Published online Feb 28, 2011. doi: 10.3748/wjg.v17.i8.996
Revised: November 3, 2010
Accepted: November 10, 2010
Published online: February 28, 2011
AIM: To determine the expression of toll-like receptor 9 (TLR9) in pancreatic tumor and the effects of cytosine phosphate-guanosine oligodeoxynucleotides 2216 (CPG ODN2216) on biological behavior of pancreatic carcinoma cell line PANC-1 and explore their clinical significance.
METHODS: The immunohistochemistry and Western blot were used to determine the expression of TLR9 protein in pancreatic cancer tissues, and immunofluorescence staining was performed to detect the TLR9 protein expression in pancreatic carcinoma cell line PANC-1. To assess the effects of CPG ODN2216 on the invasive property of Panc-1 cells, in vitro cell adhesion, wound-healing scrape, and invasion and cell colony formation were evaluated.
RESULTS: TLR9 was highly expressed in pancreatic cancer tissues and PANC-1 cells. The percentage of positive cells expressing TLR9 protein in human pancreatic tissues, paracancerous tissues and normal tissues were 73.3%, 33.3% and 20.0%, respectively, and the protein expression level of TLR9 was gradually descending (P < 0.05). In vitro tests in wound-healing scrape, cell adhesion, colony formation and matrigel invasion showed that the adhesion and motility of PANC-1 cells in CPG ODN 2216 treatment group were significantly lower than in the control group (P < 0.05). The cell growth assay showed that the proliferative ability of PANC-1 cells in treatment group was significantly decreased and CPG ODN2216 had an inhibitive effect in the growth of Panc-1 cells in a dose and time-dependent manner (P < 0.05).
CONCLUSION: The gene of TLR9 is correlated with the invasive and metastatic potential of human pancreatic carcinoma, and CPG ODN2216 induces the inhibition of migration and invasion of Panc-1 cells.
- Citation: Wu HQ, Wang B, Zhu SK, Tian Y, Zhang JH, Wu HS. Effects of CPG ODN on biological behavior of PANC-1 and expression of TLR9 in pancreatic cancer. World J Gastroenterol 2011; 17(8): 996-1003
- URL: https://www.wjgnet.com/1007-9327/full/v17/i8/996.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i8.996
Pancreatic cancer is a highly malignant tumor of digestive system, with a very poor prognosis[1,2]. In the developed countries, the pancreatic cancer ranks the fourth in the mortality of malignant tumors[3].The five-year survival rate is less than 5% and the median survival time is 5-6 mo. Although 10%-15% of patients have the opportunity for radical surgery, the median survival time is only 10-18 mo and the 5-year survival rate is about 17%-24%[4,5]. The poor prognosis of pancreatic cancer patients is mainly due to local invasion and early metastasis[6]. Most patients with pancreatic cancer are diagnosed when they are found to have local invasion or distant metastasis. Therefore, further studies of the molecular biological behavior of pancreatic cancer are necessary to improve the diagnosis of pancreatic cancer, prevention and treatment.
Toll-like receptors (TLRs) are pathogen receptors which have been recently discovered to have the ability of identifing specific types of micro-organisms, namely the pathogen-associated molecular patterns (PAMPs)[7,8]. Recent studies found that the expression of TLRs occurred not only in immune cells, but also in normal epithelial cells and tumor cells[9]. The activity of TLRs in tumor cells may delay the anti-tumor function of immune cells, interfere with the role of the immune response, leading ultimately to tumor progression[10-12].
TLR9 is an important member in this family, with an ability to induce significant secretion of cytokine and chemotactic factors by B cells and dendritic cells, such as interleukin (IL)-12, IL-6, interferon-γ (IFN-γ), monocyte profilin and metal matrix protease[13]. High-level expression of TLR9 was detected in a wide variety of tumors, however, it remains unknown whether TLR9 is expressed and what role it plays in pancreatic cancer. In order to investigate the role of TLR9 in the development of pancreatic cancer, the immunohistochemistry and Western blotting were used to determine the expression of TLR9 protein in pancreatic cancer tissues and immunofluorescence staining was also performed to detect the TLR9 protein expression in pancreatic carcinoma cell line PANC-1. And we employed TLR9-targeting ligand-sythetic CPG ODN2216 to stimulate pancreatic cancer cell Panc-1 and observe the changes in malignant phenotype of this cell line in terms of proliferation, growth, tumorigenic and invasive ability and the correlation between TLR9 gene and the pancreatic cancer cells. All these will benefit the future research of the specific mechanism of TLR9 gene.
