Published online Mar 7, 2011. doi: 10.3748/wjg.v17.i9.1167
Revised: December 22, 2010
Accepted: December 29, 2010
Published online: March 7, 2011
AIM: To assess the significance of interleukin (IL)-24 and vascular endothelial growth factor (VEGF) expression in lymph-node-positive rectal cancer.
METHODS: Between 1998 and 2005, 90 rectal adenocarcinoma patients with lymph node involvement were enrolled. All patients received radical surgery and postoperative pelvic chemoradiotherapy of 50.4-54.0 Gy. Chemotherapy of 5-fluorouracil and leucovorin or levamisole was given intravenously during the first and last week of radiotherapy, and then monthly for about 6 mo. Expression of IL-24 and VEGF was evaluated by immunohistochemical staining of surgical specimens, and their relations with patient characteristics and survival were analyzed. The median follow-up of surviving patients was 73 mo (range: 52-122 mo).
RESULTS: IL-24 expression was found in 81 out of 90 patients; 31 showed weak intensity and 50 showed strong intensity. VEGF expression was found in 64 out of 90 patients. Negative and weak intensities of IL-24 expression were classified as negative expression for analysis. IL-24 expression was significantly reduced in poorly differentiated tumors in comparison with well or moderately differentiated tumors (P = 0.004), N2b to earlier N stages (P = 0.016), and stage IIIc to stage IIIa or IIIb (P = 0.028). The number of involved lymph nodes was also significantly reduced in IL-24-positive patients in comparison with IL-24-negative ones.There was no correlation between VEGF expression and patient characteristics. Expression of IL-24 and VEGF was not correlated with survival, but N stage and stages were significantly correlated with survival.
CONCLUSION: IL-24 expression was significantly correlated with histological differentiation, and inversely correlated with the degree of lymph node involvement in stage III rectal cancer.
- Citation: Choi Y, Roh MS, Hong YS, Lee HS, Hur WJ. Interleukin-24 is correlated with differentiation and lymph node numbers in rectal cancer. World J Gastroenterol 2011; 17(9): 1167-1173
- URL: https://www.wjgnet.com/1007-9327/full/v17/i9/1167.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i9.1167
Treatment outcomes of rectal cancer have been improved with the use of adequate combination therapies and the identification of effective targets, but relapses are still frequent in advanced-stage patients. The survival rate of patients whose tumors are confined to the rectal wall at diagnosis (stage I and II) is > 75%, but those rates are reduced to 30%-60% in higher-stage patients, according to the degree of penetration into the wall, and lymph node involvement[1,2]. In order to yield more credibility and power to a study, we analyzed stage III patients for the identification of new therapeutic targets.
The melanoma differentiation associated gene-7, later renamed as interleukin (IL)-24, was identified by subtraction hybridization from human melanoma cells stimulated with interferon-β and mezerein[3]. The expression of IL-24 is inversely related to human melanoma progression. That is, it is highest in melanocytes and lowest in metastatic melanomas[3,4]. Transfection of IL-24 into melanoma cells reduces growth without a similar effect on normal cells[3], and this antiproliferative activity of IL-24 has also been detected in a variety of cancer cells, such as breast, prostate, cervix, colorectal, lung, and nasopharynx carcinomas, as well as glioblastoma[5-11]. In addition to the antiproliferative effect in cancer cell lines, favorable survival has been marginally identified in high-IL-24-expressing non small cell lung cancer (NSCLC) patients[12]. From a subset analysis, high IL-24 expression has been revealed as a significant prognostic factor in adenocarcinoma patients. Whether high IL-24 expression also has a significant impact on the survival of patients with types of cancers other than NSCLC is unknown.
Multiple anticancer mechanisms of IL-24 have been reported, including cancer-specific apoptosis induction, cell cycle regulation, an ability to inhibit angiogenesis, potent bystander antitumor activity, and a capacity to enhance the sensitivity of tumor cells to radiation and chemotherapy[13]. Among them, new vessel formation is required for tumor growth and metastasis[14]. Vascular endothelial growth factor (VEGF) is one of the most important angiogenic factors[15]. Expression of VEGF is proportional to the degree of carcinogenesis of the colorectum, ranging from 0% in dysplastic adenomas, to 62% in mucosal carcinomas, and 100% in submucosal carcinomas[16]. However, the prognostic significance of VEGF expression in rectal cancers has been inconclusive so far[17-21]. Therefore, we also analyzed VEGF expression in rectal cancer patients.
