 | 8 (LDLT) | 42 (ND) | 4 (50) | 40 (14-66) | Radiographic evidence | ND |
| Haga et al[74] | 2007 | 22 (all LDLT) | 31 (mean) (22-71) | 13 (59.1) | 31 (22-71) | Radiographic and histological evidence | ND |
| Alexander et al[69] | 2008 | 69 | 50 (1-173) | 7 (10) | 68 (24-134) | Radiographic and/or histological evidence | ACR, steroid resistant ACR, HLA-DRB1*08 in donor or recipient |
| Alabraba et al[13] | 2009 | 230 | 82.5 (0.0-239) | 54 (23.5) | 55.2 (6-155) | Radiographic and histological evidence | Intact colon at the time of LTX, use of EDC graft |
| Kashyap et al[177] | 2009 | 58 | 41.5 (mean) (ND) | 11 (19) | ND (ND) | Radiographic and/or histological evidence | ND |
| Egawa et al[73] | 2009 | 20 (all LDLT) | 63 (1-133) | 11 (55) | ND (26-71) | Radiographic or histological evidence | Cytomegalovirus infection within 3 mo, related donor |
| Moncrief et al[178] | 2009 | 59 | 68 (33-106) | 15 (25) | 40.2 (19.5-66.1) | Radiographic and/or histological evidence | ACR, cytomegalovirus mismatch |
The etiology and pathogenesis of both PSC and rPSC are currently unknown. Most studies have focused on pre-transplant (“primary”) pathogenesis, and lessons from these studies may give insight and provide hypotheses for further research even on the pathogenesis of recurrent disease. The primary disease is characterized by chronic inflammation and progressive fibrotic strictures of the bile ducts[33,34]. By the time the patient is diagnosed with PSC, the changes in the liver architecture are already quite advanced. To determine at this stadium, at a cellular level, which observations that can be of primary importance in the pathogenesis of PSC or just a secondary phenomenon for the ongoing disease is difficult to judge. So far, there has been no unified pathogenetic mechanism for PSC development.
It is important to identify risk factors for recurrence, both in the search for mechanisms involved in the pathogenesis and in improving the management of these patients after transplantation. It might also shed light on the pathogenesis of the primary disease. The pathogenesis of rPSC can be considered multifactorial and influenced by pre- and/or post-operative factors in combination with a genetic predisposition. It is also likely that it is partly related to the pathogenesis of the primary disease. Although it is beyond the scope of this review to go into details regarding PSC pathogenesis, we will briefly mention the theories that have gained the most general acceptance in recent years[7], since these mechanisms may also be involved in recurrent disease. Four hypotheses have been put forward, each is potentially relevant at different stages of the disease process.
Strong evidence indicates that genetic variants play an important role in disease susceptibility and siblings of PSC patients are 9-39 times more likely to develop PSC compared with the general population[35]. Family members of PSC patients are also at increased risk of developing UC, indicating the existence of shared genetic risk factors between these two conditions[7]. Furthermore, unbiased genome-wide association studies have demonstrated shared susceptibility loci between UC and PSC[36,37]. PSC associated variants in the human leukocyte antigen (HLA)-region were first reported almost 30 years ago[38] and have since been verified numerous times. It has so far not been possible to pin-point the exact causative genes in the HLA-region, and it is likely that more than one susceptibility gene exists at this locus. A recent genome-wide association study has also provided strong evidence for involvement of two or more non-HLA genes; in particular BCL2L11 involved in deletion of autoreactive lymphocytes and MST1 involved in macrophage activation. Variants at the MST1 locus are also associated with IBD[39,40]. The role of these genes in recurrent disease is currently unknown but it is plausible that some of these variants together with other factors determine the susceptibility to recurrent disease.
In addition to the genetic associations at loci involved in the immune response, the fact that the majority of PSC patients have IBD, an increased frequency of other autoimmune diseases[41] and the presence of multiple autoantibodies[42] further support a role for autoimmune components in the pathogenesis. The most prevalent autoantibody, which is found in more than 90% of PSC patients, is a special type of perinuclear anti-neutrophil cytoplasmatic antibody (pANCA)[43,44]. The same antibody is observed in UC and in type 1 autoimmune hepatitis[44,45]. On the other hand, the male predominance, the lack of demonstration of a specific PSC autoantigen and the missing response to immunosuppressive treatment are atypical for an autoimmune disease[46,47]. The importance of autoantibodies in both PSC and rPSC is unknown, nevertheless mechanisms related to the immune response are likely candidates for overlapping mechanistic themes between the primary and recurrent disease.
Studies in murine models with intestinal bacterial overgrowth suggest that innate immune responses to the bacterial products could initiate a PSC-like disease process[48-52], and despite the lack of convincing evidence to support a role of infectious agents in human studies[53-57], the presence of an infectious trigger or infectious modifier effects is still possible.
The strong connection to IBD has lead to the hypothesis that long-lived memory cells (lymphocytes) generated in the gut are responsible for the inflammation of the biliary tree in PSC[58]. This hypothesis is supported by the expression, in both liver and intestine in PSC patients, of vascular adhesion protein-1 (VAP-1)[59] and mucosal adressin cell adhesion molecule (MAdCAM)-1[60]. In contrast, MAdCAM-1 and VAP-1 expression in the physiological state is restricted to the gut and liver, respectively. In PSC and other inflammatory liver diseases, MAdCAM-1 may function to recruit intestinally activated T-lymphocytes to the liver[58,61]. It is an intriguing hypothesis that activated lymphocytes generated in this manner in the recipient can attack the new organ in a similar manner, and contribute to the occurrence of recurrent disease in the transplanted liver.
Several studies have focused on the composition of bile in PSC pathogenesis, this is partly based on the findings of a PSC-like disease in mice that lack proteins involved in the transportation of bile components. These changes closely resemble intrahepatic PSC in humans[62-65]. Interestingly, variants in the ABCB4 gene (also called MDR3) influence the progression of PSC[66]. ABCB4 variants are also of importance in the pathogenesis in some forms of intrahepatic cholestasis of pregnancy and type 3 progressive familiar intrahepatic cholestasis[67,68]. The influence of these variants, on disease progression, is necessarily dependent upon the genotype of the liver and it remains to be verified if variants in the donor liver potentially affect the progression of rPSC in a similar manner.
It is also important to identify the risk factors for rPSC because it can reveal essential clues in the pathogenesis of both PSC and rPSC, and potentially influence the management of PSC patients after transplantation. Since the first report on suspected recurrence in the liver graft appeared in 1988[24], potential risk factors have been sought. However, the data on specific risk factors are still limited and non-consistent, serving as an illustration of the complexity of the disease.
Several studies have shown one or more risk factors to be significantly associated with increased risk of rPSC as follows: the presence of HLA-DRB1*08 in either recipient or donor[69], absence of donor HLA DR52[70], recipient-donor gender mismatch[71], male recipient[32], older recipient age[72], younger recipient age[70], intact colon before transplantation[13,32], use of related donor[73,74], use of extended donor criteria (EDC) grafts[13], ACR[69,70], steroid-resistant ACR[69,75], use of OKT3[76], presence of UC after LTX[77], maintenance of steroid therapy for UC for more than 3 mo[77], presence of cholangiocarcinoma (CCA) before transplantation[78] and concurrent cytomegalovirus infection in the recipient[70,73]. Differences between various immunosuppressive regimes used after LTX have been hypothesized to be related to the risk of rPSC, however no such effect has been observed[13,77]. Also, no effect has been found according to post-transplant use of ursodeoxycholic acid (UDCA)[13].
Most of the studies on risk factors for rPSC have appropriately performed multivariate analysis and have found one or more factors to be significantly associated with rPSC. Nevertheless, almost none of the findings are reproduced by other groups. The discrepant results are not so surprising, considering that some of the studies included only a limited number of patients and were thus prone to both false positive and negative findings.
One of the risk factors that seem to be best documented is the link between IBD and recurrent disease in the liver allograft. This was first reported in a study by Vera et al[32] in 2002, which demonstrated a dramatic reduction in the risk of recurrence if the colon was removed before or at the time of the transplantation. This study evaluated 152 patients and found male gender and intact colon after transplantation to be the strongest predictors of rPSC. Fifty-six (37%) patients developed rPSC during follow-up, but only 1 (6%) of 17 patients who underwent colectomy before or at the time of LTX had recurrence. The importance of IBD in rPSC was considerably strengthened in a study by Cholongitas et al[77] in 2007 evaluating 69 patients receiving LTX for PSC. In this series, none of the PSC patients without UC or patients undergoing pre-LTX total colectomy developed rPSC. On the contrary, recurrence occurred in 7 (27%) of 26 patients with post-LTX UC. In their multivariate regression analysis, UC with the need for maintenance steroids for more than three months was the only risk factor significantly associated with rPSC. In 2009, Alabraba et al[13] published the largest study to date on risk factors for rPSC, of note, this study was a re-review and extension of the cohort studied by Vera et al[32]. A total of 230 consecutive adult patients who underwent liver transplantation for PSC were included. The protective effect of colectomy before or during LTX on the risk of developing rPSC was confirmed, while colectomy after LTX had no beneficial effect on the incidence of recurrent disease[13]. Taken together, these three studies give a relatively strong indication that absence of inflammation in the intestine, either due to absence of concurrent IBD or colectomy before or during LTX has a protective effect against rPSC[13,32,77]. These findings are consistent with the hypothesis of a common T-cell recruitment theme in UC and PSC, as reviewed by Grant et al[61], that may also be relevant in rPSC. Importantly, these data should not be interpreted as an advocation for pre-transplant colectomy that may in theory increase the risks for surgical complications during transplantation, but rather as input to understanding the mechanisms of rPSC development.
On the other side of arguments, Alexander et al[69] in a study on 69 patients transplanted for PSC, found no correlation of rPSC to IBD or the presence of an intact colon after transplantation. Neither did Gautam et al[28] in a systematic review from 2006 on rPSC, including only studies with available raw data. To investigate how different immunosuppressive regimens affected the natural course of PSC patients after transplantation, Kugelmas et al[76] reviewed a cohort of 71 patients with a 21% recurrence rate. No differences in the frequency of rPSC was observed in the patients with and without IBD, however they did find that OKT3 therapy for steroid-resistant ACR was associated with a higher risk of rPSC.
