Published online Mar 7, 2011. doi: 10.3748/wjg.v17.i9.1234
Revised: September 25, 2010
Accepted: October 2, 2010
Published online: March 7, 2011
Russell body gastritis is an unusual form of chronic gastritis characterized by the permeation of lamina propria by numerous plasma cells with eosinophilic cytoplasmic inclusions. Very few cases have been reported in the literature; the majority of which have shown Helicobacter Pylori (H. pylori) infection, thus suggesting a correlation between plasma cell presence and antigenic stimulation by H. pylori. We present a case of Russell body gastritis in a 78-year-old woman who was undergoing esophagogastroduodenoscopy for epigastric pain. Gastric biopsy of the gastroesophageal junction showed the presence of cells with periodic acid-Schiff-positive hyaline pink bodies. Giemsa staining for H. pylori infection was negative, as well as immunohistochemical detection. The cells with eosinophilic inclusions stained positive for CD138, CD79a, and κ and lambda light chains, which confirmed plasma cell origin. In particular, κ and lambda light chains showed a polyclonal origin and the patient was negative for immunological dyscrasia. The histological observations were confirmed by ultrastructural examination. The cases reported in the literature associated with H. pylori infection have shown regression of plasma cells after eradication of H. pylori. Nothing is known about the progression of H. pylori-negative cases. The unusual morphological appearance of this type of chronic gastritis should not be misinterpreted during routine examination, and it should be distinguished from other common forms of chronic gastritis. It is mandatory to exclude neoplastic diseases such as gastric carcinoma, lymphoma and plasmocytoma by immunohistochemistry and electron microscopy, which can help with differential diagnosis. The long-term effects of plasma cells hyperactivation are still unknown, because cases of gastric tumor that originated in patients affected by Russell body gastritis have not been described in the literature. We are of the opinion that these patients should be scheduled for endoscopic surveillance.
- Citation: Gobbo AD, Elli L, Braidotti P, Nuovo FD, Bosari S, Romagnoli S. Helicobacter pylori-negative Russell body gastritis: Case report. World J Gastroenterol 2011; 17(9): 1234-1236
- URL: https://www.wjgnet.com/1007-9327/full/v17/i9/1234.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i9.1234
Russell body gastritis is an unusual form of chronic gastritis. Its distinctive histological feature is permeation of the lamina propria by plasma cells (called Mott cells) that contain eosinophilic cytoplasmic inclusions (Russell bodies), which displace the nuclei to the periphery of the cell[1]. Patients generally present with non-specific symptoms, such as epigastric pain, dyspepsia and nausea, which suggests the presence of gastric inflammation. In the majority of the reported cases, concomitant infection with Helicobacter pylori (H. pylori) has been documented, which suggests plasma cell infiltration as the natural consequence of the infection and antigenic stimulation[2-6], or immunosuppression as in HIV infection[7,8] and Epstein-Barr virus-associated carcinoma[9].
We report a case of Russell body gastritis not associated with H. pylori infection as rarely observed previously[7,10].
A 78-year-old female patient was admitted to the Gastroenterology Unit of the Fondazione IRCCS Cà-Granda Ospedale Maggiore Policlinico to undergo esophagogastroduodenoscopy (EGDS) for epigastric pain. EGDS showed a hyperemic gastric mucosa, and biopsies were taken from the antral region and the esophagogastric junction. Histological examination showed moderate chronic gastritis in the antral region with no polymorphonuclear neutrophil activity, glandular atrophy of the gastric mucosa, or intestinal metaplasia, according to Sydney classification system[11].
