Published online Mar 7, 2011. doi: 10.3748/wjg.v17.i9.1126
Revised: December 14, 2010
Accepted: December 21, 2010
Published online: March 7, 2011
AIM: To investigate predilection sites of recurrence of pancreatic cancer by computed tomography (CT) in follow-up after surgery.
METHODS: Seventy seven patients with recurrence after pancreatic cancer surgery were retrospectively identified. The operative technique, R-status, T-stage and development of tumor markers were evaluated. Two radiologists analyzed CT scans with consensus readings. Location of local recurrence, lymph node recurrence and organ metastases were noted. Surgery and progression of findings on follow-up CT were considered as reference standard.
RESULTS: The mean follow-up interval was 3.9 ± 1.8 mo, with a mean relapse-free interval of 12.9 ± 10.4 mo. The predominant site of recurrence was local (65%), followed by lymph node (17%), liver metastasis (11%) and peritoneal carcinosis (7%). Local recurrence emerged at the superior mesenteric artery (n = 28), the hepatic artery (n = 8), in an area defined by the surrounding vessels: celiac trunk, portal vein, inferior vena cava (n = 22), and in a space limited by the mesenteric artery, portal vein and inferior vena cava (n = 17). Lymph node recurrence occurred in the mesenteric root and left lateral to the aorta. Recurrence was confirmed by surgery (n = 22) and follow-up CT (n = 55). Tumor markers [carbohydrate antigen 19-9 (CA19-9), carcinoembryonic antigen (CEA)] increased in accordance with signs of recurrence in most cases (86% CA19-9; 79.2% CEA).
CONCLUSION: Specific changes of local and lymph node recurrence can be found in the course of the cardinal peripancreatic vessels. The superior mesenteric artery is the leading structure for recurrence.
- Citation: Heye T, Zausig N, Klauss M, Singer R, Werner J, Richter GM, Kauczor HU, Grenacher L. CT diagnosis of recurrence after pancreatic cancer: Is there a pattern? World J Gastroenterol 2011; 17(9): 1126-1134
- URL: https://www.wjgnet.com/1007-9327/full/v17/i9/1126.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i9.1126
Pancreatic cancer is a disease with a poor survival rate after curative surgical therapy[1]. Local recurrence after resection with curative intent is frequently observed within 2 years for the majority of patients[2]. The median survival in resectable pancreatic cancer is reported to range from 11 mo for surgery alone to 20 mo for surgery in combination with adjuvant chemotherapy[3-6]. These data are reflected by a 5-year survival rate of 10%-25%[7,8]. The mean disease-free period in imaging studies is 267 ± 158 d with negative surgical margins but 72 ± 47 d with positive margins[9].
So far there have not been many imaging studies in the literature focusing on detection of pancreatic cancer recurrence. One reason may be that recurrence of pancreatic cancer was not treated, but in recent years radiochemotherapy and, in rare cases, surgery for local recurrence has been advocated[10]. A major problem in patients with pancreatic cancer is that extensive postoperative changes with scar tissue formation as well as lymph node enlargement are present after surgical therapy that may be mistaken for disease recurrence[11]. This study was conducted to investigate whether recurrence of pancreatic cancer shows a specific pattern of regrowth on regular follow-up computed tomography (CT) examinations after surgery. The goal was to identify predilection sites of recurrence on CT in the follow-up of pancreatic cancer surgery.
This retrospective study was approved by the local ethics committee.
A total of 641 patients, who underwent surgery for a primary malignant tumor of the pancreas in a local surgical department from January 2002 to March 2007, were identified for this study. Of these, 245 had at least one follow-up CT in our department. In all patients with CT follow-up postoperative changes such as enlarged lymph nodes, and soft tissue formation in the resection area and along the cardinal mesenteric vessels were initially present. We excluded 168 patients because further consecutive follow-up CT imaging was not available. In 77 patients (47 male, 30 female; mean age: 67.8 years; range, 41-86 years) with a baseline CT examination 3-6 mo postoperatively, follow-up imaging revealed progression of soft tissue surrounding the peripancreatic vessels, progression of lymph nodes, or appearance of liver metastasis as an indication of tumor recurrence. All these patients also showed clinical signs of tumor recurrence with rising tumor marker levels and deterioration of their physical condition. Apart from 3 patients that are alive to date, all other patients died in the course of further progression of tumor recurrence. Tumor and relevant surgical data including the operative technique, TNM stage, the R-status and the development of postoperative tumor markers were taken from the postoperative database of all pancreatic cancer patients (Table 1). If disease recurrence was detected on follow-up imaging, surgery was considered as standard of reference if performed. The surgical report as well as the histological workup was evaluated. If no surgery was performed the progression of findings on further follow-up imaging studies served as standard of reference.