The pancreatic cancer cell line Panc-1 was supplied by the laboratory of the Department of General Surgery, Wuhan Union Hospital affiliated to Tongji Medical College of Huazhong University of Science and Technology. The cells were cultivated in Dulbecco’s Modified Eagle Medium (DMEM) which contains 10% fetal bovine serum (FBS) and 100 μmoL/ml mycillin under 5% CO2 at 37°C.
Fresh human pancreatic cancer tissues (n = 30) and homologous peritumoral tissues (1-2 cm from the edge of cancer) were collected from pancreatic cancer patients. The normal human pancreatic tissues (n = 30) were obtained from non-cancer patients who had to be depancreatized for other reasons. All of the specimens were taken from patients with pancreatic cancer who were treated surgically in the Pancreatic Surgery Center of Wuhan Union Hospital and confirmed by post-operational pathology.
CpG-ODN2216 (serial number: GGGGGACGATCGTCGGGGGG) was synthesized, specifically phosphorothioate decorated and polyacrylamide gel electrophoresis (PAGE) purified by Shenggong Bio-engineering Company, Shanghai, China; TLR9 monoclonal antibody was provided by the Cell Signaling Company, USA; matrigel and dimethyl sulfoxide (DMSO) were purchased from Sigma-Aldrich Company, USA; and immunohistochemisty and immunofluorescence reagent kits were obtained from Boster Bio-engineering Co., Ltd., Wuhan, China.
Conventional methods were used to extract tissue proteins and the consistency of protein was measured by the method of Coomassie brilliant blue. Fifteen μL protein was taken for 10% polyacrylamide gel electrophoresis and transferred to a nitrocellulose filter by electrolysis. To block the non-specific antigen, the sections were incubated with 5% skim milk added with 1:750 diluted rabbit anti-human antibody TLR9 monoclonal antibody, followed by overnight incubation at 4°C. Membranes were washed with Tris-Buffered Saline Tween-20 (TBST) containing 0.1% Tween20, added with goat anti-rabbit IgG antibodies (Boster Bio-engineering Co., Ltd., Wuhan, China) tagged with horseradish peroxidase (HRP) and incubated at room temperature for 2 h. After being washed and added with EcL Plus reagent, an enhanced chemiluminescence substrate, the membranes were exposed on X-ray films for 30 s, followed by routine development and fixation. Finally, the images were scanned and analyzed by an image analysis software for gel electrophoresis (Band scan v5.0).
The streptavidin peroxidase (SP) methods were used for the immunohistochemical studies. The staining step followed the routine process. The paraffin sections were prepared, dewaxed and hydrated by alcohol. After being immersed in 3% hydrogen peroxide for 10 min, the sections underwent antigen retrieval in 0.01 mol/L citrate buffer solution (pH 6.0) and then blocked at room temperature for 30 min by 5% goat serum albumin with subsequent removal of sealing solution. The appropriately diluted primary antibody was added, and the control antibody was replaced with phosphate buffer solution (PBS) and kept at 4°C overnight. They were rinsed with PBS for 3 times, 5 min each time; biotinylated secondary antibodies were incubated at room temperature for 30 min and washed with PBS for 3 times; and horseradish peroxidase (HRP) labeled avidin was added and incubated at room temperature for 30 min. Finally, after PBS rinsing, DAB-staining, hematoxylin staining, dehydration, and septum pellucidum mounting, results were observed under microscope.
Pancreatin-digested pancreatic cancer cells were used to prepare single cell suspension and the cell suspension was dropped onto glass slides. Creeping plates were taken out after 24 h or longer according to the cell growth state and rinsed with PBS for 3 times (5 min each time), followed by 15 min fixation with 4% paraformaldehyde, PBS rinse and 20 min perforation by 0.5% Triton.
After rinsed with PBS for 3 times, the PANC-1 cell slides were blocked for 30 min by 5% bovine serum albumin (BSA). The first antibody dilution was added directly after dryness while PBS was used in the control group. Following overnight incubation at 4°C and rinsing with PBS for 3 times, the diluted second antibody was added and incubated for 1 h at 37°C. Finally, the slides were observed under a fluorescence microscope after PBS rinse for 3 times, and added with diluted Strept Avidin Biotin Complex - fluoresceine isothiocyanate (SABC-FITC) and incubated for 30 min at 37°C. PBS rinse was repeated for 3 times and sealed by water-soluble sheet.