We carried out the first analysis of the correlation between IL-24 expression and prognostic features in rectal cancer patients. To resolve the unanswered question of the effect of VEGF expression on the survival of rectal cancer patients, while limiting biases related to treatment methods and patient heterogeneity, we restricted the analysis to rectal cancer patients with lymph node metastasis who were treated at a single institution.
In this retrospective study, we reviewed 96 rectal adenocarcinoma patients with pathologic lymph node involvement, who had consecutively undergone radical surgery and postoperative chemoradiotherapy at Dong-a University Hospital, Busan, South Korea between 1998 and 2005. The analysis of these patients was approved by the Institutional Review Board. Ninety patients were included for analysis, while six patients were excluded; two for lack of surgical specimens, and four due to liver metastasis at diagnosis, familial adenomatosis polyposis, adenosquamous cell carcinoma, and mucinous adenocarcinoma, respectively (Table 1).
| n (%) | |
| Age (yr) | |
| Median | 59 |
| Range | 34 - 77 |
| Sex | |
| Male | 47 (52.2) |
| Female | 43 (47.8) |
| Histologic differentiation | |
| Well | 46 (51.1) |
| Moderately | 34 (37.8) |
| Poorly | 10 (11.1) |
| T stage | |
| T1 | 1 (1.1) |
| T2 | 8 (8.9) |
| T3 | 79 (87.8) |
| T4 | 2 (2.2) |
| N stage | |
| N1a | 24 (26.7) |
| N1b | 26 (28.9) |
| N2a | 16 (17.8) |
| N2b | 24 (26.7) |
| Stage | |
| IIIa | 8 (8.9) |
| IIIb | 57 (63.3) |
| IIIc | 25 (27.8) |
| VEGF expression | |
| Negative | 26 (28.9) |
| Positive | 64 (71.1) |
| Intensity of IL-24 expression | |
| Negative | 9 (10.0) |
| Weak | 31 (34.4) |
| Strong | 50 (55.6) |
Thirty-three patients underwent abdominoperineal resection, and 57 underwent low anterior resection. Patients received postoperative chemoradiotherapy from the fourth to sixth week after radical surgery. Patients were positioned in a prone position on a belly board for radiotherapy. Tumor beds were boosted up to 50.4-54 Gy (1.8 Gy, once daily) after 45 Gy pelvic irradiation with 15 MV X-ray using a three-field technique. Two cycles of 5-fluorouracil with levamisole or leucovorin were concurrently given for radiotherapy, and maintenance chemotherapy was done thereafter for about 6 mo. Patients were followed up at 3-mo intervals for 2 years, 4-mo intervals for the next 2 years, and then every 6 mo. The median follow-up period of the surviving patients was 73 mo (range: 52-122 mo). The seventh edition of the American Joint Committee on Cancer TNM staging system (2010) was used for patient analysis.
The immunohistochemical studies for IL-24 and VEGF were performed on formalin-fixed, paraffin-embedded, 4-μm-thick tissue sections, using the avidin-biotin-peroxidase complex method. The primary antibodies were a goat polyclonal antibody against IL-24 (Santa Cruz Biotechnology, Santa Cruz, CA, USA) used at 1:200 dilution and a rabbit polyclonal antibody against VEGF, which recognized the 121, 165 and 189 isoform (Santa Cruz Biotechnology) at a 1:100 dilution. Deparaffinization of all sections was performed through a series of xylene baths, and rehydration was performed with a series of graded alcohol solutions. To enhance the immunoreactivity, microwave antigen retrieval was performed at 750 W for 30 min in Tris EDTA (pH 9.0). After the endogenous peroxidase activity was blocked with 5% hydrogen peroxidase for 10 min, incubation with the primary antibody was performed for 1 h at room temperature. An EnvisionTMChemTM Detection Kit (DakoCytomation, Carpinteria, CA, USA) was used for the secondary antibody at room temperature for 30 min. After the tissue samples were washed in Tris-buffered saline for 10 min, 3, 3’-diaminobenzidine was used as a chromogen, and then Mayer’s hematoxylin counterstain was applied.