| Gender | 47 male, 30 female |
| Age | mean 67.8 yr; range, 41-86 yr |
| T-stage1 | 4 T2, 70 T3, 1 T4 |
| N-stage1 | 18 N0, 57 N1 |
| R-stage2 | 54 R0, 10 R1, 5 R2 |
| Histology | n |
| Adenocarcinoma of the papilla | 5 |
| Ductal adenocarcinoma | 65 |
| Mucinous-papillary carcinoma | 4 |
| Adenocarcinoma of the bile duct | 1 |
| Neuroendocrine carcinoma | 1 |
| Acinar cell carcinoma | 1 |
| Localization | |
| Papilla | 5 |
| Head | 59 |
| Body | 9 |
| Tail | 4 |
| Resection | |
| Whipple | 60 |
| Left resection | 11 |
| Total pancreatectomy | 6 |
Surgery for recurrence was performed in 22 cases confirming recurrence. In 55 cases no surgery was performed but further follow-up imaging by CT (n = 53) and by positron emission tomography (PET)-CT (n = 2) showed further progression of disease recurrence. In 9 patients a potential curative approach for surgery was pursued with 4 hemihepatectomies, 3 lymphadenectomies and 2 resections of the remnant pancreas. In 6 other patients a potential curative surgical approach was not feasible but intraoperative radiotherapy, and in one case a combination of radiochemotherapy with additional tumor reduction was performed. In all patients with a diagnosis of tumor recurrence, second line chemotherapy or radiation therapy was administered for disease control.
As tumor marker values during follow-up might have a great interindividual variability, the factor for the tumor marker increase over time was calculated from the value at the time of tumor recurrence divided by the initial postoperative (2-4 mo) value. In 48 cases tumor marker values for carcinoembryonic antigen (CEA) were measured during the initial postoperative period and the time of tumor recurrence; in 57 cases carbohydrate antigen 19-9 (CA19-9) levels were available.
All image data were evaluated by two radiologists (with 5 and 10 years experience of pancreas imaging) on diagnostic workstations (Centricity PACS, GE, USA) with consensus readings. Disease recurrence was classified into local recurrence, lymph node recurrence, liver metastasis and peritoneal carcinosis. For each case, tumor recurrence could either appear as a singular finding, such as local recurrence, or as a combination, e.g. local recurrence and liver metastasis concurrently on follow-up imaging. The criteria for local disease recurrence were defined as a soft tissue formation that increased in size over time in the resection area or along the cardinal visceral vessels around the pancreatic bed. An increase in lymph node size over multiple follow-ups was classified as lymph node recurrence. Disease recurrence was classified as liver metastasis if the appearance of new liver lesions with specific image characteristics, e.g. irregular margins, hypodensity, was observed. Peritoneal carcinosis was considered as another possible form of disease recurrence if typical, e.g. nodal peritoneal, changes were present. The exact location of each form of disease recurrence was noted.
All patients were given 1-1.5 L of water orally prior to the examination. The standard hydro-pancreas protocol consists of an unenhanced (5 mm slice thickness/4 mm increment), arterial and venous phase (3 mm slice thickness/2 mm increment, axial and coronal reconstructions) imaging after injecting 130 mL contrast agent (Ultravist 370, Bayer-Schering, Berlin, Germany) at a flow rate of 5 mL/s via a cubital vein. Scanning was performed on different CT scanner generations with 4, 16, 64 slices (Volume Zoom, Sensation, Definition; Siemens, Forchheim, Germany).
All data are presented as absolute numbers and percentages. Statistical analysis was performed using the paired Student t-test and one-way analysis of variance test for tumor marker values with commercially available software (SPSS Statistics 17.0, SPSS Inc., Chicago, USA).
The mean follow-up time between CT examinations was 3.9 ± 1.8 mo (range, 2-12 mo). The mean relapse-free time interval was 12.9 ± 10.4 mo (range, 3-76 mo).
In 55 cases a solitary presentation of tumor relapse was present and in 22 cases combinations of different types of tumor recurrence (n = 45) resulting in a total of 100 types of recurrence were present (Table 2). The predominant type of tumor recurrence was local recurrence in 65 cases (65%), with isolated manifestations in 43 cases and in combination with other types of tumor recurrence in 22 cases (Table 2). Lymph node recurrence (17%) was found in 6 cases as an isolated finding but in 11 cases in combination. Liver metastasis (11%) was commonly seen in combination with local recurrence and with isolated tumor presentation in 5 cases. Peritoneal carcinosis (7%) was, except for one case with an isolated manifestation, mainly associated with other forms of tumor recurrence. In cases where the initial resection status was R1, the most common tumor recurrence type was local recurrence in 8 cases, lymph node recurrence in one case, and in another case peritoneal carcinosis. The R2 status presented as local recurrence in all 5 cases.