Cell viability was measured using the Cell Counting Kit-8 (CCK-8) assay (Beyotime Bio., Shanghai, China) according to the method described previously[14]. Pancreatic cancer cell suspension was transferred into 96-well tissue culture polystyrene (TCP) plates with 1 × 103 cells or 100 μL per well. The cells were cultured at 37°C under 5% CO2 and saturated humidity. After cell attachment at time point of 24 h, CPG ODN2216 of various concentrations (0.1, 0.5, 1, 5 and 10 mg/L) was added to act on Panc-1 cells, followed by addition of 20 μL CCK8 of 5 mg/ml at time points of 24, 48 and 72 h, respectively and 4 h cell culture. Finally, after thorough shaking and mixing, absorbance values were determined by enzyme-linked immunosorbent assay (ELISA) reader. Inhibition rate (IC) = (A control group - A drug group)/A control group × 100%. The curve of inhibited proliferation was plotted and IC50 was obtained.
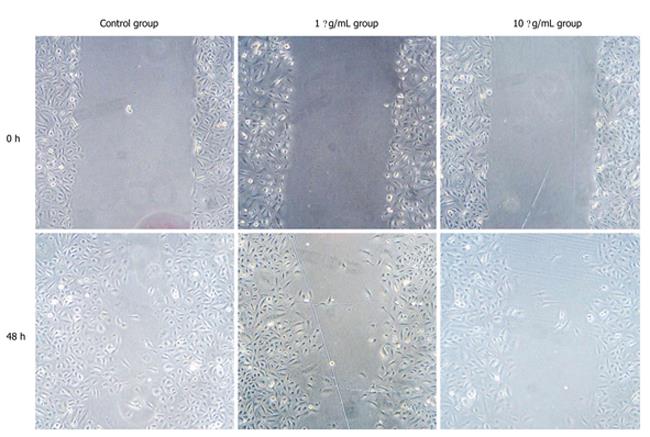
Panc-1 cellular suspension at a concentration of 2 × 105/mL was inoculated into a 6-well culture plate at 3ml per well, and cultured for 24 h. In the next step, culture medium was removed and replaced by CPG ODN2216 culture medium at concentrations of 1 and 10 mg/L, respectively after the cell growth rate reached 80%. Supernatant was abandoned and scratches were carried out in the center of wells using a scraping cutter. Four marks were used as test sites at an equal distance along the scratch edge and the average value was obtained.
Collagen gel overlays were prepared on 96-well TCP plates, followed by air dryness, BSA blocking and 60min incubation. The cell concentrations of treatment group (interfered by CPG ODN at 1 and 10 mg/L for 24 h) and control group was adjusted to 5 × 105/mL, and both cellular suspensions were inoculated in transwells, leading to 200 μL/well. With 1h incubation, PBS rinsing, addition of 5 mg/ml methyl thiazolyl tetrazolium (MTT) and 4 h culture, the culture medium was abandoned and the remainder was dissolved in DMSO for 20 min. Lastly, absorbance values were measured at 490nm wavelength. Cell adhesion inhibition rate = (A control group-A drug group)/A control group × 100%. Each group contained 3 repeated wells and the average values were obtained.
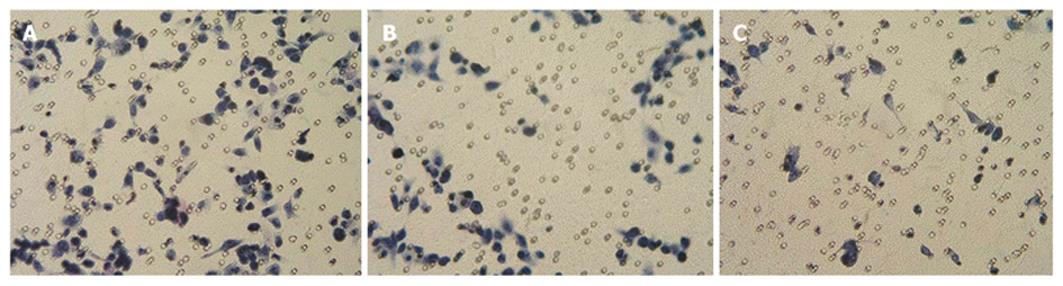
Cell invasion in vitro was performed in Matrigel Invasion Chambers (Becton Dickinson Co., Franklin Lakes, NJ) in 24-well culture plates, as described previously[15]. Thirty μL synthetic basal membrane was added into transwell upper chamber and underwent air dryness. Then, 200 μL of 24 h starvation-cultured cells was added respectively and the concentration of CPG ODN was adjusted to 1 and 10 mg/L. DMEM culture medium (500 μL) containing 10% FBS as chemokine was added into the lower chamber of invasive cabinet. It was taken out of cabinet after 24 h culture at 37°C under 5% CO2 and then cotton swab was used to rub away unmigrated cells and superfluous liquid in upper chamber. After that, the cabinet was fixed for 10 min in 4% polyoxymethylene and dyed for 30 min with 1% crystal violet, followed by thorough membrane washing with deionized water. Finally, cells were observed and counted under microscope. Each group contained 3 repeated wells and the average values were obtained.