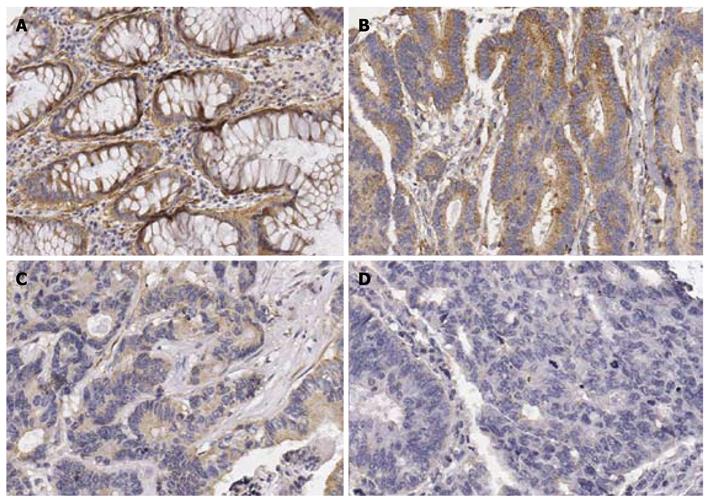
Evaluation of IL-24 expression: IL-24-positive samples were defined as those showing a cytoplasmic staining pattern of the lesional tissue. The staining intensity of IL-24 was graded as follows: 0, negative; 1, weak; 2, strong staining comparable to that seen in a positive control (adjacent normal glands of colonic mucosa) (Figure 1). The negative intensity stood for no stained cells. Weak intensity was allotted when the staining intensity was weaker than that of adjacent normal mucosa or < 5% of cells were stained, and strong intensity when the staining intensity was stronger than that of normal mucosa.
Evaluation of VEGF expression: Immunostaining of VEGF was considered to be positive if unequivocal staining of the membrane or cytoplasm was seen in > 10% of the tumor cells on the slides of the largest section of the tumor.
Survival was calculated from the date of surgery for rectal adenocarcinoma. The Kaplan-Meier method was used for survival analysis, and a log rank test was used for survival difference analysis. Correlation between patient characteristics and IL-24 or VEGF expression was evaluated by Fisher’s exact test. The number of involved lymph nodes according to IL-24 intensity or expression states was analyzed by one-way ANOVA and t test, respectively. Differences were considered significant at P < 0.05. Statistical analyses were carried out with SPSS version 18 (Chicago, IL, USA).
IL-24 expression was found in 81 out of 90 patients: nine negative, 31 weak intensity, and 50 strong intensity. Most cancer cells belonged to the strong intensity group were stained diffusely, so the proportion of immunoreactions was not analyzed. VEGF expression was observed in 64 out of 90 patients. The staining intensity of VEGF was strong in most stained cells, so the difference in terms of intensity of immunoreaction was not assessed.
IL-24 expression was weaker in poorly differentiated tumors compared to well or moderately differentiated tumors. When the negative and weak intensities of IL-24 expression were categorized as negative expression for analysis (Table 2), IL-24 expression was significantly reduced in poorly differentiated tumors compared to well or moderately differentiated tumors, N2b to earlier N stages, and stage IIIc to stage IIIa or IIIb. These significant findings were maintained when another cut-off value of IL-24 was used for analysis (data not shown). There was no significant association between VEGF expression and patients’ characteristics. This non-significance was sustained when other cut-off values of VEGF positivity were applied (data not shown).