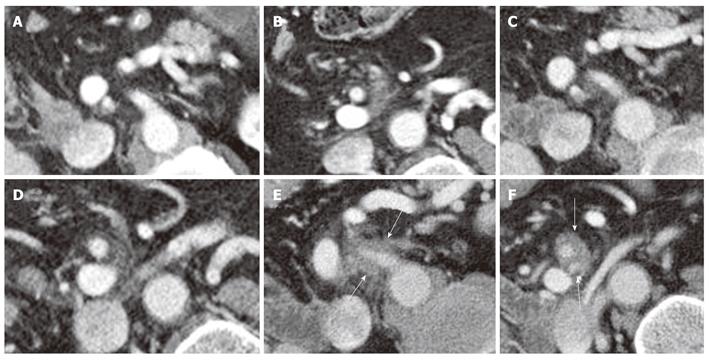
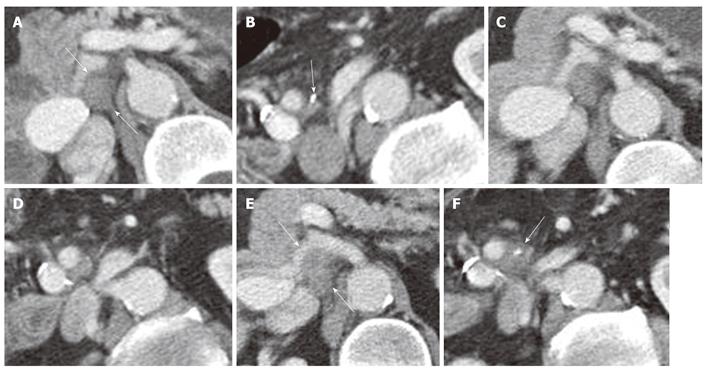
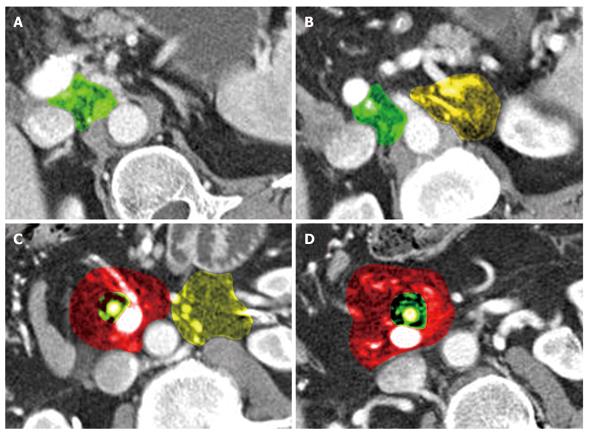
Local recurrence was found most often along the superior mesenteric artery (SMA, n = 28), either as a localized tumor mass or as a diffuse cuff-like tissue formation (Figure 1). The second most common site for tumor recurrence (n = 22) was an area defined by the celiac trunk (cT) as the medial border, the portal vein (PV) as the anterior border and the inferior vena cava (IVC) as the dorsal border (Figure 2). This is followed by an area (n = 17) which is just caudal to the aforementioned space where the medial boundary is the SMA while the other limiting structures, the PV and IVC, remain the same. In 10 cases, tumor regrowth or a secondary tumor occurred at the resection margin of the residual pancreatic parenchyma. In 8 cases the tumor presented as a cuff-like tissue formation along the common or proper hepatic artery (HA). One case was identified with tumor recurrence in the very distal mesenteric root.
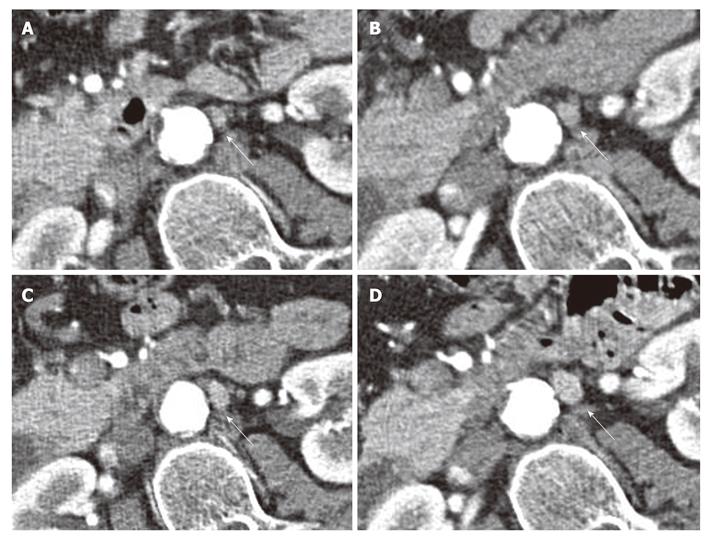
Lymph node recurrence was found in 12 cases in the mesenteric root mainly in close proximity to the superior mesenteric artery. In 5 cases, lymph node recurrence was found to the left of the aorta and in one case anterior to the aorta (Figure 3).