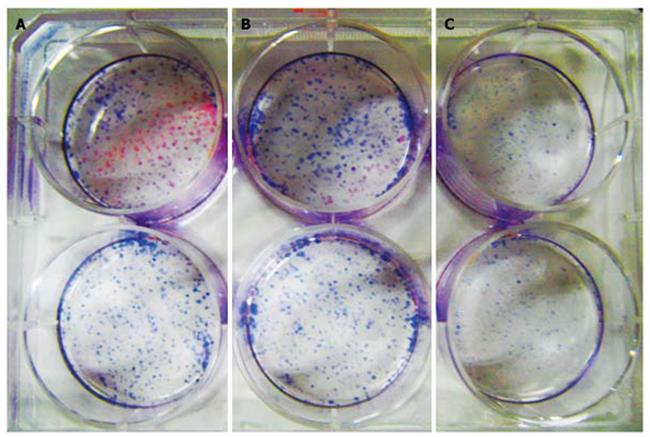
Panc-1 cells were digested with pancreatin and single cell suspension was prepared by blowing method, and then inoculated into a 6-well plate at about 200/well. Three groups were set up, one group without CPG ODN2216, the other two containing 1 and 10 mg/L CPG ODN2216, respectively. Each group contained two repeated wells. Then, the inoculated cells were cultured in the incubator till d 14. Cell culture was suspended when visible clone appeared and then culture medium was removed, rinsed by PBS, dyed for 30 min with hematoxylin and PBS rinse was repeated. Finally, the formation rate of cell clone was calculated and photographed by digital camera. The test was repeated 3 times.
Experimental data was analyzed by Statistical Package for Social Sciences(SPSS) software(SPSS, Inc., Version 16.0,USA), All of the data were represented by mean ± SD and T test was performed for statistical analysis, and P values < 0.05 were considered statistically significant.
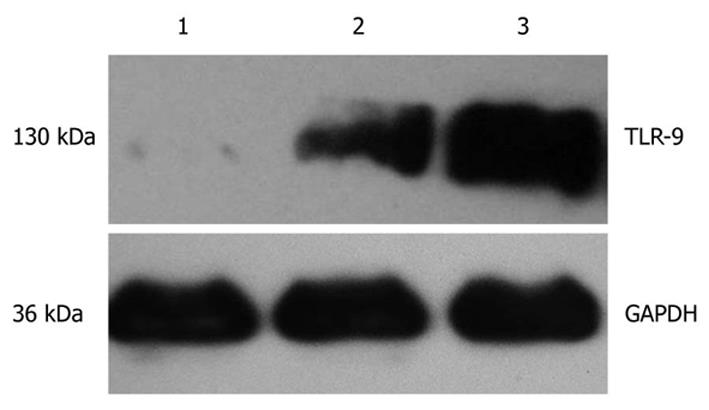
As shown in Figure 1, the expression of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) protein was detected in cancer tissues, homologous peritumoral tissues and normal pancreatic tissues and protein band was located at 36 kDa. TLR-9 was expressed at 130 kDa. The expression level of TLR9 in cancer tissues was significantly higher than in both peritumoral tissues and normal pancreatic tissues.
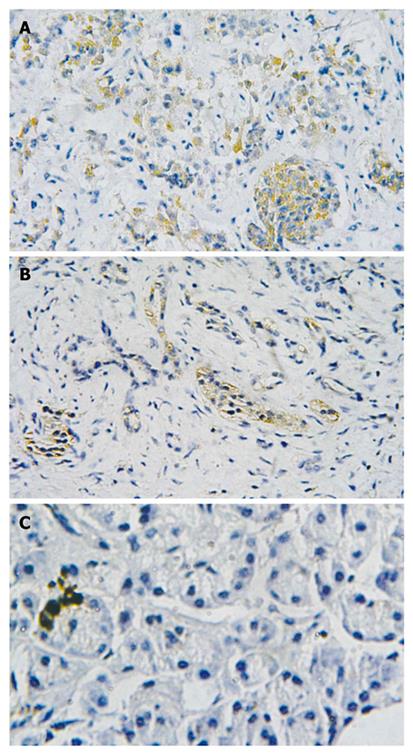
Immunohistochemistry showed that TLR9 protein was mainly expressed in the cytoplasm of cancer cells, exhibiting yellowish and reddish brownness in an even manner. The expression of TLR9 protein was detected in both peritumoral tissues and normal pancreatic tissues, showing light yellowness and the expression level was lower than in the pancreatic cancer tissues. The total positive rates of TLR9 protein were 73.3% in human pancreatic cancer (22/30) and 33.3% (10/30) and 20% (2/10) in pancreatic peritumoral tissues and normal pancreatic tissues, respectively. There were statistical differences among the three groups (χ2 = 13.99, P < 0.01), (Figure 2).