| IL-24 | VEGF | |||||
| Negative1 (%) | Positive1 (%) | P value | Negative (%) | Positive (%) | P value | |
| Differentiation | 0.009 | 0.634 | ||||
| Well | 19 (41.3) | 27 (58.7) | 12 (26.1) | 34 (73.9) | ||
| Moderate | 12 (35.3) | 22 (64.7) | 10 (29.4) | 24 (70.6) | ||
| Poor | 9 (90.0) | 1 (10.0) | 0.004 (poor vs others) | 4 (40.0) | 6 (60.0) | |
| N stage | 0.055 | 0.463 | ||||
| N1a | 9 (37.5) | 15 (62.5) | 4 (16.7) | 20 (83.3) | ||
| N1b | 11 (42.3) | 15 (57.7) | 8 (30.8) | 18 (69.2) | ||
| N2a | 4 (25.0) | 12 (75.0) | 6 (37.5) | 10 (62.5) | ||
| N2b | 16 (66.7) | 8 (33.3) | 0.016 (N1~N2a vs N2b) | 8 (33.3) | 16 (66.7) | |
| Stage | 0.055 | 0.164 | ||||
| IIIa | 3 (37.5) | 5 (62.5) | 0 (0.0) | 8 (100.0) | ||
| IIIb | 22 (37.3) | 37 (62.7) | 18 (30.5) | 41 (69.5) | ||
| IIIc | 15 (65.2) | 8 (34.8) | 0.028 (IIIa, IIIb vs IIIc) | 8 (34.8) | 15 (65.2) | |
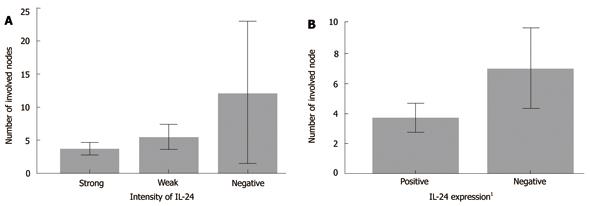
IL-24 expression was inversely proportional to the N stages, so we compared the IL-24 expression status with the number of lymph nodes. The mean numbers of involved lymph nodes in the patients with negative, weak, and strong intensities of IL-24 expression were 12.11 ± 13.878, 5.48 ± 5.253, and 3.70 ± 3.346, respectively (P < 0.05) (Table 3, Figure 2). The numbers of involved lymph nodes in patients with weak and strong intensities were not different after a multiple comparison test using the Tukey B method.
| n | No. of lymph nodes | P value | |
| Intensity of IL-24 expression | 0.001 | ||
| Negative | 9 | 12.11 ± 13.878 | |
| Weak | 31 | 5.48 ± 5.253 | |
| Strong | 50 | 3.70 ± 3.346 | |
| IL-24 expression | 0.012 | ||
| Negative (negative & weak intensity) | 40 | 6.98 ± 8.282 | |
| Positive | 50 | 3.70 ± 3.346 | |
| IL-24 expression | 0.000 | ||
| Negative | 9 | 12.11 ± 13.878 | |
| Positive (weak & strong intensity) | 81 | 4.38 ± 4.238 |
There were significant differences in disease-specific survival (DSS), disease-free survival (DFS), local-recurrence-free survival (LRFS), and distant-metastasis-free survival (DMFS) with regard to N stages (Table 4). DSS, DFS and DMFS were different according to stages. A significant difference in LRFS was found according to histological differentiation. The expressions of IL-24 and VEGF had no effect on survival.
| n | 5-yr disease specific survival | P value | 5-yr disease free survival | P value | 5-yr local recurrence free survival | P value | 5-yr distant metastasis free survival | P value | |
| Age (yr) | |||||||||
| Less than 60 | 48 | 60.4 | 0.963 | 51.8 | 0.918 | 60.3 | 0.683 | 57 | 0.483 |
| 60 or older | 42 | 60.7 | 53.1 | 59.5 | 66.3 | ||||
| Sex | |||||||||
| Male | 47 | 60.3 | 0.742 | 49.2 | 0.528 | 57.4 | 0.903 | 60.3 | 0.760 |
| Female | 43 | 60.8 | 55.8 | 62.8 | 62.6 | ||||
| Differentiation | |||||||||
| Well | 46 | 74.9 | 0.060 | 59.3 | 0.333 | 73.8 | 0.008 | 64.7 | 0.459 |
| Moderately | 34 | 48.5 | 45.7 | 50 | 59.8 | ||||
| Poorly | 10 | 33.3 | 44.4 | 30 | 55.6 | ||||
| T stage | |||||||||
| T1 | 1 | 100 | 0.441 | 100 | 0.427 | 100 | 0.385 | 100 | 0.298 |
| T2 | 8 | 87.5 | 87.5 | 75 | 87.5 | ||||
| T3 | 79 | 57.6 | 48.2 | 58.2 | 57.3 | ||||
| T4 | 2 | 50 | 50 | 50 | 100 | ||||
| N stage | |||||||||
| N1a | 24 | 82.6 | 0.004 | 73.7 | 0.003 | 74.8 | 0.001 | 77 | 0.015 |
| N1b | 26 | 59.4 | 55.7 | 69.2 | 68.5 | ||||
| N2a | 16 | 68.8 | 50 | 68.8 | 56.3 | ||||
| N2b | 24 | 34.2 | 29.3 | 29.2 | 40.8 | ||||
| Stage | |||||||||
| IIIa | 8 | 100 | 0.002 | 100 | 0.002 | 100 | 0.230 | 100 | 0.009 |
| IIIb | 59 | 64.6 | 54.1 | 80.1 | 63 | ||||
| IIIc | 23 | 35.8 | 30.7 | 64.5 | 42.7 | ||||
| VEGF expression | |||||||||
| Negative | 26 | 53.1 | 0.801 | 50 | 0.851 | 73.3 | 0.629 | 64.1 | 0.791 |
| Positive | 64 | 61.9 | 53.5 | 81.1 | 60.5 | ||||
| IL-24 expression | |||||||||
| Negative | 40 | 59.2 | 0.759 | 50.7 | 0.890 | 60 | 0.496 | 60.7 | 0.849 |
| Positive | 50 | 34.7 | 53.9 | 59.9 | 61.6 |