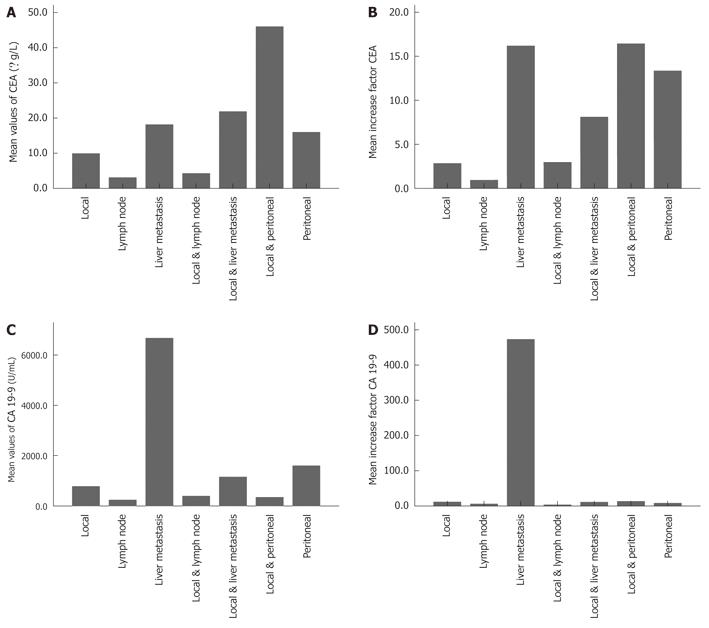
The mean initial postoperative tumor marker level for CEA was 3.12 ± 2.7 μg/L and for CA19-9 was 66.98 ± 501.2 U/mL. At the time of tumor recurrence, the mean value for CEA was 12.3 ± 18.1 μg/L and for CA19-9 was 587.65 ± 4475.4 U/mL. CEA tumor marker values at the time of tumor recurrence were significantly different from the mean initial values (P = 0.001). The mean values of CA19-9 showed no significant differences in this comparison (P = 0.067). The mean factor for the tumor marker increase was 5.6 ± 1.6 for CEA and 51.5 ± 309.6 for CA19-9. There was no significant difference (P > 0.05) for mean tumor marker values of CEA and CA19-9 stratified by type of recurrence (Table 3), except for the level of CA19-9 in the case of an isolated appearance of liver metastasis compared with mean values in local recurrence (P = 0.025). The mean factor for the increase in CEA and CA19-9 values did not show any significant differences stratified by type of recurrence (Figure 4B and D). In a direct comparison of local recurrence with liver metastasis, there was a significant difference for both CEA and CA19-9 increase factors (P < 0.05). In 8 of 57 (14.0%) cases CA19-9 did not increase, and in 10 of 48 (20.8%) CEA values did not increase with detection of recurrence on CT scans.
| Type of recurrence | Tumor marker | |||
| n | CA 19-9 (U/mL) | n | CEA (μg/L) | |
| Local | 32 | 781.67 ± 2090.28 | 26 | 9.95 ± 15.57 |
| Lymph node | 5 | 244.02 ± 413.03 | 4 | 3.13 ± 2.35 |
| Liver metastasis | 5 | 6674.80 ± 14338.02 | 5 | 18.1 ± 18.26 |
| Local & lymph node | 7 | 399 ± 738.15 | 6 | 4.28 ± 4.03 |
| Local & liver metastasis | 5 | 1157.0 ± 1802.0 | 5 | 21.88 ± 26.76 |
| Local & peritoneal carcinosis | 3 | 351.0 ± 257.66 | 2 | 46.0 ± 39.6 |
This study indicated that a specific pattern of disease recurrence in pancreatic cancer exists, especially of local recurrence, and that regular follow-up CT examinations are able to identify this pattern and detect recurrence in accordance with an increase in tumor markers (Figure 5).
To improve the prognosis of pancreatic cancer it is necessary to focus on the situation when tumor recurrence takes place. Second-line chemotherapy or radiotherapy in case of disease relapse seek to control tumor progression and improve the survival rate[12]. In rare cases a potentially curative surgical approach for liver metastasis or local tumor recurrence was shown to be successful[13-22].