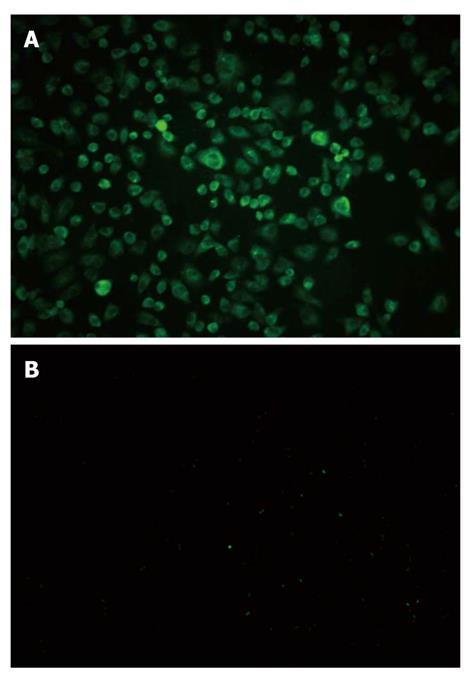
The high expression of TLR9 in Panc-1 cells was found by immune fluorescence method. Upon excitation by ultraviolet, cytoplasm displayed green fluorescence whereas control group did not show the fluorescence (Figure 3).
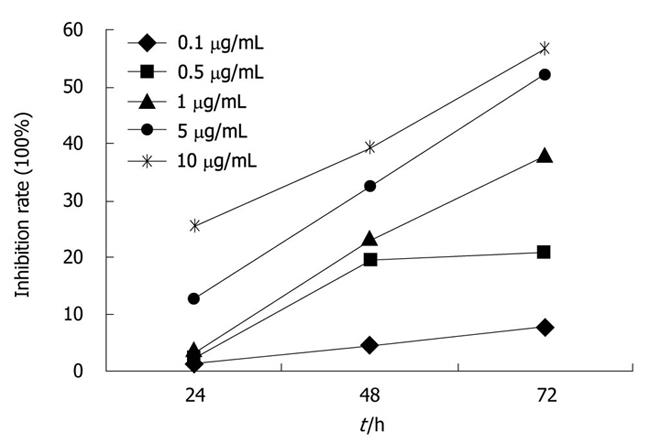
CPG ODN2216 of different concentrations (0.1, 0.5 , 5 and 10 mg/mL) showed inhibitory effects on Panc-1 cell line, and the growth inhibition rate increased markedly with concentration and time. Growth inhibition curve was plotted and the IC50 at 24, 48 and 72 h was 65.1, 16.43 and 4.47 mg/mL, respectively. The inhibitory effect was time and dose dependant (Figure 4).
The results of scratch test showed that in contrast with control group, migration ability of Panc-1 cell was decreased with addition of CPG ODN2216 and higher CPG ODN2216 concentration led to significantly lower migration ability. Migration distance after 48 h was 20.20 ± 1.06 mm at 10 mg/L and 17.37 ± 0.70 mm at 1 mg/L and 13.27 ± 1.11 mm in the control group, the difference being statistically significant (P < 0. 05), (Table 1, Figure 5).
The adhesive inhibition rates were 12.5% and 2.5% respectively in 1 mg/mL CPG ODN treated group and 10 mg/L CPG ODN group, respectively. Cell adhesion strength was significantly lower than control group (t = 25.4, 14.9 P < 0.01) and the inhibitory effect appeared dose-dependent (t = 6.32, P < 0.01). The control group had higher invasive ability, and the higher the concentration of CPG ODN2216, the fewer cells crossed the membrane. Invasion inhibitory rates were 21.6% and 59.5%, respectively (t = 6.59, 13.79 P < 0.01), showing a dose-effect relationship (t = 18.29 P < 0.01), (Table 2, Figure 6).