The stage has been known as the most important prognostic factor in colorectal cancer patients until now. The seventh AJCC TNM classification (2010) subdivides N stages according to the number of involved lymph nodes[22]. N1a is metastasis in one regional node, N1b in two or three, N2a in four to six, and N2b in seven or more. In this study, patients were distributed throughout the N stages. That is, the proportions of the patient study group with cancer in the N1a, N1b, N2a and N2b stages were 26%, 29%, 18% and 26%, respectively. However, patients were not distributed evenly in terms of T stages: 79% were in stage T3. Therefore, to gain reasonable results from the T stage analysis was not possible, unlike the N stage analysis. The higher stages correlated significantly with poorer outcomes in terms of DSS, DFS, LRFS and DMFS (P < 0.05). In addition, higher N stages also had poorer survival rates compared to lower ones. The N stages and stages from the seventh AJCC staging system seem to be applicable, although the subgroup patient numbers were not large in our analysis.
IL-24 promotes growth suppression and induces apoptosis in a broad array of human cancers, after forced expression by means of a plasmid or a replication-competent adenovirus, but it does not induce growth suppressive or toxic effects in normal cells[23]. To the best of our knowledge, the clinical significance of IL-24 expression in rectal cancer patients has not been assessed. This is believed to be the first study to analyze the association between IL-24 expression and patient prognostic features in lymph-node-involved rectal cancer patients.
IL-24 expression was weaker in the poorly differentiated tumors, N2b stage, and stage IIIc compared to well or moderately differentiated tumors, N1 or N2a stage, and stage IIIa or IIIb, respectively (Table 2). Moreover, the number of involved lymph nodes was significantly lower in patients with IL-24 expression compared to those without (Table 3). Even though IL-24 expression showed a significant correlation with these prognostic features, the status of IL-24 expression did not affect survival. This discrepancy may in part be explained by an inadequate patient number in the poor prognostic subgroups, and undetermined appropriate cut-off values for IL-24 positivity. Therefore, further studies with larger numbers of patients are necessary in order to develop an adequate grading system of IL-24 expression, and to verify whether IL-24 expression has a prognostic value.
The clinical significance of VEGF expression in rectal cancer is still open to debate. Several studies have insisted that increased VEGF expression is associated with poor prognosis, but some studies have shown that VEGF expression is not related to survival. Casinu et al [21]have claimed from their analysis of lymph-node-positive rectal cancer patients that patients with VEGF-positive tumors have lower event-free survival rates and more frequent distant metastases. However, Bertolini et al[18] have found from their study of locally advanced rectal cancer patients that VEGF expression obtained from pretreatment and post-chemoradiotherapy specimens does not show any significant correlation with DFS and overall survival (OS). In the study of Soumaoro et al[24], OS was worse in colorectal cancer patients with VEGF expression, but this prognostic independence disappeared after multivariate analysis. Our study used unhampered surgical specimens without exposure to any chemoradiotherapy for immunohistochemical staining. VEGF expression was found in 64 out of 90 lymph-node-involved rectal cancer patients. There was no significant survival difference in the rectal cancer patients with regard to VEGF expression in spite of applying several cut-off values for VEGF expression. To verify the influence of VEGF expression on survival in advanced rectal cancer patients, further studies with large numbers of patients are required.