It is essential to identify tumor recurrence early in order to offer patients further disease controlling measures or potentially curative options. Early intervention with chemotherapy in case of tumor recurrence has a higher chance of improving survival. The case for follow-up imaging is to identify tumor recurrence as early as possible in order to intervene appropriately. A major problem is that extensive postoperative changes, such as scar tissue formation, and enlarged and increased lymph nodes in the resection area are difficult to distinguish from real tumor relapse[23].
Ruf et al[24] compared 18F-fluorodeoxyglucose (FDG)-PET with CT/magnetic resonance imaging (MRI) in 25 patients with suspected disease recurrence and reported a 96% detection rate for FDG-PET in comparison to 39% with CT/MRI. Mortelé et al[25] reported a diagnostic accuracy of 93.5% for CT in detecting recurrent pancreatic cancer, but pointed out that no predilection site for tumor recurrence was found in their series of 32 patients. Coombs et al[26] investigated 19 patients after pancreatoduodenectomy and detected tumor recurrence by follow-up CT in 12 patients. Larger studies on the diagnostic accuracy of follow-up imaging after surgical treatment for pancreatic cancer are lacking.
CT is a morphology-based imaging method and does not offer functional or metabolic information such as FDG-PET or PET-CT. Regular postoperative PET-CT imaging may be the optimal method to identify early tumor relapse but this modality is costly and not widely available. CT imaging does offer high resolution and artifact-free imaging which is widely available, safe and fast. In order to render CT the method of choice for follow-up of patients after surgery for pancreatic cancer there is a need to gather morphological information from follow-up studies to determine the pattern of disease recurrence.
Local pancreatic tumor re-growth spreads along the cardinal visceral vessels, mainly the SMA and the HA, but can also present as a mass in specific spaces that are defined by the surrounding vasculature (SMA, HA, PV, IVC, cT). This behavior is consistent with the fact that pancreatic cancer is known to propagate along neurovascular structures on a histological level[27]. Makino et al[28] found a pattern of invasion into pancreatic and extrapancreatic nerve plexuses depending on the ventral or dorsal location of the tumor in the pancreatic head. Interestingly, in cases of invasion into the extrapancreatic nerve plexus of the SMA and HA, invasion to the adventitia of these vessels was a frequent finding. The distribution of local recurrence in the current study, which is primarily defined by the cardinal visceral vessels, may support speculations that tumor recurrence follows the same patterns of spread as the primary tumor. Noto et al[29] investigated the SMA in 6 patients with en bloc resection of the pancreatic head for cancer, and found that the SMA was directly invaded in 3 cases and in 4 cases there was invasion to the perivascular nerve plexus. Furthermore invasion extended upwards along the SMA for the celiac nerve plexus. This corresponds with our results of the SMA being the most frequent site for tumor recurrence but also the space defined by the celiac trunk as the second most frequent site of recurrence. Additionally, Noto et al[29], Kayahara et al[30] found nodal metastasis around the SMA in all cases. Again this correlates with our findings that lymph node recurrence was mainly seen to the left of the aorta just superior or inferior to the left renal vein, as well as in the mesenteric root within a certain boundary of the SMA. Thus, for primary cancer of the pancreatic head, the SMA seems to serve as the main leading structure for disease propagation. As a hypothesis one could transfer these facts from initial tumor spread to tumor recurrence. This concept is even more convincing looking at recent discussions that the high rate of local tumor recurrence in pancreatic cancer is due to the fact that neurovascular tumor invasion may be left behind during the initial surgery. In other words, most pancreatic cancer resections are R1 (= microscopic residual tumor) resections, as recent studies with standardized pathological examinations have shown[31,32]. According to recent articles there is no international consensus on the resection margins, and pathological reporting of pancreatic cancer specimens has resulted in a false low rate of R1 status[32]. Following Verbeke[32], the critical resection margins for microscopic tumor residuals, the posterior margin but especially the medial margin, are very close to the SMA. In the case of R1 status, the remaining tumor cells would regrow along these vascular structures and coincide with the locations of macroscopic tumor relapse on follow-up imaging. The results of our study seem to support this thesis since all sites of recurrence are mainly defined by vascular structures and locations of extrapancreatic nerve plexuses. These results are supported by the work of Ishigami et al[33] showing malignant perivascular soft tissue along the HA and SMA. However, it must be mentioned that most of the included patients were initially operated on before standardized pathological reporting was introduced at the local pathology institute, explaining the low rate of R1 resections[31].