Hematoxylin stained cells in 10 mg/mL CPG ODN2216 treated group showed significantly fewer colonies per square centimeter (0.27 ± 0.13/cm2) than in group 1 mg/L (1.08 ± 0.11) and control group (1.17 ± 0.12), with statistical difference between the latter two groups (t = 8.29, 8.67 P < 0.01) but without significant difference between treatment group and control group (P > 0.05), (Figure 7).
Pancreatic cancer has a high degree of malignancy and develops stealthily, seriously threatening the human health. Therapeutic effects have been low by the available treatment methods and prognosis is poor. Epidemiological studies showed that both the incidence and mortality of pancreatic cancer are hiking in recent years[16]. Currently, in China pancreatic cancer jumped from 16th to 6th in the death rate caused by a variety of cancer. Therefore, search for target of gene therapy for pancreatic cancer has always been the focus in this field. Substantial findings demonstrate that signaling pathway induced by TLRs and TLR9 may play an important role in the development of tumors and the up-regulation of TLRs expression may be closely connected with the stomach, lung, bowel cancers and so on[17]. The expression of TLR9 in tumor is expected to provide new regimens for tumor chemotherapy and immunotherapy. In this study, we used Western blotting and immunohistochemical analysis to detect the expression of TLR9 gene in pancreatic cancer tissues and found that the expression of TLR9 gene in pancreatic cancer tissues was significantly higher than in pancreatic peritumoral tissue and normal pancreatic tissues. This is in agreement with Droemann’s[18] findings in lung cancer study. We also determined the expression of TLR9 gene in pancreatic cancer cells using immunofluorescence method and found high level expression of TLR9 protein in pancreatic cancer cells, mainly in endochylema. In order to investigate the significance of high expression of TLR9 gene in pancreatic cancer cells and clarify its effects, we applied specific ligands of TLR9, namely CPG ODN2216 and pancreatic cancer cell Panc-1, to study the function of CPG ODN and its influence on TLR9 gene in human pancreatic cancer cells.
In recent years it has been verified that CPG ODN has a strong immunoregulatory effect in Th1 direction, mainly through its combination with TLR9 and induction of secretion of Th1 polarizing cell factors such as IFN-7, IL-12 and so on, promoting the differentiation of Th0 to Th1[19,20]. For example, CPG DNA penetrates into dendritic cells (DCs) by means of sequence independent, receptor-mediated endocytosis, then acts on TLR9 specifically in lysosomal area and finally activates signal transduction through interleukin-1 receptor associated kinase (IRAK1), interferon regulatory factor 7 (IRF7), TNF receptor-associated factor 6 (TRAF6), mitogen-activated protein kinases (MAPK) and nuclear factor kB(NF-KB) pathway and adaptor myeloid differentiation primary response gene (88) (MyD88)[21-24]. However, at present, CpG ODN, which is used as an immunoadjuvant to induce Th1 immune response on human lung adenocarcinoma A549 cell by means of TLR9, may be adopted for monotherapy or supplement to immunization therapy to treat cancers[25]. As for the direct effect of CPG ODN on pancreatic cancer, there has been no report in the literature so far. We combined CPG ODN2216 and pancreatic cancer cell and found by CCK-8 assay that the growth and proliferation of pancreatic cancer cells were inhibited in vitro and the inhibitory effect was time-dose dependent.
One of the important biological characteristics of malignant tumor is its invasion into adjacent tissues and subsequent distant metastasis. Invasion is recognized as the foundation and precondition of distant metastasis and distant metastasis is the continuation and further development of invasion. Factually, they are two stages of the same process, and pancreatic cancer invasion and metastasis into adjacent tissues occur in the early stage. Only by preventing the two biological behaviors at proper time can we defeat pancreatic cancer. Like other tumors, invasion and metastasis of pancreatic cancer is a process characterized by polygenic participation, multi-steps and multi-stages[26]. At the same time, the adhesion and invasion process increases the malignant tumor survival and metastasis ability, that is a committed step in the tumor transition process[27]. To further investigate the role of TLR9 gene in the process of tumor invasion and metastasis, TLR9 specifically ligand-CPG ODN2216 and pancreatic cancer cell line Panc-1 were applied in the subsequent tests such as scratch adhesion test, adhesion test, Transwells in vitro invasion assay, cell clone test and so on. Scratch adhesion test is considered as one of the classic ways to determine cell immigration ability and adhesion and invasion test can relatively ideally simulate and reflect the invasion behavior and ability of tumor cells. Cell clone test can also be used to detect the proliferation ability of cancer cells. CPG ODN2216 had weakened in vitro migration, membrane anchor ability and clone proliferation ability of Panc-1 cells compared with the cells in the control group. Therefore, the expression of TLR9 can influence the degree of malignancy of human pancreatic cancer cell line.