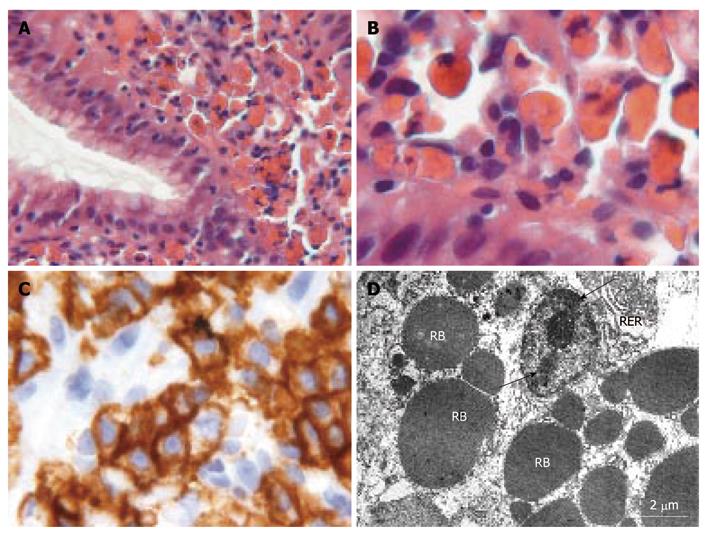
The lamina propria of the gastroesophageal junction mucosa showed the presence of cells with hyaline pink bodies that were periodic acid-Schiff (PAS)-positive and PAS-diastase-resistant (Figure 1A and B). No mitotic activity or atypia was observed. Giemsa staining for H. pylori infection in the antral and cardiac regions was negative, as was immunohistochemical detection.
The cells with eosinophilic inclusions stained positive for CD138 (Figure 1C), CD79a, and κ and lambda light chains, and negative for cytokeratin pool and leukocyte common antigen. κ and lambda light chains showed a polyclonal origin of plasma cells. Evaluation for immunological dyscrasia was negative. Ultrastructural examination showed the presence of plasma cells with an abundance of round and electron-dense material, up to 5 μm in diameter, in the rough endoplasmic reticulum (RER) (Figure 1D). These findings were suggestive of a diagnosis of Russell body gastritis.
The patient performed a 13C urea breath test (UBT), which gave a negative result; as did abdominal ultrasonographic examination, chest X-ray, electrocardiographic study and routine biochemical analysis. In the absence of confirmed H. pylori infection, the patient was treated with proton pump inhibitors, which led to resolution of epigastric pain, and long-term clinical endoscopic follow-up was scheduled.
The first case of Russell body gastritis was described in 1998, when Tazawa and Tsutsumi reported a localized accumulation of plasma cells with Russell bodies in the gastric mucosa, in association with H. pylori infection[3].
Many authors have suggested that chronic antigenic stimulation caused by H. pylori infection can result in overproduction of immunoglobulins by plasma cells. In contrast with the majority of case reports that have been positive for H. pylori[1-3,5,6,8], our patient, at the time of examination, was negative by histology and UBT. Although we could not exclude a precedent infection, it is possible to hypothesize a different etiology for Russell body gastritis, as in the case described by Erbersdobler et al[10], which was associated with ethanol and analgesic abuse and a history of fungal esophagitis. Recently, vacA and cagA H. pylori genotypes, which are characterized by increased pathogenicity, have been associated with the development of Russell bodies and Mott cells in the antral mucosa[12]. However, we think that a direct link between H. pylori infection and Mott cells in the gastric mucosa has yet to be demonstrated because the high frequency of H. pylori infection in western countries is not associated with an increase in Russell body gastritis, which is still a rare event.
As in our patient, subjects affected by Russell body gastritis are generally women, from 47 to 80 years old (median: 60 years), with non-specific symptoms (abdominal and epigastric pain, dyspepsia and/or nausea) that overlap with those of irritable bowel syndrome, and without specific endoscopic markers. These could be the reasons for the rare diagnosis of Russell body gastritis, which is probably underestimated.
Russell body gastritis must be clearly differentiated from neoplastic diseases, such as signet ring carcinoma, MALToma and plasmacytoma[3]. Most often, the differential diagnosis is with monoclonal gammopathy of undetermined significance, which develops after chronic antigen stimulation (in the present case, H. pylori antigens) in subjects with a genetic predisposition. Immunohistochemistry is essential to exclude a monoclonal origin of these plasma cells[4]. Absence of nuclear atypia and mitosis, lack of lymphoepithelial lesions, and a polyclonal pattern of the plasma cells are factors that favor diagnosis of Russell body gastritis when negative staining for cytokeratins rules out carcinoma.