In our study, 9 patients underwent surgical resection of the recurrent tumor with potential curative intent. Three of these patients, with initial surgery performed in 2000, 2003 and 2004 and second-line surgery in 2004 and 2007, are alive to date and are seen for regular follow-up. In all patients with the diagnosis of recurrence, second-line chemotherapy was recommended. This strongly emphasizes that there are multiple therapy options for disease recurrence at hand. In the future it will be necessary to develop new multi-modal approaches to therapy for the scenario of disease recurrence in order to improve survival even in cases with advanced disease presentations.
Tumor marker (CEA, CA19-9) measurement showed that the increase in levels were concordant with the detection of recurrence on CT in most cases. The mean values for CEA and CA19-9 increased from local and lymph node recurrence to liver metastasis and combinations of recurrence, indicating an association between tumor burden and tumor markers (Figure 4A-D). This gives an estimation of the type of recurrence but does underline the need for imaging at this point if an increase in tumor marker levels is detected. However, this deduction from the present data is limited since local recurrence is over-represented compared to other types of recurrence, and the variability of the tumor marker values is high.
Another limitation of this study is a possible selection bias as it was not possible for practical reasons to include all patients after surgery, e.g. follow-up at their local hospitals, etc. Therefore it cannot be concluded that the remaining 168 patients with initial CT imaging did not develop tumor recurrence at some point. Also pathologic proof was not obtained in all cases owing to the retrospective nature of the study and also for ethical and practical reasons. The deductions of this work reflect mainly experience with ductal adenocarcinoma of the pancreatic head after Whipple’s procedure. However this is the natural distribution of pancreatic tumors.
CT is a valuable tool that allows identification of tumor recurrence in accordance with an increase in tumor marker level. If tumor marker levels do not increase, recurrence can only be detected by imaging studies. We suggest a protocol for postoperative follow-up for pancreatic cancer that includes regular CT imaging and tumor marker measurement as a cost-effective and secure way to monitor these patients. A baseline CT examination 3 mo postoperatively is a prerequisite for follow-up imaging. This baseline examination is the template for comparisons with follow-up studies to distinguish postoperative changes from local tumor growth. PET-CT should be performed in addition if clinical suspicion of tumor recurrence is high, tumor markers increase and CT findings are inconclusive or negative for disease recurrence.
In conclusion, specific changes in local and lymph node recurrence of pancreatic cancer after surgery are present along the path of the peripancreatic cardinal vessels. These findings may also help differentiate non-specific postoperative changes from actual recurrence. Local tumor regrowth spreads along the cardinal visceral vessels but can also present as a mass-like recurrence at specific spaces that are defined by the surrounding vasculature. This is consistent with the known behavior of pancreatic cancer propagating along neurovascular structures. The SMA plays a major role as a leading structure for tumor regrowth. CT follow-up examinations in combination with tumor marker measurement are crucial diagnostic tools to identify local recurrence and lymph node metastasis at an early stage, allowing as many patients as possible to have second, potentially curative, surgical therapy or second-line radio/chemotherapy.
Pancreatic cancer has a poor 5-year survival rate of 10%-25%. Local recurrence is observed within 2 years after surgery for the majority of patients. Detection of recurrence of pancreatic cancer by imaging is challenging since extensive postoperative changes are present in the resection area after pancreatic surgery. It is crucial to identify signs of recurrence early in order to provide second line chemotherapy or even reoperation.
Detection of recurrence patterns by CT imaging of pancreatic cancer after surgery to facilitate differentiation of postoperative changes from true recurrence. Development of a follow-up imaging protocol for pancreatic cancer after surgery.
This study describes a recurrence pattern of pancreatic cancer on CT imaging that has not been identified so far. Detection of recurrence by imaging was concordant with significant rise in tumor markers. The pattern demonstrates that pancreatic cancer reoccurs mainly along the cardinal neurovascular structures such as the superior mesenteric artery and hepatic artery. This is the same propagation pattern as the primary pancreatic cancer exhibits during tumor extension. The high rate of tumor recurrence is thought to arise because of residual tumor at the medial resection margin which is very close to the superior mesenteric artery. Previous articles have shown that many tumor resections were R1 resections as there was no international consensus on the histopathological workup. As a consequence a standardized histopathological workup for pancreatic cancer was recently introduced. This study confirms the relationship between residual microscopic tumor after surgery and its presentation as macroscopic recurrence on CT imaging.
The results of this study, demonstrating a distinct pattern of tumor recurrence, can be applied to optimize the radiological follow-up of pancreatic cancer after surgery. A baseline CT imaging study 3 mo after surgery is recommended for further follow-up imaging. It remains to be determined if early detection of recurrence leads to new therapy options and ultimately an improved survival rate.