In conclusion, there is high expression of TLR9 in both human pancreatic tissues and Panc-1 cells. TLR9 may increase the cell abilities of invasion, metastasis and adhesion to promote the occurrence and development of pancreatic neoplasm, and play an important role in invasion and metastasis of pancreatic cancer. However, the questions of how the gene regulates cell migration and invasion, and what transconduction system ligand CPG ODN relies on to influence TLR9, remain to be answered and the specific mechanism need to be further studied.
Pancreatic cancer is a highly malignant digestive tumor with a very poor prognosis. Recently, an increasing number of studies reported that toll-like receptors (TLRs) were related to malignancies and involved in tumor progression, but whether TLRs, such as TLR9, is expressed in pancreatic cells remains unknown. Regulation of TLR9 signaling pathway in pancreatic cancer cells, and the relationship between the biological impact of behavior change are also unclear.
Activating TLRs pathway in tumor cells could promote the proliferation and inhibit the apoptosis, leading to migration, invasion and angiogenesis of tumor, but it remains unknown whether TLR9 is expressed and what role it plays in PANC-1. There is no report about the direct effect of cytosine phosphate-guanosine oligodeoxynucleotides (CPG ODN) on pancreatic cancer in the literature so far. The authors demonstrate that the high expression of TLR9 exists in both human pancreatic tissues and Panc-1 cells. TLR9 may increase the cell abilities of invasion, metastasis and adhesion to promote the occurrence and development of pancreatic neoplasm, and play an important role in invasion and metastasis of pancreatic cancer.
This is the first study to report that TLR9 is expressed in both human pancreatic tissues and Panc-1 cells, and first demonstrate that TLR9 may increase the cell abilities of invasion, metastasis and adhesion to promote the occurrence and development of pancreatic neoplasm, and play an important role in invasion and metastasis of pancreatic cancer.
This study demonstrated that TLR9 may be a novel marker for the progression and prognosis of pancreatic cancer, and may provide a new strategy for the treatment of patients with pancreatic cancer.
CPG ODN 2216 (cytosine phosphate-guanosine oligodeoxynucleotides 2216): the TLR9-targeting ligand-synthetic. It has a strong immunoregulatory effect in Th1 direction, mainly through its combination with TLR9 and induction of secretion of Th1 polarizing cell factors such as IFN-7 and IL-12, promoting the differentiation of Th0 to Th1.
The authors examined the expression of TLR9 in both human pancreatic tissue and Panc-1 cells, and demonstrated a role for TLR9 in adhesion, migration and invasion of human Panc-1 cells. This is an interesting article in which the experimental design is well thought out and the results are intriguing.
Peer reviewer: Seong Gyu Hwang, Professor, MD, Department of Internal Medicine, CHA Bundang Medical Center, CHA university, #351, Yatap-Dong, Bundang-Gu, Seongnam, Gyeonggi-Do, 463-712, South Korea
S- Editor Sun H L- Editor Ma JY E- Editor Ma WH
| 1. | Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43-66. |
| 2. | Hawes RH, Xiong Q, Waxman I, Chang KJ, Evans DB, Abbruzzese JL. A multispecialty approach to the diagnosis and management of pancreatic cancer. Am J Gastroenterol. 2000;95:17-31. |
| 3. | Real FX, Cibrián-Uhalte E, Martinelli P. Pancreatic cancer development and progression: remodeling the model. Gastroenterology. 2008;135:724-728. |
| 4. | Tanase CP, Dima S, Mihai M, Raducan E, Nicolescu MI, Albulescu L, Voiculescu B, Dumitrascu T, Cruceru LM, Leabu M. Caveolin-1 overexpression correlates with tumour progression markers in pancreatic ductal adenocarcinoma. J Mol Histol. 2009;40:23-29. |
| 5. | Neoptolemos JP, Dunn JA, Stocken DD, Almond J, Link K, Beger H, Bassi C, Falconi M, Pederzoli P, Dervenis C. Adjuvant chemoradiotherapy and chemotherapy in resectable pancreatic cancer: a randomised controlled trial. Lancet. 2001;358:1576-1585. |