The cases reported in literature associated with H. pylori infection show that eradication of H. pylori leads to regression of the activated plasma cells, with the disappearance of Russell bodies and Mott cells[6]. Nothing is known about the progression of H. pylori-negative cases.
Russell body gastritis is an unusual and rare form of chronic gastritis; it can be associated with H. pylori infection, and it can be misinterpreted during ordinary routine examination.
The peculiar presence of Mott cells in the lamina propria of the gastric mucosa needs to be distinguished from other common forms of chronic gastritis, and it is mandatory to exclude neoplastic diseases such as gastric carcinoma, lymphoma and plasmocytoma by means of immunohistochemistry and electron microscopy, which can help in the differential diagnosis.
The long-term effects of plasma cell hyperactivation are still unknown. Gastric tumors that originate in patients with Russell body gastritis are not described in the literature; however, since the etiopathogenesis of this entity is not completely understood, we are of the opinion that these patients should be scheduled for endoscopic surveillance.
Peer reviewers: Dr. T Choli-Papadopoulou, Associate Professor, Department of Biochemistry, Aristotle University of Thessaloniki, School of Chemistry, Thessaloniki, 55124, Greece; Dr. Nawfal Hussein, PhD, Centre for Biomolecular Sciences, University of Nottingham, University Park, Nottingham, NG7 2RD, United Kingdom
S- Editor Sun H L- Editor Kerr C E- Editor Zheng XM
| 1. | Tazawa K, Tsutsumi Y. Localized accumulation of Russell body-containing plasma cells in gastric mucosa with Helicobacter pylori infection: 'Russell body gastritis'. Pathol Int. 1998;48:242-244. |
| 2. | Ensari A, Savas B, Okcu Heper A, Kuzu I, Idilman R. An unusual presentation of Helicobacter pylori infection: so-called "Russell body gastritis". Virchows Arch. 2005;446:463-466. |
| 3. | Paik S, Kim SH, Kim JH, Yang WI, Lee YC. Russell body gastritis associated with Helicobacter pylori infection: a case report. J Clin Pathol. 2006;59:1316-1319. |
| 4. | Wolkersdörfer GW, Haase M, Morgner A, Baretton G, Miehlke S. Monoclonal gammopathy of undetermined significance and Russell body formation in Helicobacter pylori gastritis. Helicobacter. 2006;11:506-510. |
| 5. | Stewart CJ, Spagnolo DV. Crystalline plasma cell inclusions in helicobacter-associated gastritis. J Clin Pathol. 2006;59:851-854. |
| 6. | Pizzolitto S, Camilot D, DeMaglio G, Falconieri G. Russell body gastritis: expanding the spectrum of Helicobacter pylori - related diseases? Pathol Res Pract. 2007;203:457-460. |
| 7. | Drut R, Olenchuk AB. Images in pathology. Russell body gastritis in an HIV-positive patient. Int J Surg Pathol. 2006;14:141-142. |
| 8. | Licci S, Sette P, Del Nonno F, Ciarletti S, Antinori A, Morelli L. Russell body gastritis associated with Helicobacter pylori infection in an HIV-positive patient: case report and review of the literature. Z Gastroenterol. 2009;47:357-360. |
| 9. | Shinozaki A, Ushiku T, Fukayama M. Prominent Mott cell proliferation in Epstein-Barr virus-associated gastric carcinoma. Hum Pathol. 2010;41:134-138. |
| 10. | Erbersdobler A, Petri S, Lock G. Russell body gastritis: an unusual, tumor-like lesion of the gastric mucosa. Arch Pathol Lab Med. 2004;128:915-917. |
| 11. | Dixon MF, Genta RM, Yardley JH, Correa P. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol. 1996;20:1161-1181. |
| 12. | Soltermann A, Koetzer S, Eigenmann F, Komminoth P. Correlation of Helicobacter pylori virulence genotypes vacA and cagA with histological parameters of gastritis and patient's age. Mod Pathol. 2007;20:878-883. |