This well written and well structured paper reports the results of a retrospective single-center study on the role of CT imaging after surgical resection of pancreatic cancer. Adjuvant treatment strategies as well as novel methods for the follow-up of patients with this high risk disease are currently topics of a high clinical and scientific interest.
Peer reviewer: TC He, MD, PhD, Associate Professor, Director , Molecular Oncology Laboratory ,The University of Chicago Medical Center , 5841 South Maryland Avenue, MC3079, SBRI Room J-611 ,Chicago, IL 60637, United States
S- Editor Tian L L- Editor Cant MR E- Editor Ma WH
| 1. | Carpelan-Holmström M, Nordling S, Pukkala E, Sankila R, Lüttges J, Klöppel G, Haglund C. Does anyone survive pancreatic ductal adenocarcinoma? A nationwide study re-evaluating the data of the Finnish Cancer Registry. Gut. 2005;54:385-387. |
| 2. | Barugola G, Falconi M, Bettini R, Boninsegna L, Casarotto A, Salvia R, Bassi C, Pederzoli P. The determinant factors of recurrence following resection for ductal pancreatic cancer. JOP. 2007;8:132-140. |
| 3. | Rocha-Lima CM. New directions in the management of advanced pancreatic cancer: a review. Anticancer Drugs. 2008;19:435-446. |
| 4. | Neoptolemos JP. Clinical evidence does not support the use of adjuvant radiotherapy in pancreatic cancer. Nat Clin Pract Oncol. 2008;5:431. |
| 5. | Neoptolemos JP, Stocken DD, Friess H, Bassi C, Dunn JA, Hickey H, Beger H, Fernandez-Cruz L, Dervenis C, Lacaine F. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med. 2004;350:1200-1210. |
| 6. | Valentini V, Morganti AG, Macchia G, Mantini G, Mattiucci GC, Brizi MG, Alfieri S, Bossola M, Pacelli F, Sofo L. Intraoperative radiation therapy in resected pancreatic carcinoma: long-term analysis. Int J Radiat Oncol Biol Phys. 2008;70:1094-1099. |
| 7. | Kleeff J, Michalski C, Friess H, Büchler MW. Pancreatic cancer: from bench to 5-year survival. Pancreas. 2006;33:111-118. |
| 8. | Saif MW. Pancreatic cancer: highlights from the 42nd annual meeting of the American Society of Clinical Oncology, 2006. JOP. 2006;7:337-348. |
| 9. | Bluemke DA, Abrams RA, Yeo CJ, Cameron JL, Fishman EK. Recurrent pancreatic adenocarcinoma: spiral CT evaluation following the Whipple procedure. Radiographics. 1997;17:303-313. |
| 10. | Wilkowski R, Thoma M, Bruns C, Dühmke E, Heinemann V. Combined chemoradiotherapy for isolated local recurrence after primary resection of pancreatic cancer. JOP. 2006;7:34-40. |
| 11. | Lepanto L, Gianfelice D, Déry R, Dagenais M, Lapointe R, Roy A. Postoperative changes, complications, and recurrent disease after Whipple's operation: CT features. AJR Am J Roentgenol. 1994;163:841-846. |
| 12. | Reni M, Berardi R, Mambrini A, Pasetto L, Cereda S, Ferrari VD, Cascinu S, Cantore M, Mazza E, Grisanti S. A multi-centre retrospective review of second-line therapy in advanced pancreatic adenocarcinoma. Cancer Chemother Pharmacol. 2008;62:673-678. |
| 13. | Tajima Y, Kuroki T, Ohno T, Furui J, Tsuneoka N, Adachi T, Mishima T, Kosaka T, Haraguchi M, Kanematsu T. Resectable carcinoma developing in the remnant pancreas 3 years after pylorus-preserving pancreaticoduodenectomy for invasive ductal carcinoma of the pancreas. Pancreas. 2008;36:324-327. |
| 14. | Eriguchi N, Aoyagi S, Imayama H, Okuda K, Hara M, Fukuda S, Tamae T, Kanazawa N, Noritomi T, Hiraki M. Resectable carcinoma of the pancreatic head developing 7 years and 4 months after distal pancreatectomy for carcinoma of the pancreatic tail. J Hepatobiliary Pancreat Surg. 2000;7:316-320. |
| 15. | Wada K, Takada T, Yasuda H, Amano H, Yoshida M. A repeated pancreatectomy in the remnant pancreas 22 months after pylorus-preserving pancreatoduodenectomy for pancreatic adenocarcinoma. J Hepatobiliary Pancreat Surg. 2001;8:174-178. |