| 6. | Jungert K, Buck A, von Wichert G, Adler G, König A, Buchholz M, Gress TM, Ellenrieder V. Sp1 is required for transforming growth factor-beta-induced mesenchymal transition and migration in pancreatic cancer cells. Cancer Res. 2007;67:1563-1570. |
| 7. | Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783-801. |
| 8. | O’Neill LA. How Toll-like receptors signal: what we know and what we don’t know. Curr Opin Immunol. 2006;18:3-9. |
| 9. | Cook DN, Pisetsky DS, Schwartz DA. Toll-like receptors in the pathogenesis of human disease. Nat Immunol. 2004;5:975-979. |
| 10. | Goto Y, Arigami T, Kitago M, Nguyen SL, Narita N, Ferrone S, Morton DL, Irie RF, Hoon DS. Activation of Toll-like receptors 2, 3, and 4 on human melanoma cells induces inflammatory factors. Mol Cancer Ther. 2008;7:3642-3653. |
| 11. | Kelly MG, Alvero AB, Chen R, Silasi DA, Abrahams VM, Chan S, Visintin I, Rutherford T, Mor G. TLR-4 signaling promotes tumor growth and paclitaxel chemoresistance in ovarian cancer. Cancer Res. 2006;66:3859-3868. |
| 12. | Jurk M, Vollmer J. Therapeutic applications of synthetic CpG oligodeoxynucleotides as TLR9 agonists for immune modulation. BioDrugs. 2007;21:387-401. |
| 13. | Theiner G, Rössner S, Dalpke A, Bode K, Berger T, Gessner A, Lutz MB. TLR9 cooperates with TLR4 to increase IL-12 release by murine dendritic cells. Mol Immunol. 2008;45:244-252. |
| 14. | Ishiyama M, Tominaga H, Shiga M, Sasamoto K, Ohkura Y, Ueno K. A combined assay of cell viability and in vitro cytotoxicity with a highly water-soluble tetrazolium salt, neutral red and crystal violet. Biol Pharm Bull. 1996;19:1518-1520. |
| 15. | Albini A, Iwamoto Y, Kleinman HK, Martin GR, Aaronson SA, Kozlowski JM, McEwan RN. A rapid in vitro assay for quantitating the invasive potential of tumor cells. Cancer Res. 1987;47:3239-3245. |
| 16. | Lowenfels AB, Maisonneuve P. Epidemiology and prevention of pancreatic cancer. Jpn J Clin Oncol. 2004;34:238-244. |
| 18. | Droemann D, Albrecht D, Gerdes J, Ulmer AJ, Branscheid D, Vollmer E, Dalhoff K, Zabel P, Goldmann T. Human lung cancer cells express functionally active Toll-like receptor 9. Respir Res. 2005;6:1. |
| 19. | Sun S, Zhang X, Tough DF, Sprent J. Type I interferon-mediated stimulation of T cells by CpG DNA. J Exp Med. 1998;188:2335-2342. |
| 20. | Bendigs S, Salzer U, Lipford GB, Wagner H, Heeg K. CpG-oligodeoxynucleotides co-stimulate primary T cells in the absence of antigen-presenting cells. Eur J Immunol. 1999;29:1209-1218. |
| 21. | Underhill DM. Toll-like receptors: networking for success. Eur J Immunol. 2003;33:1767-1775. |
| 22. | Ahmad-Nejad P, Häcker H, Rutz M, Bauer S, Vabulas RM, Wagner H. Bacterial CpG-DNA and lipopolysaccharides activate Toll-like receptors at distinct cellular compartments. Eur J Immunol. 2002;32:1958-1968. |
| 23. | Kerkmann M, Rothenfusser S, Hornung V, Towarowski A, Wagner M, Sarris A, Giese T, Endres S, Hartmann G. Activation with CpG-A and CpG-B oligonucleotides reveals two distinct regulatory pathways of type I IFN synthesis in human plasmacytoid dendritic cells. J Immunol. 2003;170:4465-4474. |
| 24. | Takauji R, Iho S, Takatsuka H, Yamamoto S, Takahashi T, Kitagawa H, Iwasaki H, Iida R, Yokochi T, Matsuki T. CpG-DNA-induced IFN-alpha production involves p38 MAPK-dependent STAT1 phosphorylation in human plasmacytoid dendritic cell precursors. J Leukoc Biol. 2002;72:1011-1019. |
| 25. | Krieg AM. Toll-like receptor 9 (TLR9) agonists in the treatment of cancer. Oncogene. 2008;27:161-167. |
| 26. | Celetti A, Testa D, Staibano S, Merolla F, Guarino V, Castellone MD, Iovine R, Mansueto G, Somma P, De Rosa G. Overexpression of the cytokine osteopontin identifies aggressive laryngeal squamous cell carcinomas and enhances carcinoma cell proliferation and invasiveness. Clin Cancer Res. 2005;11:8019-8027. |
| 27. | Eble JA, Haier J. Integrins in cancer treatment. Curr Cancer Drug Targets. 2006;6:89-105. |