A few other groups have also independently reported an association between ACR and recurrent disease. In a study of 118 consecutive liver transplantations for PSC, Jeyarajah et al[70] found a significantly higher incidence of ACR in recipients that later developed rPSC or chronic rejection. Alexander et al[69] found ACR and steroid-resistant ACR to be predictive of an increased risk of rPSC and in a study of 49 patients transplanted for PSC evaluated by magnetic resonance cholangiography (MRC), steroid-resistant ACR was the only significant predictor for rPSC[75].
Up to date, the possible mechanism behind the association between ACR and an increased risk of recurrent disease is unknown. The biliary epithelium is one of the components that is attacked and injured in ACR, and it has been postulated that this can result in an increase in autoimmune epitopes, leading to ductal damage mediated by the immune system[70]. Others have suggested the existence of a common factor predisposing to both ACR and recurrent disease. Especially, the fact that PSC patients seem to be more prone to ACR than most other patient groups, has led to the speculation of a common link between the pathophysiology of rPSC and ACR[69]. One hypothesis is the existence of a hyperactive component of the immune system, and another theory is that the increased risk of both ACR and rPSC results from a defective mechanism in the immune regulation.
What is also worth mentioning is a reported higher rate of recurrence in recipients of grafts from living related donors, with rPSC occurring in 55% and 59% of the transplanted PSC patients[73,74]. Both studies involved a relatively low number of patients, but should urgently inspire further investigations in larger cohorts to confirm important pathogenic mechanisms.
Since the report by Lerut et al[24], a number of studies have indicated that biliary complications, especially nonanastomotic biliary strictures occurred more frequently after liver transplantation for PSC than for other end-stage liver diseases[79-85].
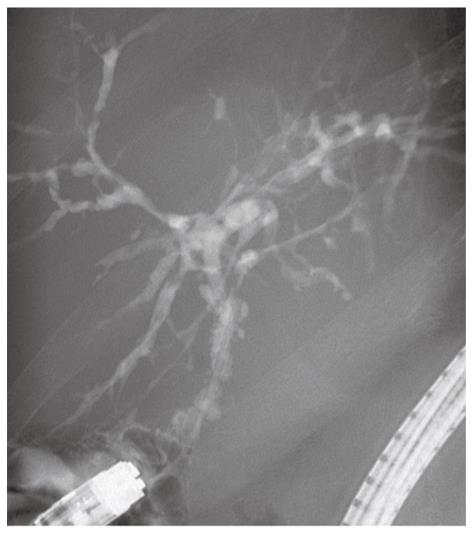
As with the diagnosis of PSC in the native liver, rPSC is diagnosed based on the combination of radiological, histological and biochemical findings. The diagnosis is considered problematic because of the difficulty in distinguishing rPSC from other conditions with similar biliary changes. A variety of potential insults to the liver graft can all result in biliary injury and stricturing[28,86]. In 1999, Graziadei et al[87] established specific criteria for diagnosing rPSC. This is a useful definition that has been followed since (Table 2). Strict biochemical indices or typical clinical symptoms are frequently absent in patients with rPSC. These patients often present with a cholestatic biochemical profile, and further examinations with cholangiography with either endoscopic retrograde cholangiopancreatography (ERCP), MRC or percutaneous cholangiography (PTC) show characteristic changes of the bile ducts with multifocal strictures and segmental dilatations[88]. However, emphasis must be put on exclusion of other causes that can cause similar radiographic and histological changes. Accordingly, the diagnosis is made in patients with a confirmed diagnosis of PSC prior to transplantation, and by either typical histological features on biopsy or by radiological demonstration of multiple nonanastomotic strictures occurring more than 90 d after LTX (Table 2 and Figure 1).
| Diagnosis | ||
| Confirmed diagnosis of primary sclerosing cholangitis prior to liver transplantation | ||
| And | ||
| Cholangiography | Histology | |
| Intrahepatic and/or extrahepatic biliary stricturing, beading, and irregularity > 90 d | Or | Fibrous cholangitis and/or fibro-obliterative lesions with or without ductopenia, biliary fibrosis, or biliary cirrhosis |
| Exclusion criteria | ||
| Hepatic artery thrombosis/stenosis | ||
| Established ductopenic rejection | ||
| Anastomotic strictures alone | ||
| Non Anastomotic strictures before post-transplantation day 90 | ||
| ABO incompatibility between donor and recipient | ||
As PSC affects both the intra- and extra-hepatic bile ducts, most recipients will have a Roux-en-Y loop after LTX[89,90]. This renders access to the bile ducts via the endoscopic route technically challenging[91]. Some centers have addressed this challenge using single or double balloon enteroscopy and in this way are able to perform ERCP in patients with complex postsurgical gastrointestinal anatomy[92,93]. It is also worth mentioning that some centers have reported preliminary good results after bile duct reconstruction by direct choledochoduodenostomy[94,95], resulting in an anastomosis that have the advantage of easier access by conventional ERCP. Still, PTC or MRC is most widely used in the post-LTX population[92,96,97]. The typical findings on cholangiography in rPSC involve multifocal nonanastomotic strictures in both intra- and extrahepatic bile ducts, with intervening segments of normal or dilated ducts[86]. Sheng et al[85] compared the radiological signs of rPSC with other conditions after LTX. They included 32 patients transplanted for PSC who had developed biliary strictures in the graft and a control group of 32 non-PSC grafts who also had developed biliary strictures. This study demonstrated that intrahepatic strictures were much more common in patients with rPSC and the appearance of the strictures was different, mural irregularity and diverticular outpouchings being more common in rPSC. Even though these changes occurred significantly more frequently in patients with rPSC [15 (47%) patients and 6 (19%) patients, respectively], in the majority of the cases, it was impossible to distinguish rPSC from other conditions based solely on cholangiographic findings.
Several studies have evaluated the diagnostic accuracy of MRC in PSC patients[98-100]. A meta-analysis published last year found that MRC had a sensitivity and specificity of 86% and 94%, respectively in detecting PSC[100]. The latest guidelines by both the European Association for the Study of the Liver (EASL) and the American Association for the Study of Liver Diseases (AASLD) recommend MRC rather than ERCP in patients with suspected PSC[101,102]. Nevertheless, some have raised concerns regarding the sensitivity of MRC when it comes to subtle intrahepatic changes[103] or in detailed characterization of extrahepatic biliary changes[104]. There has been no study so far comparing ERCP or PTC with MRC in rPSC and it is thus not clear whether the sensitivity/specificity figures are similar to those in the primary disease. Importantly, the quality of the MRC imaging is continuously and rapidly evolving and with the noninvasive nature and reduced cost, MRC has become the first choice for most clinicians to evaluate abnormalities of the extra- and intrahepatic bile ducts after LTX.
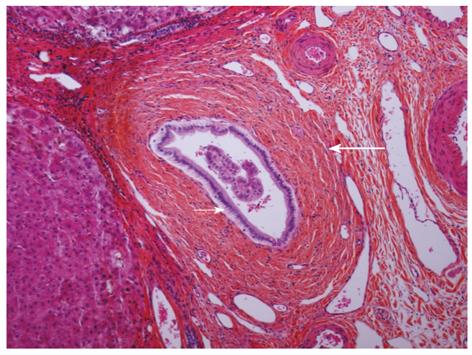
The use of liver biopsies in diagnosing both PSC and rPSC is usually regarded supplementary to cholangiography. This is partly due to the relative lack of specificity and the patchy involvement resulting in a certain degree of sample variability[27]. Histopathologically, the changes in rPSC (Figure 2) are identical to what is described in the native liver with PSC and can be extremely difficult to distinguish from biliary strictures of other causes in allografts, including recurrent biliary infections, ischemia due to arterial problems, chronic rejection, small-for-size syndrome, blood group incompatibility or reperfusion injury[86,105]. The early changes in rPSC are characterized by mild, nonspecific (peri) cholangitis and loss of small bile ducts. As the disease progresses, periductal lamellar edema, increased neutrophil and eosinophil inflammation in the portal tracts, periportal edema and an increased ductular reaction are becoming apparent. Marked deposits of copper with Mallory’s hyaline in periportal hepatocytes can often be seen[26]. In medium and small bile ducts, the typical features of fibro-obliterative lesions are observed at this stage together with focal loss of medium and small bile ducts. Lobular changes in the early stages of rPSC include mild nodular regenerative hyperplasia. In the later stages, the characteristic changes of cholestasis, biliary cirrhosis, foam cell clusters and marked deposition of copper in the periportal hepatocytes are observed[105]. In the extrahepatic and large bile ducts of the hilum, ulceration, bile sludge, periductal fibrosis and foam cell accumulation are often seen[105].
The distinction between chronic rejection and rPSC is often challenging, as both conditions can cause loss of bile ducts and a cholestatic pattern of liver enzyme elevation[106]. The diagnosis of both rPSC and chronic rejection should therefore be based on a combination of histopathological, laboratory, radiological and clinical findings. In both conditions, it is important to review prior biopsies combined with the clinical course, and hence for the pathologist to take the clinical history into account. Often there is a history of failed compliance or episodes of severe rejection with unsatisfactory response to treatment in chronic rejection[105].
There is currently no proven medical therapy to halt or slow the progression of PSC or rPSC. The major focus regarding pharmacological treatment in PSC has been a possible effect of UDCA on the disease course. Some of the first studies could document an improvement in both hepatic, biochemical and histological parameters[107-109], but this effect did not significantly benefit transplant-free survival. In the three largest studies[110-112] using UDCA at different doses, no beneficial effect was found on the risk of liver transplantation or death. This conclusion is further supported by a recent meta-analysis by Shi et al[113]. In rPSC, no effect has been shown from any specific treatment. Some centers recommend discontinuation of corticosteroid therapy[26] and several centers prescribe UDCA[25]. Patients with coexisting UC may benefit from UDCA in reducing the risk of developing carcinoma in the colon[114], but regarding a possible effect on the progression of rPSC, documentation is so far lacking. The choice of immunosuppression seems to have no influence on the incidence or progression to rPSC[76]. This is, however, a complicated research field and difficult to review in retrospective studies, because detailed information for each patient is needed, including any potential changes in immunosuppressive medication and the disease stage where the change of medication took place. In this study, OKT3 was found associated with a higher risk of recurrence[76], but it is difficult to determine if it is the treatment or the reason for giving the treatment that had a deleterious effect.