VEGF expression has been reported to be significantly correlated with tumor size, lymph node metastasis, lymphatic invasion, and TNM stage in colorectal cancer patients[24,25]. It is also significantly associated with lymph node involvement in patients with locally advanced rectal cancer[26]. However, these associations with VEGF expression were not found in present study.
In addition to the VEGF analysis with tumor tissues, soluble VEGF in the serum or plasma of patients has also been investigated. Werther et al[27] have reported that preoperative soluble VEGF is of independent prognostic value in patients with colon cancer, but not in those with rectal cancer. Tsai et al[28] have found that patients with plasma VEGF elevation have worse DFS than those without plasma VEGF elevation in lymph-node negative colorectal cancer, but not in lymph-node-positive patients. Therefore, further studies are necessary to assess the role of soluble VEGF in rectal cancer patients.
In conclusion, we observed that IL-24 expression had a significant inverse relationship with N stage, overall stage, and the number of involved lymph nodes, but the status of IL-24 expression did not affect survival. Therefore, further studies with larger numbers of patients are needed in order to verify whether IL-24 expression has a prognostic value.
The survival rate of rectal cancer patients has been improved with the addition of chemoradiotherapy to surgery. However, that is still inadequate in stage III patients. Therefore, much endeavor is needed to increase the outcome of those through identification of new therapeutic targets. Anticancer activity of interleukin (IL)-24 has been reported in various cancer cells, but it is not known whether IL-24 has clinical importance in rectal cancer patients. In addition, vascular endothelial growth factor (VEGF) is considered to be essential in tumorigenesis, but its prognostic significance is still inconclusive in rectal cancer patients.
Favorable survival is marginally identified in high-IL-24-expressing non-small cell lung cancer (NSCLC) patients. From a subset analysis, high IL-24 expression has been revealed as a significant prognostic factor in adenocarcinoma patients. However, whether high IL-24 expression has a significant impact on the survival of patients with types of cancers other than NSCLC is unknown.
Selective anticancer effects of IL-24 have been reported in vitro and in vivo without significant toxic effects on normal cells. These interesting properties may make IL-24 a candidate therapeutic target.
From this study, correlation of IL-24 expression with histological differentiation and the degree of lymph node involvement in rectal cancer patients was found, but IL-24 expression was not significantly correlated with survival. Therefore, further studies with larger numbers of patients are required in order to assess the prognostic value of IL-24 expression in rectal cancer patients.
The melanoma differentiation associated gene-7 was identified by subtraction hybridization from human melanoma cells stimulated with interferon-β and mezerein, and was renamed later as IL-24. Multiple anticancer properties have been identified in a variety of cancer cells without injury to normal cells. Therefore, IL-24 has been emerging as an interesting candidate treatment target in many cancers.
Choi et al studied immunohistochemically the clinicopathological significance of IL-24 expression in rectal carcinoma. The experiments were conducted appropriately and the results were reasonable.