| 16. | D'Amato A, Gentili V, Santella S, Boschetto A, Pronio A, Montesani C. [Carcinoma of the pancreatic remnant developing after pancreaticoduodenectomy for adenocarcinoma of the head of pancreas]. Chir Ital. 2002;54:539-544. |
| 17. | Doi R, Ikeda H, Kobayashi H, Kogire M, Imamura M. Carcinoma in the remnant pancreas after distal pancreatectomy for carcinoma. Eur J Surg Suppl. 2003;62-65. |
| 18. | Takamatsu S, Ban D, Irie T, Noguchi N, Kudoh A, Nakamura N, Kawamura T, Igari T, Teramoto K, Arii S. Resection of a cancer developing in the remnant pancreas after a pancreaticoduodenectomy for pancreas head cancer. J Gastrointest Surg. 2005;9:263-269. |
| 19. | Dalla Valle R, Mancini C, Crafa P, Passalacqua R. Pancreatic carcinoma recurrence in the remnant pancreas after a pancreaticoduodenectomy. JOP. 2006;7:473-477. |
| 20. | Miura F, Takada T, Amano H, Yoshida M, Isaka T, Toyota N, Wada K, Takagi K, Kato K. Repeated pancreatectomy after pancreatoduodenectomy. J Gastrointest Surg. 2007;11:179-186. |
| 21. | Ibusuki M, Hiraoka T, Kanemitsu K, Takamori H, Tsuji T. Complete remission of pancreatic cancer after multiple resections of locally pancreatic recurrent sites and liver metastasis: report of a case. Surg Today. 2008;38:563-566. |
| 22. | Yamada H, Hirano S, Tanaka E, Shichinohe T, Kondo S. Surgical treatment of liver metastases from pancreatic cancer. HPB (Oxford). 2006;8:85-88. |
| 23. | Barkin JS, Goldstein JA. Diagnostic approach to pancreatic cancer. Gastroenterol Clin North Am. 1999;28:709-722, xi. |
| 24. | Ruf J, Lopez Hänninen E, Oettle H, Plotkin M, Pelzer U, Stroszczynski C, Felix R, Amthauer H. Detection of recurrent pancreatic cancer: comparison of FDG-PET with CT/MRI. Pancreatology. 2005;5:266-272. |
| 25. | Mortelé KJ, Lemmerling M, de Hemptinne B, De Vos M, De Bock G, Kunnen M. Postoperative findings following the Whipple procedure: determination of prevalence and morphologic abdominal CT features. Eur Radiol. 2000;10:123-128. |
| 26. | Coombs RJ, Zeiss J, Howard JM, Thomford NR, Merrick HW. CT of the abdomen after the Whipple procedure: value in depicting postoperative anatomy, surgical complications, and tumor recurrence. AJR Am J Roentgenol. 1990;154:1011-1014. |
| 27. | Kayahara M, Nakagawara H, Kitagawa H, Ohta T. The nature of neural invasion by pancreatic cancer. Pancreas. 2007;35:218-223. |
| 28. | Makino I, Kitagawa H, Ohta T, Nakagawara H, Tajima H, Ohnishi I, Takamura H, Tani T, Kayahara M. Nerve plexus invasion in pancreatic cancer: spread patterns on histopathologic and embryological analyses. Pancreas. 2008;37:358-365. |
| 29. | Noto M, Miwa K, Kitagawa H, Kayahara M, Takamura H, Shimizu K, Ohta T. Pancreas head carcinoma: frequency of invasion to soft tissue adherent to the superior mesenteric artery. Am J Surg Pathol. 2005;29:1056-1061. |
| 30. | Kayahara M, Nagakawa T, Ohta T, Kitagawa H, Ueno K, Tajima H, Elnemr A, Miwa K. Analysis of paraaortic lymph node involvement in pancreatic carcinoma: a significant indication for surgery? Cancer. 1999;85:583-590. |
| 31. | Esposito I, Kleeff J, Bergmann F, Reiser C, Herpel E, Friess H, Schirmacher P, Büchler MW. Most pancreatic cancer resections are R1 resections. Ann Surg Oncol. 2008;15:1651-1660. |
| 32. | Verbeke CS. Resection margins and R1 rates in pancreatic cancer--are we there yet? Histopathology. 2008;52:787-796. |
| 33. | Ishigami K, Yoshimitsu K, Irie H, Tajima T, Asayama Y, Hirakawa M, Kakihara D, Shioyama Y, Nishihara Y, Yamaguchi K. Significance of perivascular soft tissue around the common hepatic and proximal superior mesenteric arteries arising after pancreaticoduodenectomy: evaluation with serial MDCT studies. Abdom Imaging. 2008;33:654-661. |