Three retrospective studies on the effect of endoscopic treatment of dominant strictures in PSC patients have all suggested improved 3- and 5-year survival rates[115-117]. Whether endoscopic treatment influences the progression of rPSC is currently unknown. There have been no published studies with this specific focus in patients with recurrent disease, but based on the effect observed in PSC patients before LTX, further studies of endoscopic treatment modalities should be encouraged.
Earlier studies reported no difference in graft or patient survival among recipients with or without rPSC[10,11]. Long-term data, however, indicate that recurrent disease has a significant impact on graft survival, rate of retransplantations and perhaps also patient survival[12,13]. A study from the United Network for Organ Sharing (UNOS) database on LTX in more than 3000 PSC patients compared with a similar number of patients with primary biliary cirrhosis (PBC), who have a very good outcome after liver transplantation found the retransplantation rate to be significantly higher in PSC patients than in PBC (12.4% vs 8.5%). PSC patients had significantly lower graft and patient survival than PBC patients after adjusting for other risk factors and a diagnosis of PSC was an independent predictor for retransplantation[118]. The reduced survival did not become evident until 7 years after the primary operation. In a retrospective study performed by Rowe et al[12] analyzing graft loss due to recurrence in 1840 patients undergoing primary LTX between 1982 and 2004, the risk of graft loss from recurrent disease was significantly higher in PSC patients than in PBC patients (hazard ratio 6.0; 95% CI 2.5-14.2). Cholongitas et al[77] reported in 2008 a recurrence rate of 13.5% after transplantation for PSC, and 43% of the patients with recurrence required retransplantation. In this study, no difference in survival was seen, but the number was relatively small with a total of 69 patients transplanted for PSC. In the largest series reported to date[13], rPSC developed in 61 grafts in 54 patients, of which, 23 grafts in 20 patients were lost and 13 retransplantations were carried out. After exclusion of all patients surviving less than 6 mo and adjustment for age, there was significantly better survival in patients without rPSC. This is not surprising, considering that retransplantation usually is a much more complicated procedure than the primary operation. A range of reports has shown that retransplantations, independent of the underlying disease, have a significantly worse outcome than the primary procedure[119-124]. In a review of the outcome after retransplantations (for any underlying cause) in 196 patients over a 25-year period at Queen Elisabeth Hospital, the 5- and 10-year survival after retransplantation were 57% and 47%, respectively[119]. The five-year survival after a second retransplantation was 40%. The risk of perioperative death increases significantly from less than 5% in the primary procedure to almost 20% in retransplantations[120,121].
There is little doubt that the incidence of rejection after liver transplantation has been declining, yet still 20%-40% of the recipients will experience at least one episode of ACR requiring additional immunosuppressive treatment[125]. PSC patients have been suggested to have a higher risk of ACR compared with other liver recipients[10,126,127]. There are nevertheless relatively few published studies with specific focus on the incidence of rejection after LTX according to underlying disease, and the reported incidence varies substantially in different studies. In an attempt to clarify whether this patient population really is at an increased risk of rejection, we identified eleven publications containing specific data on the incidence of acute rejection in patients transplanted for PSC. These studies include a total of 656 patients transplanted for PSC, of whom, 373 (57%) recipients (range, 17%-100% in individual studies) had one or more episodes of acute rejection (Table 3) In the largest study including 150 consecutive transplantations for PSC, at least one episode of acute rejection occurred in 103 (69%) of the recipients, while the incidence in the control group was 59% (261/440)[10]. The reported incidence of ACR after LTX shows a large discrepancy between different centers and time periods. This is the case regarding ACR after LTX for PSC, as well as for other underlying diseases. Evolving immunosuppressive strategy clearly has a significant influence. No data regarding immunosuppression trends are currently available from Europe, but based on individual series and data from the UNOS registry, the majority of centers use triple drug therapy, including calcineurin inhibitors (CNIs), corticosteroids and antimetabolite drugs. However, the immunosuppressive regimens vary among centers and the use of induction therapy is inconsistent. Another important factor influencing the reported number of ACR is the use of protocol biopsies or not. An additional confounder is the fact that diagnostic criteria for rejection vary largely in earlier reports. This situation improved considerably with the introduction of the Banff classification in 1997[128]. Based on the current data, and the general difficulties in reporting rejection rates as discussed above, it is hard to state with certainty that transplanted PSC patients carry a higher risk of ACR than other patient groups. There are for instance several publications that show an equally high or even higher incidence of acute rejection in patients transplanted for PBC[84,127,129-131] and autoimmune hepatitis (AIH)[127,130,131]. What seems to be clear though is that there is a tendency that patients with an underlying “autoimmune” condition have an increased risk of ACR after LTX. Whether this is due to a generally more “active” immune system in these patients is unclear. Another interesting and relatively consistent finding in many reports supporting this hypothesis, is that patients treated for non-immunological conditions, like alcoholic liver disease or fulminant hepatic failure from paracetamol intoxication, seem to be at an especially low risk for acute rejection[127,130-133].
| Ref. | Yr | No. ofPSC patients | Median follow-up(range) in months | ACRn (%) | Diagnostic criteria for rejection | Immunosuppression |
| Klintmalm et al[179] | 1988 | 9 | ND (ND) | 4 (44) | Biopsy | CyA, CS and azathioprine |
| McEntee et al[180] | 1991 | 44 | ND (ND) | 44 (100) | ND | ND |
| Shaked et al[181] | 1992 | 36 | ND (ND) | 6 (17) | Biopsy | CyA/Tac, CS and azathioprine |
| Miki et al[3] | 1994 | 55 | ND (ND) | 37 (67) | Biopsy | CyA/Tac, CS and azathioprine |
| Narumi et al[30] | 1995 | 33 | 37.8 (6-73) | 19(57) | Biopsy | ATG for 3-5 d, CS, azathioprine and delayed CyA |
| Farges et al[131] | 1996 | 23 | Minimal follow-up 18 mo | 12 (52) | Biopsy | CyA/Tac, CS and azathioprine |
| Jeyarajah et al[70] | 1998 | 115 | Minimal follow-up 12 mo | 45 (39) | Biopsy | CyA/Tac, CS and azathioprine |
| Wiesner et al[130] | 1998 | 126 | 34 | 58 (46) | Biopsy | CyA/Tac, CS and azathioprine (1 group received induction with ATG) |
| Graziadei et al[10] | 1999 | 150 | 55 (10-138) | 103 (69.7) | Biopsy | CyA/Tac, CS and azathioprine |
| Bathgate et al[182] | 2000 | 16 | ND (ND) | 10 (63) | Biopsy | ND |
| Brandsaeter et al[75] | 2005 | 49 | 77 (17-182) | 35 (71) | Biopsy | CyA/Tac, CS and azathioprine/ MMF |
In general, rejection of an allograft can be defined as “an immunologic reaction to the presence of a foreign tissue or organ that has the potential to result in graft dysfunction and failure”[134]. Since the start of experimental solid organ transplantation, it has been obvious that the liver has an immunological advantage over other organs. In several animal transplant models, spontaneous tolerance was achieved after LTX, while skin, heart or kidney transplants were aggressively rejected in the same models[135-139]. Tolerance following liver transplantation is rare in humans, however ACR is usually easily reversed.
Findings in studies on factors that increase the risk of acute rejection after LTX include: use of living donor[140], lack of induction treatment with anti-IL-2 receptor antibody[141,142], severe preservation injury[143], younger age of the recipient[130], lack of renal failure[130], fewer HLA-DR matches[130,144], cold ischemia time greater than 15 h[130] and being transplanted for PSC[10,30,70,87], PBC[129], autoimmune hepatitis[127] or HCV infection[127]. In addition, a meta-analysis of cytokine gene polymorphisms and acute liver graft rejection suggests a role for genetic variation at the IL-10 locus[145].
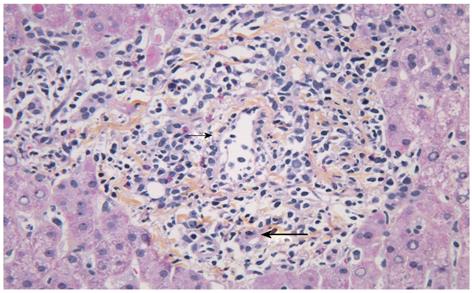
For PSC, both disease recurrence and the presumed increased incidence of ACR have been speculated to be caused by a continuum of ongoing immunological attack against the bile ducts and the liver[10]. Whether different immune mechanisms are involved in the rejection process in PSC patients vs patients with other underlying disease is not known. To date, no study has focused on characterizing histopathological differences in the inflammatory infiltrates of ACR according to the underlying disease. The typical histological features described in ACR in the liver allograft (Figure 3) are similar to that in other organs, the dominant cell types being CD4+ and CD8+ T cells and macrophages[128,146]. B-cells, neutrophils and eosinophils have been observed in varying proportions, and scattered cells positive for the natural killer (NK) marker CD56+ have been described[147]. NK cells have emerged as a particular focus of interest in the transplant setting because of their ability to recognize allogenic major histo-compatibility complex (MHC) antigens and their potent cytolytic activity[148]. They possess a variety of inhibitory and activating receptors, such as killer immunoglobulin-like receptors (KIRs), which recognize MHC class I molecules, and based on the “missing self” hypothesis, kill target cells that display reduced levels of MHC class I antigens. Interestingly, it has been shown that particular combinations of KIRs and HLA class I ligands that reduce NK cell inhibition increase the susceptibility to autoimmune diseases[149], and that certain genetic variants of ligands for NK cell receptors might contribute to the risk of PSC[150,151]. An increase in NK cells in the liver has been observed in PSC as compared with other liver diseases[152,153]. The effect of NK epitope mismatching on acute rejection after liver transplantation is uncertain. The few studies conducted so far have given conflicting results[154-156]. A recent paper by Hanvesakul et al[155] on 416 liver transplant recipients showed a striking influence of donor HLA-C genotype on graft survival and chronic rejection, but no effect on ACR. To what extent such phenomena may be restricted to patients with PSC has not been determined.
As previously addressed, there is conflicting data about whether the presence of IBD has an adverse impact on the risk of recurrence of PSC after the transplantation. Some reports also suggest that PSC patients with concomitant IBD may be at increased risk of ACR. In a study by Narumi et al[30], the incidence of moderate or severe rejection in patients with IBD was 70% vs 36% in PSC patients without IBD, and 37 % in a matched control group. This was further supported by a study by Miki et al[3] in which 87% of the patients with IBD developed ACR while only 41% of the patients without IBD developed ACR. Consistent with these studies, Gradziadei et al[10] also reported a significantly increased risk of ACR in patients with IBD in their large cohort of 150 transplanted PSC patients. On the other hand, in the study by Jeyarajah et al[70], including 118 transplantations for PSC, no association between concomitant IBD and ACR was found.