Peer reviewer: Hitoshi Tsuda, MD, PhD, Diagnostic Pathology Section, Clinical Laboratory Division, National Cancer Center Hospital, 5-1-1 Tsukiji, Chuo-ku, Tokyo 104-0045, Japan
S- Editor Tian L L- Editor Kerr C E- Editor Ma WH
| 1. | Gunderson LL, Jessup JM, Sargent DJ, Greene FL, Stewart A. Revised tumor and node categorization for rectal cancer based on surveillance, epidemiology, and end results and rectal pooled analysis outcomes. J Clin Oncol. 2010;28:256-263. |
| 2. | Smalley SR, Benedetti JK, Williamson SK, Robertson JM, Estes NC, Maher T, Fisher B, Rich TA, Martenson JA, Kugler JW. Phase III trial of fluorouracil-based chemotherapy regimens plus radiotherapy in postoperative adjuvant rectal cancer: GI INT 0144. J Clin Oncol. 2006;24:3542-3547. |
| 3. | Jiang H, Lin JJ, Su ZZ, Goldstein NI, Fisher PB. Subtraction hybridization identifies a novel melanoma differentiation associated gene, mda-7, modulated during human melanoma differentiation, growth and progression. Oncogene. 1995;11:2477-2486. |
| 4. | Ellerhorst JA, Prieto VG, Ekmekcioglu S, Broemeling L, Yekell S, Chada S, Grimm EA. Loss of MDA-7 expression with progression of melanoma. J Clin Oncol. 2002;20:1069-1074. |
| 5. | Su ZZ, Madireddi MT, Lin JJ, Young CS, Kitada S, Reed JC, Goldstein NI, Fisher PB. The cancer growth suppressor gene mda-7 selectively induces apoptosis in human breast cancer cells and inhibits tumor growth in nude mice. Proc Natl Acad Sci USA. 1998;95:14400-14405. |
| 6. | Lebedeva IV, Sarkar D, Su ZZ, Kitada S, Dent P, Stein CA, Reed JC, Fisher PB. Bcl-2 and Bcl-x(L) differentially protect human prostate cancer cells from induction of apoptosis by melanoma differentiation associated gene-7, mda-7/IL-24. Oncogene. 2003;22:8758-8773. |
| 7. | Shi H, Wei LL, Yuan CF, Yang JX, Yi FP, Ma YP, Song FZ. Melanoma differentiation-associated gene-7/interleukin 24 inhibits invasion and migration of human cervical cancer cells in vitro. Saudi Med J 2007; 28: 1671-1675 . . |
| 8. | Zhao L, Gu J, Dong A, Zhang Y, Zhong L, He L, Wang Y, Zhang J, Zhang Z, Huiwang J. Potent antitumor activity of oncolytic adenovirus expressing mda-7/IL-24 for colorectal cancer. Hum Gene Ther. 2005;16:845-858. |
| 9. | Saeki T, Mhashilkar A, Chada S, Branch C, Roth JA, Ramesh R. Tumor-suppressive effects by adenovirus-mediated mda-7 gene transfer in non-small cell lung cancer cell in vitro. Gene Ther. 2000;7:2051-2057. |
| 10. | Jiang H, Su ZZ, Lin JJ, Goldstein NI, Young CS, Fisher PB. The melanoma differentiation associated gene mda-7 suppresses cancer cell growth. Proc Natl Acad Sci USA. 1996;93:9160-9165. |
| 11. | Yacoub A, Mitchell C, Lister A, Lebedeva IV, Sarkar D, Su ZZ, Sigmon C, McKinstry R, Ramakrishnan V, Qiao L. Melanoma differentiation-associated 7 (interleukin 24) inhibits growth and enhances radiosensitivity of glioma cells in vitro and in vivo. Clin Cancer Res. 2003;9:3272-3281. |
| 12. | Ishikawa S, Nakagawa T, Miyahara R, Kawano Y, Takenaka K, Yanagihara K, Otake Y, Katakura H, Wada H, Tanaka F. Expression of MDA-7/IL-24 and its clinical significance in resected non-small cell lung cancer. Clin Cancer Res. 2005;11:1198-1202. |
| 13. | Lebedeva IV, Emdad L, Su ZZ, Gupta P, Sauane M, Sarkar D, Staudt MR, Liu SJ, Taher MM, Xiao R. mda-7/IL-24, novel anticancer cytokine: focus on bystander antitumor, radiosensitization and antiangiogenic properties and overview of the phase I clinical experience (Review). Int J Oncol. 2007;31:985-1007. |
| 14. | Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285:1182-1186. |
| 15. | Baeriswyl V, Christofori G. The angiogenic switch in carcinogenesis. Semin Cancer Biol. 2009;19:329-337. |