As opposed to kidney transplantation[157-159], there is no convincing data indicating that ACR in the early phase after liver transplantation (for any underlying condition) affects long-term graft or patient survival. After the introduction of more potent and specific immunosuppression, it is easier to prevent episodes of ACR and a major challenge in LTX today is the morbidity and mortality related to side effects of long- term use of immunosuppressive medication[157-160]. In animal models with spontaneous tolerance, the liver allograft undergoes an initial and transient acute rejection-like reaction[161-163], that could indicate that the histopathological diagnosis of ACR does not necessarily always require treatment, but as some argue that it might be a first step on the way to a degree of spontaneous tolerance.
Data regarding the incidence and significance of late acute rejection (LAR) are scarce, but an interpretation might indicate a risk of more serious consequences than with early ACR. LAR has been variably defined in different reports ranging from one, three, six or twelve months after the transplantation. The incidence of LAR after liver transplantation in general varies from 7% to 23% in the published reports[126,164-168]. In a study of more than 1600 LTX patients, where LAR was defined as ACR occurring later than six months after LTX, the incidence was 19%[126]. Interestingly, the study also showed that patients with a primary diagnosis of autoimmune hepatitis, PBC and PSC had the highest incidence of LAR and that patient and graft survival was significantly lower in the LAR group. Another study also reported an increased risk of LAR in PSC patients but did not find any negative effect on patient survival[168].
ACR represents an immunological insult towards the graft and could influence the risk of developing rPSC since more reactive lymphocytes are likely to be present. As previously mentioned, ACR or steroid resistant ACR were found to have a significant effect on the risk of developing recurrent disease[69,70,75,76]. In contrast, most studies evaluating risk factors for rPSC have not found an association with ACR. There is little data supporting the conclusion that mild or moderate ACR, affecting approximately 50% of the transplanted PSC patients, has any effect on the risk of recurrent disease. The question is if severe rejection is associated with recurrence or not. As of today, there is not enough data to answer this question with certainty.
Rejection and recurrence of the primary disease in the liver allograft remain as two major challenges in the clinical care of post-transplant PSC patients. Even as there are huge discrepancies in the reported incidence of rPSC, it is at least present in approximately 1/5 of the transplanted patients. The pathogenesis of rPSC remains enigmatic and is believed to have similar features as the primary disease. There is so far an underutilized potential in studying the pathogenesis of the primary disease in parallel with recurrent disease, an approach that potentially can uncover shared mechanisms relevant to both conditions. Several studies have identified one or more risk factors for rPSC, but few have been confirmed from one study to another. The most convincing data seem to be the link with concurrent IBD and a protective effect on the development of rPSC of colectomy before or at the time of transplantation[13,32,77]. There is, importantly, no reason to advocate colectomy prior to liver transplantation for PSC on this basis. There seems also to be an increased incidence of acute rejection in PSC patients with a potential relevance to the development of recurrence. Rejection and recurrence might therefore represent a continuum of immunological affection of the graft in transplanted PSC patients.
We are specially thankful to Grzyb Krzysztof for immunohistochemical images and Audun E Berstad for radiological images.
Peer reviewer: Teng-Yu Lee, MD, Division of Gastroenterology and Hepatology, Department of Internal Medicine, Taichung Veterans General Hospital, 160, Sec. 3, Taichung Harbor Road, Taichung 407, Taiwan, China
S- Editor Tian L L- Editor Ma JY E- Editor Zhang DN
| 1. | Farrant JM, Hayllar KM, Wilkinson ML, Karani J, Portmann BC, Westaby D, Williams R. Natural history and prognostic variables in primary sclerosing cholangitis. Gastroenterology. 1991;100:1710-1717. [PubMed] |
| 2. | Tischendorf JJ, Hecker H, Krüger M, Manns MP, Meier PN. Characterization, outcome, and prognosis in 273 patients with primary sclerosing cholangitis: A single center study. Am J Gastroenterol. 2007;102:107-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 288] [Cited by in RCA: 271] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 3. | Miki C, Harrison JD, Gunson BK, Buckels JA, McMaster P, Mayer AD. Inflammatory bowel disease in primary sclerosing cholangitis: an analysis of patients undergoing liver transplantation. Br J Surg. 1995;82:1114-1117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 46] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 4. | Shorbagi A, Bayraktar Y. Primary sclerosing cholangitis--what is the difference between east and west? World J Gastroenterol. 2008;14:3974-3981. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 26] [Cited by in RCA: 26] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 5. | Aadland E, Schrumpf E, Fausa O, Elgjo K, Heilo A, Aakhus T, Gjone E. Primary sclerosing cholangitis: a long-term follow-up study. Scand J Gastroenterol. 1987;22:655-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 181] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 6. | Broomé U, Olsson R, Lööf L, Bodemar G, Hultcrantz R, Danielsson A, Prytz H, Sandberg-Gertzén H, Wallerstedt S, Lindberg G. Natural history and prognostic factors in 305 Swedish patients with primary sclerosing cholangitis. Gut. 1996;38:610-615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 596] [Cited by in RCA: 544] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 7. | Karlsen TH, Schrumpf E, Boberg KM. Update on primary sclerosing cholangitis. Dig Liver Dis. 2010;42:390-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 78] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 8. | Weismüller TJ, Wedemeyer J, Kubicka S, Strassburg CP, Manns MP. The challenges in primary sclerosing cholangitis--aetiopathogenesis, autoimmunity, management and malignancy. J Hepatol. 2008;48 Suppl 1:S38-S57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 109] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 9. | Adam R, Hoti E. Liver transplantation: the current situation. Semin Liver Dis. 2009;29:3-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 91] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 10. | Graziadei IW, Wiesner RH, Marotta PJ, Porayko MK, Hay JE, Charlton MR, Poterucha JJ, Rosen CB, Gores GJ, LaRusso NF. Long-term results of patients undergoing liver transplantation for primary sclerosing cholangitis. Hepatology. 1999;30:1121-1127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 231] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 11. | Goss JA, Shackleton CR, Farmer DG, Arnaout WS, Seu P, Markowitz JS, Martin P, Stribling RJ, Goldstein LI, Busuttil RW. Orthotopic liver transplantation for primary sclerosing cholangitis. A 12-year single center experience. Ann Surg. 1997;225:472-481; discussion 481-483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 213] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 12. | Rowe IA, Webb K, Gunson BK, Mehta N, Haque S, Neuberger J. The impact of disease recurrence on graft survival following liver transplantation: a single centre experience. Transpl Int. 2008;21:459-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 128] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 13. | Alabraba E, Nightingale P, Gunson B, Hubscher S, Olliff S, Mirza D, Neuberger J. A re-evaluation of the risk factors for the recurrence of primary sclerosing cholangitis in liver allografts. Liver Transpl. 2009;15:330-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 196] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 14. | Clesca P, Dirlando M, Park SI, García R, Ferraz E, Pinheiro-Machado PG, Kushnaroff L, Tedesco-Silva H, Medina-Pestana JO. Thymoglobulin and rate of infectious complications after transplantation. Transplant Proc. 2007;39:463-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 15. | Guimarães-Souza NK, Dalboni MA, Câmara NC, Medina-Pestana JO, Paheco-Silva A, Cendoroglo M. Infectious complications after deceased kidney donor transplantation. Transplant Proc. 2010;42:1137-1141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 16. | Selzner N, Grant DR, Shalev I, Levy GA. The immunosuppressive pipeline: meeting unmet needs in liver transplantation. Liver Transpl. 2010;16:1359-1372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 17. | Bambha K, Kim WR, Talwalkar J, Torgerson H, Benson JT, Therneau TM, Loftus EV, Yawn BP, Dickson ER, Melton LJ. Incidence, clinical spectrum, and outcomes of primary sclerosing cholangitis in a United States community. Gastroenterology. 2003;125:1364-1369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 18. | Boberg KM, Aadland E, Jahnsen J, Raknerud N, Stiris M, Bell H. Incidence and prevalence of primary biliary cirrhosis, primary sclerosing cholangitis, and autoimmune hepatitis in a Norwegian population. Scand J Gastroenterol. 1998;33:99-103. [PubMed] |
| 19. | Kingham JG, Kochar N, Gravenor MB. Incidence, clinical patterns, and outcomes of primary sclerosing cholangitis in South Wales, United Kingdom. Gastroenterology. 2004;126:1929-1930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 91] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 20. | Kochhar R, Goenka MK, Das K, Nagi B, Bhasin DK, Chawla YK, Vaiphei K, Singh K, Dilawari JB. Primary sclerosing cholangitis: an experience from India. J Gastroenterol Hepatol. 1996;11:429-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 39] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 21. | Ang TL, Fock KM, Ng TM, Teo EK, Chua TS, Tan JY. Clinical profile of primary sclerosing cholangitis in Singapore. J Gastroenterol Hepatol. 2002;17:908-913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 60] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 22. | Escorsell A, Parés A, Rodés J, Solís-Herruzo JA, Miras M, de la Morena E. Epidemiology of primary sclerosing cholangitis in Spain. Spanish Association for the Study of the Liver. J Hepatol. 1994;21:787-791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 102] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 23. | Bjøro K, Brandsaeter B, Foss A, Schrumpf E. Liver transplantation in primary sclerosing cholangitis. Semin Liver Dis. 2006;26:69-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 63] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 24. | Lerut J, Demetris AJ, Stieber AC, Marsh JW, Gordon RD, Esquivel CO, Iwatsuki S, Starzl TE. Intrahepatic bile duct strictures after human orthotopic liver transplantation. Recurrence of primary sclerosing cholangitis or unusual presentation of allograft rejection? Transpl Int. 1988;1:127-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 25. | Schreuder TC, Hübscher SG, Neuberger J. Autoimmune liver diseases and recurrence after orthotopic liver transplantation: what have we learned so far? Transpl Int. 2009;22:144-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 39] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 26. | Duclos-Vallee JC, Sebagh M. Recurrence of autoimmune disease, primary sclerosing cholangitis, primary biliary cirrhosis, and autoimmune hepatitis after liver transplantation. Liver Transpl. 2009;15 Suppl 2:S25-S34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 69] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 27. | Li KK, Neuberger J. Recurrent nonviral liver disease following liver transplantation. Expert Rev Gastroenterol Hepatol. 2009;3:257-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 28. | Gautam M, Cheruvattath R, Balan V. Recurrence of autoimmune liver disease after liver transplantation: a systematic review. Liver Transpl. 2006;12:1813-1824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 168] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 29. | Harrison RF, Davies MH, Neuberger JM, Hubscher SG. Fibrous and obliterative cholangitis in liver allografts: evidence of recurrent primary sclerosing cholangitis? Hepatology. 1994;20:356-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 30. | Narumi S, Roberts JP, Emond JC, Lake J, Ascher NL. Liver transplantation for sclerosing cholangitis. Hepatology. 1995;22:451-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 31. | Saldeen K, Friman S, Olausson M, Olsson R. Follow-up after liver transplantation for primary sclerosing cholangitis: effects on survival, quality of life, and colitis. Scand J Gastroenterol. 1999;34:535-540. [PubMed] |
| 32. | Vera A, Moledina S, Gunson B, Hubscher S, Mirza D, Olliff S, Neuberger J. Risk factors for recurrence of primary sclerosing cholangitis of liver allograft. Lancet. 2002;360:1943-1944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 138] [Article Influence: 6.0] [Reference Citation Analysis (36)] |
| 33. | Schwartz SI, Dale WA. Primary sclerosing cholangitis; review and report of six cases. AMA Arch Surg. 1958;77:439-451. [PubMed] |
| 34. | Chapman RW, Arborgh BA, Rhodes JM, Summerfield JA, Dick R, Scheuer PJ, Sherlock S. Primary sclerosing cholangitis: a review of its clinical features, cholangiography, and hepatic histology. Gut. 1980;21:870-877. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 35. | Bergquist A, Montgomery SM, Bahmanyar S, Olsson R, Danielsson A, Lindgren S, Prytz H, Hultcrantz R, Lööf LA, Sandberg-Gertzén H. Increased risk of primary sclerosing cholangitis and ulcerative colitis in first-degree relatives of patients with primary sclerosing cholangitis. Clin Gastroenterol Hepatol. 2008;6:939-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 126] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 36. | Karlsen TH, Franke A, Melum E, Kaser A, Hov JR, Balschun T, Lie BA, Bergquist A, Schramm C, Weismüller TJ. Genome-wide association analysis in primary sclerosing cholangitis. Gastroenterology. 2010;138:1102-1111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 260] [Cited by in RCA: 246] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 37. | Melum E, Franke A, Schramm C, Weismüller TJ, Gotthardt DN, Offner FA, Juran BD, Laerdahl JK, Labi V, Björnsson E. Genome-wide association analysis in primary sclerosing cholangitis identifies two non-HLA susceptibility loci. Nat Genet. 2011;43:17-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 180] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 38. | Schrumpf E, Fausa O, Førre O, Dobloug JH, Ritland S, Thorsby E. HLA antigens and immunoregulatory T cells in ulcerative colitis associated with hepatobiliary disease. Scand J Gastroenterol. 1982;17:187-191. [PubMed] |
| 39. | Anderson CA, Boucher G, Lees CW, Franke A, D'Amato M, Taylor KD, Lee JC, Goyette P, Imielinski M, Latiano A. Meta-analysis identifies 29 additional ulcerative colitis risk loci, increasing the number of confirmed associations to 47. Nat Genet. 2011;43:246-252. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1095] [Cited by in RCA: 1048] [Article Influence: 74.9] [Reference Citation Analysis (1)] |
| 40. | Franke A, Balschun T, Karlsen TH, Hedderich J, May S, Lu T, Schuldt D, Nikolaus S, Rosenstiel P, Krawczak M. Replication of signals from recent studies of Crohn's disease identifies previously unknown disease loci for ulcerative colitis. Nat Genet. 2008;40:713-715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 263] [Cited by in RCA: 285] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 41. | Saarinen S, Olerup O, Broomé U. Increased frequency of autoimmune diseases in patients with primary sclerosing cholangitis. Am J Gastroenterol. 2000;95:3195-3199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 125] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 42. | Hov JR, Boberg KM, Karlsen TH. Autoantibodies in primary sclerosing cholangitis. World J Gastroenterol. 2008;14:3781-3791. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 109] [Cited by in RCA: 117] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 43. | Terjung B, Herzog V, Worman HJ, Gestmann I, Bauer C, Sauerbruch T, Spengler U. Atypical antineutrophil cytoplasmic antibodies with perinuclear fluorescence in chronic inflammatory bowel diseases and hepatobiliary disorders colocalize with nuclear lamina proteins. Hepatology. 1998;28:332-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 60] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 44. | Terjung B, Spengler U, Sauerbruch T, Worman HJ. "Atypical p-ANCA" in IBD and hepatobiliary disorders react with a 50-kilodalton nuclear envelope protein of neutrophils and myeloid cell lines. Gastroenterology. 2000;119:310-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 105] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 45. | Klein R, Eisenburg J, Weber P, Seibold F, Berg PA. Significance and specificity of antibodies to neutrophils detected by western blotting for the serological diagnosis of primary sclerosing cholangitis. Hepatology. 1991;14:1147-1152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 47] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 46. | O'Mahony CA, Vierling JM. Etiopathogenesis of primary sclerosing cholangitis. Semin Liver Dis. 2006;26:3-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 93] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 47. | Worthington J, Cullen S, Chapman R. Immunopathogenesis of primary sclerosing cholangitis. Clin Rev Allergy Immunol. 2005;28:93-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 48. | Lichtman SN, Okoruwa EE, Keku J, Schwab JH, Sartor RB. Degradation of endogenous bacterial cell wall polymers by the muralytic enzyme mutanolysin prevents hepatobiliary injury in genetically susceptible rats with experimental intestinal bacterial overgrowth. J Clin Invest. 1992;90:1313-1322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 59] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 49. | Lichtman SN, Keku J, Schwab JH, Sartor RB. Evidence for peptidoglycan absorption in rats with experimental small bowel bacterial overgrowth. Infect Immun. 1991;59:555-562. [PubMed] |
| 50. | Lichtman SN, Keku J, Schwab JH, Sartor RB. Hepatic injury associated with small bowel bacterial overgrowth in rats is prevented by metronidazole and tetracycline. Gastroenterology. 1991;100:513-519. [PubMed] |
| 51. | Lichtman SN, Keku J, Clark RL, Schwab JH, Sartor RB. Biliary tract disease in rats with experimental small bowel bacterial overgrowth. Hepatology. 1991;13:766-772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 52. | Lichtman SN, Sartor RB, Keku J, Schwab JH. Hepatic inflammation in rats with experimental small intestinal bacterial overgrowth. Gastroenterology. 1990;98:414-423. [PubMed] |
| 53. | Boomkens SY, de Rave S, Pot RG, Egberink HF, Penning LC, Rothuizen J, Zondervan PE, Kusters JG. The role of Helicobacter spp. in the pathogenesis of primary biliary cirrhosis and primary sclerosing cholangitis. FEMS Immunol Med Microbiol. 2005;44:221-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 54. | Krasinskas AM, Yao Y, Randhawa P, Dore MP, Sepulveda AR. Helicobacter pylori may play a contributory role in the pathogenesis of primary sclerosing cholangitis. Dig Dis Sci. 2007;52:2265-2270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 31] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 55. | Mehal WZ, Hattersley AT, Chapman RW, Fleming KA. A survey of cytomegalovirus (CMV) DNA in primary sclerosing cholangitis (PSC) liver tissues using a sensitive polymerase chain reaction (PCR) based assay. J Hepatol. 1992;15:396-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 21] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 56. | Minuk GY, Rascanin N, Paul RW, Lee PW, Buchan K, Kelly JK. Reovirus type 3 infection in patients with primary biliary cirrhosis and primary sclerosing cholangitis. J Hepatol. 1987;5:8-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 32] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 57. | Kulaksiz H, Rudolph G, Kloeters-Plachky P, Sauer P, Geiss H, Stiehl A. Biliary candida infections in primary sclerosing cholangitis. J Hepatol. 2006;45:711-716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 45] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 58. | Adams DH, Eksteen B. Aberrant homing of mucosal T cells and extra-intestinal manifestations of inflammatory bowel disease. Nat Rev Immunol. 2006;6:244-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 220] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 59. | Salmi M, Jalkanen S. Endothelial ligands and homing of mucosal leukocytes in extraintestinal manifestations of IBD. Inflamm Bowel Dis. 1998;4:149-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 60. | Grant AJ, Lalor PF, Hübscher SG, Briskin M, Adams DH. MAdCAM-1 expressed in chronic inflammatory liver disease supports mucosal lymphocyte adhesion to hepatic endothelium (MAdCAM-1 in chronic inflammatory liver disease). Hepatology. 2001;33:1065-1072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 207] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 61. | Grant AJ, Lalor PF, Salmi M, Jalkanen S, Adams DH. Homing of mucosal lymphocytes to the liver in the pathogenesis of hepatic complications of inflammatory bowel disease. Lancet. 2002;359:150-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 167] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 62. | Fickert P, Zollner G, Fuchsbichler A, Stumptner C, Weiglein AH, Lammert F, Marschall HU, Tsybrovskyy O, Zatloukal K, Denk H. Ursodeoxycholic acid aggravates bile infarcts in bile duct-ligated and Mdr2 knockout mice via disruption of cholangioles. Gastroenterology. 2002;123:1238-1251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 238] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 63. | Fickert P, Fuchsbichler A, Wagner M, Zollner G, Kaser A, Tilg H, Krause R, Lammert F, Langner C, Zatloukal K. Regurgitation of bile acids from leaky bile ducts causes sclerosing cholangitis in Mdr2 (Abcb4) knockout mice. Gastroenterology. 2004;127:261-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 365] [Cited by in RCA: 349] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 64. | Popov Y, Patsenker E, Fickert P, Trauner M, Schuppan D. Mdr2 (Abcb4)-/- mice spontaneously develop severe biliary fibrosis via massive dysregulation of pro- and antifibrogenic genes. J Hepatol. 2005;43:1045-1054. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 198] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 65. | Nakken KE, Nygård S, Haaland T, Berge KE, Arnkvaern K, Ødegaard A, Labori KJ, Raeder MG. Multiple inflammatory-, tissue remodelling- and fibrosis genes are differentially transcribed in the livers of Abcb4 (-/ - ) mice harbouring chronic cholangitis. Scand J Gastroenterol. 2007;42:1245-1255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 66. | Melum E, Boberg KM, Franke A, Bergquist A, Hampe J, Karlsen TH. Variation in the MDR3 gene influences disease progression in PSC patients and disease susceptibility in epistatic interaction with a polymorphism in the OST-alpha gene. Hepatology. 2007;46:265A. |