| 16. | Kondo Y, Arii S, Furutani M, Isigami S, Mori A, Onodera H, Chiba T, Imamura M. Implication of vascular endothelial growth factor and p53 status for angiogenesis in noninvasive colorectal carcinoma. Cancer. 2000;88:1820-1827. |
| 17. | Toiyama Y, Inoue Y, Saigusa S, Okugawa Y, Yokoe T, Tanaka K, Miki C, Kusunoki M. Gene expression profiles of epidermal growth factor receptor, vascular endothelial growth factor and hypoxia-inducible factor-1 with special reference to local responsiveness to neoadjuvant chemoradiotherapy and disease recurrence after rectal cancer surgery. Clin Oncol (R Coll Radiol). 2010;22:272-280. |
| 18. | Bertolini F, Bengala C, Losi L, Pagano M, Iachetta F, Dealis C, Jovic G, Depenni R, Zironi S, Falchi AM. Prognostic and predictive value of baseline and posttreatment molecular marker expression in locally advanced rectal cancer treated with neoadjuvant chemoradiotherapy. Int J Radiat Oncol Biol Phys. 2007;68:1455-1461. |
| 19. | Theodoropoulos GE, Lazaris AC, Theodoropoulos VE, Papatheodosiou K, Gazouli M, Bramis J, Patsouris E, Panoussopoulos D. Hypoxia, angiogenesis and apoptosis markers in locally advanced rectal cancer. Int J Colorectal Dis. 2006;21:248-257. |
| 20. | Giralt J, Navalpotro B, Hermosilla E, de Torres I, Espin E, Reyes V, Cerezo L, de las Heras M, Ramon y Cajal S, Armengol M. Prognostic significance of vascular endothelial growth factor and cyclooxygenase-2 in patients with rectal cancer treated with preoperative radiotherapy. Oncology. 2006;71:312-319. |
| 21. | Cascinu S, Graziano F, Catalano V, Staccioli MP, Rossi MC, Baldelli AM, Barni S, Brenna A, Secondino S, Muretto P. An analysis of p53, BAX and vascular endothelial growth factor expression in node-positive rectal cancer. Relationships with tumUse recurrence and event-free survival of patients treated with adjuvant chemoradiation. Br J Cancer. 2002;86:744-749. |
| 22. | Stephen , EB , David , BR , Carolyn , CC , April , FG , Frederick , GL . Colon and Rectum. Colon and Rectum. AJCC Cancer Staging Manual. New York:. Springer-Verlag. 2010;143-164. |
| 23. | Lebedeva IV, Emdad L, Su ZZ, Gupta P, Sauane M, Sarkar D, Staudt MR, Liu SJ, Taher MM, Xiao R. mda-7/IL-24, novel anticancer cytokine: focus on bystander antitumor, radiosensitization and antiangiogenic properties and overview of the phase I clinical experience (Review). Int J Oncol. 2007;31:985-1007. |
| 24. | Soumaoro LT, Uetake H, Takagi Y, Iida S, Higuchi T, Yasuno M, Enomoto M, Sugihara K. Coexpression of VEGF-C and Cox-2 in human colorectal cancer and its association with lymph node metastasis. Dis Colon Rectum. 2006;49:392-398. |
| 25. | Cao D, Hou M, Guan YS, Jiang M, Yang Y, Gou HF. Expression of HIF-1alpha and VEGF in colorectal cancer: association with clinical outcomes and prognostic implications. BMC Cancer. 2009;9:432. |
| 26. | Yang D, Schneider S, Azuma M, Iqbal S, El-Khoueiry A, Groshen S, Agafitei D, Danenberg KD, Danenberg PV, Ladner RD. Gene expression levels of epidermal growth factor receptor, survivin, and vascular endothelial growth factor as molecular markers of lymph node involvement in patients with locally advanced rectal cancer. Clin Colorectal Cancer. 2006;6:305-11. |
| 27. | Werther K, Christensen IJ, Brünner N, Nielsen HJ. Soluble vascular endothelial growth factor levels in patients with primary colorectal carcinoma. The Danish RANX05 Colorectal Cancer Study Group. Eur J Surg Oncol. 2000;26:657-662. |
| 28. | Tsai WS, Changchien CR, Yeh CY, Chen JS, Tang R, Chiang JM, Hsieh PS, Fan CW, Wang JY. Preoperative plasma vascular endothelial growth factor but not nitrite is a useful complementary tumor marker in patients with colorectal cancer. Dis Colon Rectum. 2006;49:883-894. |