| 67. | Smit JJ, Schinkel AH, Oude Elferink RP, Groen AK, Wagenaar E, van Deemter L, Mol CA, Ottenhoff R, van der Lugt NM, van Roon MA. Homozygous disruption of the murine mdr2 P-glycoprotein gene leads to a complete absence of phospholipid from bile and to liver disease. Cell. 1993;75:451-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1080] [Cited by in RCA: 1044] [Article Influence: 32.6] [Reference Citation Analysis (0)] |
| 68. | Trauner M, Fickert P, Wagner M. MDR3 (ABCB4) defects: a paradigm for the genetics of adult cholestatic syndromes. Semin Liver Dis. 2007;27:77-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 144] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 69. | Alexander J, Lord JD, Yeh MM, Cuevas C, Bakthavatsalam R, Kowdley KV. Risk factors for recurrence of primary sclerosing cholangitis after liver transplantation. Liver Transpl. 2008;14:245-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 98] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 70. | Jeyarajah DR, Netto GJ, Lee SP, Testa G, Abbasoglu O, Husberg BS, Levy MF, Goldstein RM, Gonwa TA, Tillery GW. Recurrent primary sclerosing cholangitis after orthotopic liver transplantation: is chronic rejection part of the disease process? Transplantation. 1998;66:1300-1306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 124] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 71. | Khettry U, Keaveny A, Goldar-Najafi A, Lewis WD, Pomfret EA, Pomposelli JJ, Jenkins RL, Gordon FD. Liver transplantation for primary sclerosing cholangitis: a long-term clinicopathologic study. Hum Pathol. 2003;34:1127-1136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 49] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 72. | Abu-Elmagd K, Demetris J, Rakela J, Kang Y, Martin D, Dvorchik I, McMicheal J, Balan V, Starzl T, Fung J. Recurrence of primary sclerosing cholangitis (PSC) after hepatic transplantation (HTx): single center experience with 380 grafts. Abstract of the 18th Annual Meeting of the American Society of Transplantation (AST), May 15-19, 1999. Transplantation. 1999;67:S236. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 73. | Egawa H, Taira K, Teramukai S, Haga H, Ueda Y, Yonezawa A, Masuda S, Tsuji H, Ashihara E, Takada Y. Risk factors for recurrence of primary sclerosing cholangitis after living donor liver transplantation: a single center experience. Dig Dis Sci. 2009;54:1347-1354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 44] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 74. | Haga H, Miyagawa-Hayashino A, Taira K, Morioka D, Egawa H, Takada Y, Manabe T, Uemoto S. Histological recurrence of autoimmune liver diseases after living-donor liver transplantation. Hepatol Res. 2007;37 Suppl 3:S463-S469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 38] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 75. | Brandsaeter B, Schrumpf E, Bentdal O, Brabrand K, Smith HJ, Abildgaard A, Clausen OP, Bjoro K. Recurrent primary sclerosing cholangitis after liver transplantation: a magnetic resonance cholangiography study with analyses of predictive factors. Liver Transpl. 2005;11:1361-1369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 75] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 76. | Kugelmas M, Spiegelman P, Osgood MJ, Young DA, Trotter JF, Steinberg T, Wachs ME, Bak T, Kam I, Everson GT. Different immunosuppressive regimens and recurrence of primary sclerosing cholangitis after liver transplantation. Liver Transpl. 2003;9:727-732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 86] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 77. | Cholongitas E, Shusang V, Papatheodoridis GV, Marelli L, Manousou P, Rolando N, Patch D, Rolles K, Davidson B, Burroughs AK. Risk factors for recurrence of primary sclerosing cholangitis after liver transplantation. Liver Transpl. 2008;14:138-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 119] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 78. | Campsen J, Zimmerman MA, Trotter JF, Wachs M, Bak T, Steinberg T, Kam I. Clinically recurrent primary sclerosing cholangitis following liver transplantation: a time course. Liver Transpl. 2008;14:181-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 111] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 79. | Sheng R, Zajko AB, Campbell WL, Abu-Elmagd K. Biliary strictures in hepatic transplants: prevalence and types in patients with primary sclerosing cholangitis vs those with other liver diseases. AJR Am J Roentgenol. 1993;161:297-300. [PubMed] |
| 80. | Campbell WL, Sheng R, Zajko AB, Abu-Elmagd K, Demetris AJ. Intrahepatic biliary strictures after liver transplantation. Radiology. 1994;191:735-740. [PubMed] |
| 81. | Letourneau JG, Day DL, Hunter DW, Ascher NL, Najarian JS, Thompson WM, Castaneda-Zuniga WR. Biliary complications after liver transplantation in patients with preexisting sclerosing cholangitis. Radiology. 1988;167:349-351. [PubMed] |
| 82. | Ward EM, Kiely MJ, Maus TP, Wiesner RH, Krom RA. Hilar biliary strictures after liver transplantation: cholangiography and percutaneous treatment. Radiology. 1990;177:259-263. [PubMed] |
| 83. | McDonald V, Matalon TA, Patel SK, Brunner MC, Sankary H, Foster P, Williams J. Biliary strictures in hepatic transplantation. J Vasc Interv Radiol. 1991;2:533-538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 40] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 84. | McEntee G, Wiesner RH, Rosen C, Cooper J, Wahlstrom E. A comparative study of patients undergoing liver transplantation for primary sclerosing cholangitis and primary biliary cirrhosis. Transplant Proc. 1991;23:1563-1564. [PubMed] |
| 85. | Sheng R, Campbell WL, Zajko AB, Baron RL. Cholangiographic features of biliary strictures after liver transplantation for primary sclerosing cholangitis: evidence of recurrent disease. AJR Am J Roentgenol. 1996;166:1109-1113. [PubMed] |
| 86. | Graziadei IW. Recurrence of primary sclerosing cholangitis after liver transplantation. Liver Transpl. 2002;8:575-581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 73] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 87. | Graziadei IW, Wiesner RH, Batts KP, Marotta PJ, LaRusso NF, Porayko MK, Hay JE, Gores GJ, Charlton MR, Ludwig J. Recurrence of primary sclerosing cholangitis following liver transplantation. Hepatology. 1999;29:1050-1056. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 277] [Cited by in RCA: 229] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 88. | Abdalian R, Heathcote EJ. Sclerosing cholangitis: a focus on secondary causes. Hepatology. 2006;44:1063-1074. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 134] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 89. | Welsh FK, Wigmore SJ. Roux-en-Y Choledochojejunostomy is the method of choice for biliary reconstruction in liver transplantation for primary sclerosing cholangitis. Transplantation. 2004;77:602-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 46] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 90. | Akamatsu N, Sugawara Y, Hashimoto D. Biliary reconstruction, its complications and management of biliary complications after adult liver transplantation: a systematic review of the incidence, risk factors and outcome. Transpl Int. 2011;24:379-392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 247] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 91. | Ostroff JW. Management of biliary complications in the liver transplant patient. Gastroenterol Hepatol (. NY). 2010;6:264-272. [PubMed] |
| 92. | Saleem A, Baron TH, Gostout CJ, Topazian MD, Levy MJ, Petersen BT, Wong Kee Song LM. Endoscopic retrograde cholangiopancreatography using a single-balloon enteroscope in patients with altered Roux-en-Y anatomy. Endoscopy. 2010;42:656-660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 102] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 93. | Koornstra JJ. Double balloon enteroscopy for endoscopic retrograde cholangiopancreaticography after Roux-en-Y reconstruction: case series and review of the literature. Neth J Med. 2008;66:275-279. [PubMed] |
| 94. | Bennet W, Zimmerman MA, Campsen J, Mandell MS, Bak T, Wachs M, Kam I. Choledochoduodenostomy is a safe alternative to Roux-en-Y choledochojejunostomy for biliary reconstruction in liver transplantation. World J Surg. 2009;33:1022-1025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 95. | Campsen J, Zimmerman MA, Mandell MS, Wachs M, Bak T, Forman L, Steinberg T, Kam I. Hepaticoduodenostomy is an alternative to Roux-en-Y hepaticojejunostomy for biliary reconstruction in live donor liver transplantation. Transplantation. 2009;87:1842-1845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 96. | Weber C, Kuhlencordt R, Grotelueschen R, Wedegaertner U, Ang TL, Adam G, Soehendra N, Seitz U. Magnetic resonance cholangiopancreatography in the diagnosis of primary sclerosing cholangitis. Endoscopy. 2008;40:739-745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 50] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 97. | Dave M, Elmunzer BJ, Dwamena BA, Higgins PD. Primary sclerosing cholangitis: meta-analysis of diagnostic performance of MR cholangiopancreatography. Radiology. 2010;256:387-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 156] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 98. | Vitellas KM, El-Dieb A, Vaswani KK, Bennett WF, Tzalonikou M, Mabee C, Kirkpatrick R, Bova JG. MR cholangiopancreatography in patients with primary sclerosing cholangitis: interobserver variability and comparison with endoscopic retrograde cholangiopancreatography. AJR Am J Roentgenol. 2002;179:399-407. [PubMed] |
| 99. | Textor HJ, Flacke S, Pauleit D, Keller E, Neubrand M, Terjung B, Gieseke J, Scheurlen C, Sauerbruch T, Schild HH. Three-dimensional magnetic resonance cholangiopancreatography with respiratory triggering in the diagnosis of primary sclerosing cholangitis: comparison with endoscopic retrograde cholangiography. Endoscopy. 2002;34:984-990. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 57] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 100. | Dave M, Elmunzer BJ, Dwamena BA, Higgins PD. Primary sclerosing cholangitis: meta-analysis of diagnostic performance of MR cholangiopancreatography. Radiology. 2010;256:387-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 156] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 101. | Chapman R, Fevery J, Kalloo A, Nagorney DM, Boberg KM, Shneider B, Gores GJ. Diagnosis and management of primary sclerosing cholangitis. Hepatology. 2010;51:660-678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 888] [Cited by in RCA: 833] [Article Influence: 55.5] [Reference Citation Analysis (0)] |
| 102. | EASL Clinical Practice Guidelines: management of cholestatic liver diseases. J Hepatol. 2009;51:237-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1382] [Cited by in RCA: 1202] [Article Influence: 75.1] [Reference Citation Analysis (1)] |
| 103. | Angulo P, Pearce DH, Johnson CD, Henry JJ, LaRusso NF, Petersen BT, Lindor KD. Magnetic resonance cholangiography in patients with biliary disease: its role in primary sclerosing cholangitis. J Hepatol. 2000;33:520-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 40] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 104. | Moff SL, Kamel IR, Eustace J, Lawler LP, Kantsevoy S, Kalloo AN, Thuluvath PJ. Diagnosis of primary sclerosing cholangitis: a blinded comparative study using magnetic resonance cholangiography and endoscopic retrograde cholangiography. Gastrointest Endosc. 2006;64:219-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 65] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 105. | Demetris AJ. Distinguishing between recurrent primary sclerosing cholangitis and chronic rejection. Liver Transpl. 2006;12:S68-S72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 106. | Adeyi O, Fischer SE, Guindi M. Liver allograft pathology: approach to interpretation of needle biopsies with clinicopathological correlation. J Clin Pathol. 2010;63:47-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 39] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 107. | Stiehl A, Walker S, Stiehl L, Rudolph G, Hofmann WJ, Theilmann L. Effect of ursodeoxycholic acid on liver and bile duct disease in primary sclerosing cholangitis. A 3-year pilot study with a placebo-controlled study period. J Hepatol. 1994;20:57-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 141] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 108. | Mitchell SA, Bansi DS, Hunt N, Von Bergmann K, Fleming KA, Chapman RW. A preliminary trial of high-dose ursodeoxycholic acid in primary sclerosing cholangitis. Gastroenterology. 2001;121:900-907. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 201] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 109. | Beuers U, Spengler U, Kruis W, Aydemir U, Wiebecke B, Heldwein W, Weinzierl M, Pape GR, Sauerbruch T, Paumgartner G. Ursodeoxycholic acid for treatment of primary sclerosing cholangitis: a placebo-controlled trial. Hepatology. 1992;16:707-714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 324] [Cited by in RCA: 297] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 110. | Lindor KD. Ursodiol for primary sclerosing cholangitis. Mayo Primary Sclerosing Cholangitis-Ursodeoxycholic Acid Study Group. N Engl J Med. 1997;336:691-695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 421] [Cited by in RCA: 380] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 111. | Olsson R, Boberg KM, de Muckadell OS, Lindgren S, Hultcrantz R, Folvik G, Bell H, Gangsøy-Kristiansen M, Matre J, Rydning A. High-dose ursodeoxycholic acid in primary sclerosing cholangitis: a 5-year multicenter, randomized, controlled study. Gastroenterology. 2005;129:1464-1472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 262] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 112. | Lindor KD, Kowdley KV, Luketic VA, Harrison ME, McCashland T, Befeler AS, Harnois D, Jorgensen R, Petz J, Keach J. High-dose ursodeoxycholic acid for the treatment of primary sclerosing cholangitis. Hepatology. 2009;50:808-814. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 572] [Cited by in RCA: 490] [Article Influence: 30.6] [Reference Citation Analysis (0)] |
| 113. | Shi J, Li Z, Zeng X, Lin Y, Xie WF. Ursodeoxycholic acid in primary sclerosing cholangitis: meta-analysis of randomized controlled trials. Hepatol Res. 2009;39:865-873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 50] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 114. | Pardi DS, Loftus EV, Kremers WK, Keach J, Lindor KD. Ursodeoxycholic acid as a chemopreventive agent in patients with ulcerative colitis and primary sclerosing cholangitis. Gastroenterology. 2003;124:889-893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 423] [Cited by in RCA: 355] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 115. | Stiehl A, Rudolph G, Klöters-Plachky P, Sauer P, Walker S. Development of dominant bile duct stenoses in patients with primary sclerosing cholangitis treated with ursodeoxycholic acid: outcome after endoscopic treatment. J Hepatol. 2002;36:151-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 180] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 116. | Baluyut AR, Sherman S, Lehman GA, Hoen H, Chalasani N. Impact of endoscopic therapy on the survival of patients with primary sclerosing cholangitis. Gastrointest Endosc. 2001;53:308-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 127] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 117. | Gluck M, Cantone NR, Brandabur JJ, Patterson DJ, Bredfeldt JE, Kozarek RA. A twenty-year experience with endoscopic therapy for symptomatic primary sclerosing cholangitis. J Clin Gastroenterol. 2008;42:1032-1039. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 72] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 118. | Maheshwari A, Yoo HY, Thuluvath PJ. Long-term outcome of liver transplantation in patients with PSC: a comparative analysis with PBC. Am J Gastroenterol. 2004;99:538-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 63] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 119. | Marudanayagam R, Shanmugam V, Sandhu B, Gunson BK, Mirza DF, Mayer D, Buckels J, Bramhall SR. Liver retransplantation in adults: a single-centre, 25-year experience. HPB (. Oxford). 2010;12:217-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 79] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 120. | Reese PP, Yeh H, Thomasson AM, Shults J, Markmann JF. Transplant center volume and outcomes after liver retransplantation. Am J Transplant. 2009;9:309-317. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 50] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 121. | Akpinar E, Selvaggi G, Levi D, Moon J, Nishida S, Island E, DeFaria W, Pretto E, Ruiz P, Tzakis AG. Liver retransplantation of more than two grafts for recurrent failure. Transplantation. 2009;88:884-890. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 122. | Postma R, Haagsma EB, Peeters PM, van den Berg AP, Slooff MJ. Retransplantation of the liver in adults: outcome and predictive factors for survival. Transpl Int. 2004;17:234-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 123. | Facciuto M, Heidt D, Guarrera J, Bodian CA, Miller CM, Emre S, Guy SR, Fishbein TM, Schwartz ME, Sheiner PA. Retransplantation for late liver graft failure: predictors of mortality. Liver Transpl. 2000;6:174-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 124. | Markmann JF, Markowitz JS, Yersiz H, Morrisey M, Farmer DG, Farmer DA, Goss J, Ghobrial R, McDiarmid SV, Stribling R. Long-term survival after retransplantation of the liver. Ann Surg. 1997;226:408-418; discussion 418-420. [PubMed] |
| 125. | Neil DA, Hübscher SG. Current views on rejection pathology in liver transplantation. Transpl Int. 2010;23:971-983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 102] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 126. | Uemura T, Ikegami T, Sanchez EQ, Jennings LW, Narasimhan G, McKenna GJ, Randall HB, Chinnakotla S, Levy MF, Goldstein RM. Late acute rejection after liver transplantation impacts patient survival. Clin Transplant. 2008;22:316-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 66] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 127. | Neuberger J. Incidence, timing, and risk factors for acute and chronic rejection. Liver Transpl Surg. 1999;5:S30-S36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 128. | Banff schema for grading liver allograft rejection: an international consensus document. Hepatology. 1997;25:658-663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1016] [Cited by in RCA: 1001] [Article Influence: 35.8] [Reference Citation Analysis (1)] |
| 129. | Seiler CA, Dufour JF, Renner EL, Schilling M, Büchler MW, Bischoff P, Reichen J. Primary liver disease as a determinant for acute rejection after liver transplantation. Langenbecks Arch Surg. 1999;384:259-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 130. | Wiesner RH, Demetris AJ, Belle SH, Seaberg EC, Lake JR, Zetterman RK, Everhart J, Detre KM. Acute hepatic allograft rejection: incidence, risk factors, and impact on outcome. Hepatology. 1998;28:638-645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 383] [Cited by in RCA: 338] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 131. | Farges O, Saliba F, Farhamant H, Samuel D, Bismuth A, Reynes M, Bismuth H. Incidence of rejection and infection after liver transplantation as a function of the primary disease: possible influence of alcohol and polyclonal immunoglobulins. Hepatology. 1996;23:240-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 17] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 132. | Garcia RF, Garcia CE, McMaster P. Chronic rejection of the liver: the role of immunosuppression. BioDrugs. 2000;14:283-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 133. | Burra P, Senzolo M, Adam R, Delvart V, Karam V, Germani G, Neuberger J. Liver transplantation for alcoholic liver disease in Europe: a study from the ELTR (European Liver Transplant Registry). Am J Transplant. 2010;10:138-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 248] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 134. | Terminology for hepatic allograft rejection. International Working Party. Hepatology. 1995;22:648-654. [PubMed] |
| 135. | Knechtle SJ, Kwun J. Unique aspects of rejection and tolerance in liver transplantation. Semin Liver Dis. 2009;29:91-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 66] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 136. | Calne R. Immunological tolerance: the liver effect. J Gastroenterol Hepatol. 2002;17 Suppl:S488-S490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 137. | Flye MW, Pennington L, Kirkman R, Weber B, Sindelar W, Sachs DH. Spontaneous acceptance or rejection of orthotopic liver transplants in outbred and partially inbred miniature swine. Transplantation. 1999;68:599-607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 138. | Kamada N, Calne RY. A surgical experience with five hundred thirty liver transplants in the rat. Surgery. 1983;93:64-69. [PubMed] |
| 139. | Jahr H, Wolff H. [The liver--an immunologically privileged organ?]. Allerg Immunol (Leipz). 1989;35:155-166. [PubMed] |
| 140. | Shaked A, Ghobrial RM, Merion RM, Shearon TH, Emond JC, Fair JH, Fisher RA, Kulik LM, Pruett TL, Terrault NA. Incidence and severity of acute cellular rejection in recipients undergoing adult living donor or deceased donor liver transplantation. Am J Transplant. 2009;9:301-308. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 140] [Cited by in RCA: 128] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 141. | Tippner C, Nashan B, Hoshino K, Schmidt-Sandte E, Akimaru K, Böker KH, Schlitt HJ. Clinical and subclinical acute rejection early after liver transplantation: contributing factors and relevance for the long-term course. Transplantation. 2001;72:1122-1128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 142. | Randomised trial comparing tacrolimus (FK506) and cyclosporin in prevention of liver allograft rejection. European FK506 Multicentre Liver Study Group. Lancet. 1994;344:423-428. [PubMed] |
| 143. | Howard TK, Klintmalm GB, Cofer JB, Husberg BS, Goldstein RM, Gonwa TA. The influence of preservation injury on rejection in the hepatic transplant recipient. Transplantation. 1990;49:103-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 218] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 144. | Lan X, Zhang MM, Pu CL, Guo CB, Kang Q, Li YC, Dai XK, Deng YH, Xiong Q, Ren ZM. Impact of human leukocyte antigen mismatching on outcomes of liver transplantation: a meta-analysis. World J Gastroenterol. 2010;16:3457-3464. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 61] [Cited by in RCA: 56] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 145. | Warlé MC, Metselaar HJ, Hop WC, Tilanus HW. Cytokine gene polymorphisms and acute liver graft rejection: a meta-analysis. Liver Transpl. 2005;11:19-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 46] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 146. | Adams D. Mechanisms of liver allograft rejection in man. Clin Sci (Lond). 1990;78:343-350. [PubMed] |
| 147. | Jain A, Ryan C, Mohanka R, Orloff M, Abt P, Romano J, Bryan L, Batzold P, Mantry P, Bozorgzadeh A. Characterization of CD4, CD8, CD56 positive lymphocytes and C4d deposits to distinguish acute cellular rejection from recurrent hepatitis C in post-liver transplant biopsies. Clin Transplant. 2006;20:624-633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 41] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 148. | Pratschke J, Stauch D, Kotsch K. Role of NK and NKT cells in solid organ transplantation. Transpl Int. 2009;22:859-868. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 36] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 149. | Parham P. MHC class I molecules and KIRs in human history, health and survival. Nat Rev Immunol. 2005;5:201-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 873] [Cited by in RCA: 902] [Article Influence: 45.1] [Reference Citation Analysis (0)] |
| 150. | Karlsen TH, Boberg KM, Olsson M, Sun JY, Senitzer D, Bergquist A, Schrumpf E, Thorsby E, Lie BA. Particular genetic variants of ligands for natural killer cell receptors may contribute to the HLA associated risk of primary sclerosing cholangitis. J Hepatol. 2007;46:899-906. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 67] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 151. | Hov JR, Lleo A, Selmi C, Woldseth B, Fabris L, Strazzabosco M, Karlsen TH, Invernizzi P. Genetic associations in Italian primary sclerosing cholangitis: heterogeneity across Europe defines a critical role for HLA-C. J Hepatol. 2010;52:712-717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 40] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 152. | Hashimoto E, Lindor KD, Homburger HA, Dickson ER, Czaja AJ, Wiesner RH, Ludwig J. Immunohistochemical characterization of hepatic lymphocytes in primary biliary cirrhosis in comparison with primary sclerosing cholangitis and autoimmune chronic active hepatitis. Mayo Clin Proc. 1993;68:1049-1055. [PubMed] |
| 153. | Hata K, Van Thiel DH, Herberman RB, Whiteside TL. Phenotypic and functional characteristics of lymphocytes isolated from liver biopsy specimens from patients with active liver disease. Hepatology. 1992;15:816-823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 39] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 154. | Bishara A, Brautbar C, Zamir G, Eid A, Safadi R. Impact of HLA-C and Bw epitopes disparity on liver transplantation outcome. Hum Immunol. 2005;66:1099-1105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 155. | Hanvesakul R, Spencer N, Cook M, Gunson B, Hathaway M, Brown R, Nightingale P, Cockwell P, Hubscher SG, Adams DH. Donor HLA-C genotype has a profound impact on the clinical outcome following liver transplantation. Am J Transplant. 2008;8:1931-1941. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 44] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 156. | Moya-Quiles MR, Alvarez R, Miras M, Gomez-Mateo J, Lopez-Alvarez MR, Marin-Moreno I, Martínez-Barba E, Sanchez-Mozo MP, Gomez M, Arnal F. Impact of recipient HLA-C in liver transplant: a protective effect of HLA-Cw*07 on acute rejection. Hum Immunol. 2007;68:51-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 157. | Moreso F, Ibernon M, Gomà M, Carrera M, Fulladosa X, Hueso M, Gil-Vernet S, Cruzado JM, Torras J, Grinyó JM. Subclinical rejection associated with chronic allograft nephropathy in protocol biopsies as a risk factor for late graft loss. Am J Transplant. 2006;6:747-752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 245] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 158. | Emiroğlu R, Yagmurdur MC, Karakayali F, Haberal C, Ozcelik U, Colak T, Haberal M. Role of donor age and acute rejection episodes on long-term graft survival in cadaveric kidney transplantations. Transplant Proc. 2005;37:2954-2956. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 159. | Meier-Kriesche HU, Ojo AO, Hanson JA, Cibrik DM, Punch JD, Leichtman AB, Kaplan B. Increased impact of acute rejection on chronic allograft failure in recent era. Transplantation. 2000;70:1098-1100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 145] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 160. | Hübscher S. Diagnosis and grading of liver allograft rejection: a European perspective. Transplant Proc. 1996;28:504-507. [PubMed] |
| 161. | Calne RY, Sells RA, Pena JR, Davis DR, Millard PR, Herbertson BM, Binns RM, Davies DA. Induction of immunological tolerance by porcine liver allografts. Nature. 1969;223:472-476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 162. | Kamada N. The immunology of experimental liver transplantation in the rat. Immunology. 1985;55:369-389. [PubMed] |
| 163. | Qian S, Demetris AJ, Murase N, Rao AS, Fung JJ, Starzl TE. Murine liver allograft transplantation: tolerance and donor cell chimerism. Hepatology. 1994;19:916-924. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 325] [Cited by in RCA: 346] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 164. | Mor E, Gonwa TA, Husberg BS, Goldstein RM, Klintmalm GB. Late-onset acute rejection in orthotopic liver transplantation--associated risk factors and outcome. Transplantation. 1992;54:821-824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 78] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 165. | Cakaloglu Y, Devlin J, O'Grady J, Sutherland S, Portmann BC, Heaton N, Tan KC, Williams R. Importance of concomitant viral infection during late acute liver allograft rejection. Transplantation. 1995;59:40-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 42] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 166. | Anand AC, Hubscher SG, Gunson BK, McMaster P, Neuberger JM. Timing, significance, and prognosis of late acute liver allograft rejection. Transplantation. 1995;60:1098-1103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 68] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 167. | Ramji A, Yoshida EM, Bain VG, Kneteman NM, Scudamore CH, Ma MM, Steinbrecher UP, Gutfreund KS, Erb SR, Partovi N. Late acute rejection after liver transplantation: the Western Canada experience. Liver Transpl. 2002;8:945-951. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 36] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 168. | Florman S, Schiano T, Kim L, Maman D, Levay A, Gondolesi G, Fishbein T, Emre S, Schwartz M, Miller C. The incidence and significance of late acute cellular rejection (> 1000 days) after liver transplantation. Clin Transplant. 2004;18:152-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 169. | Kubota T, Thomson A, Clouston AD, Nakazawa Y, Steadman C, Kerlin P, Shimada H, Balderson GA, Lynch SV, Strong RW. Clinicopathologic findings of recurrent primary sclerosing cholangitis after orthotopic liver transplantation. J Hepatobiliary Pancreat Surg. 1999;6:377-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 170. | Lidén H, Norrby J, Gäbel M, Friman S, Olausson M. Outcome after liver transplantation for primary sclerosing cholangitis. Transplant Proc. 2001;33:2452-2453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 171. | Renz JF, Ascher NL. Liver transplantation for nonviral, nonmalignant diseases: problem of recurrence. World J Surg. 2002;26:247-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 172. | Yusoff IF, House AK, De Boer WB, Ferguson J, Garas G, Heath D, Mitchell A, Jeffrey G. Disease recurrence after liver transplantation in Western Australia. J Gastroenterol Hepatol. 2002;17:203-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 173. | Khuroo MS, Al Ashgar H, Khuroo NS, Khan MQ, Khalaf HA, Al-Sebayel M, El Din Hassan MG. Biliary disease after liver transplantation: the experience of the King Faisal Specialist Hospital and Research Center, Riyadh. J Gastroenterol Hepatol. 2005;20:217-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 174. | Ołdakowska-Jedynak U, Nowak M, Mucha K, Foroncewicz B, Nyckowski P, Zieniewicz K, Ziarkiewicz-Wróblewska B, Patkowski W, Górnicka B, Paczkowska A. Recurrence of primary sclerosing cholangitis in patients after liver transplantation. Transplant Proc. 2006;38:240-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 175. | Yamagiwa S, Ichida T. Recurrence of primary biliary cirrhosis and primary sclerosing cholangitis after liver transplantation in Japan. Hepatol Res. 2007;37 Suppl 3:S449-S454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 176. | Tamura S, Sugawara Y, Kaneko J, Matsui Y, Togashi J, Makuuchi M. Recurrence of primary sclerosing cholangitis after living donor liver transplantation. Liver Int. 2007;27:86-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 50] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 177. | Kashyap R, Mantry P, Sharma R, Maloo MK, Safadjou S, Qi Y, Jain A, Maliakkal B, Ryan C, Orloff M. Comparative analysis of outcomes in living and deceased donor liver transplants for primary sclerosing cholangitis. J Gastrointest Surg. 2009;13:1480-1486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 50] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 178. | Moncrief KJ, Savu A, Ma MM, Bain VG, Wong WW, Tandon P. The natural history of inflammatory bowel disease and primary sclerosing cholangitis after liver transplantation--a single-centre experience. Can J Gastroenterol. 2010;24:40-46. [PubMed] |
| 179. | Klintmalm GB, Nery JR, Husberg BS, Gonwa TA, Tillery GW. Rejection in liver transplantation. Hepatology. 1989;10:978-985. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 114] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 180. | McEntee G, Wiesner RH, Rosen C, Cooper J, Wahlstrom E. A comparative study of patients undergoing liver transplantation for primary sclerosing cholangitis and primary biliary cirrhosis. Transplant Proc. 1991;23:1563-1564. [PubMed] |
| 181. | Shaked A, Colonna JO, Goldstein L, Busuttil RW. The interrelation between sclerosing cholangitis and ulcerative colitis in patients undergoing liver transplantation. Ann Surg. 1992;215:598-603; discussion 604-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 58] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 182. | Bathgate AJ, Pravica V, Perrey C, Therapondos G, Plevris JN, Hayes PC, Hutchinson IV. The effect of polymorphisms in tumor necrosis factor-alpha, interleukin-10, and transforming growth factor-beta1 genes in acute hepatic allograft rejection. Transplantation. 2000;69:1514-1517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 82] [Article Influence: 3.3] [Reference Citation Analysis (0)] |