Published online Feb 28, 2011. doi: 10.3748/wjg.v17.i8.1076
Revised: November 30, 2010
Accepted: December 7, 2010
Published online: February 28, 2011
AIM: To investigate the differences in cultivable gut bacteria and peroxisome proliferator-activated receptor γ2 (PPAR-γ2) gene Pro12Ala variation in obese and normal-weight Chinese people.
METHODS: Using culture methods, the amounts of Escherichia coli, Enterococci, Bacteroides, Lactobacilli, Bifidobacteria and Clostridium perfringens (C. perfringens) in the feces of 52 obese participants [body mass index (BMI): ≥ 28 kg/m2] and 52 participants of normal-weight (BMI: 18.5-24 kg/m2) were obtained. Study participants completed comprehensive questionnaires and underwent clinical laboratory tests. The polymerase chain reaction-restriction fragment length polymorphism (PCR-PFLP) assay was used to analyze PPAR-γ2 gene Pro12Ala variation.
RESULTS: The obese group exhibited a lower amount of C. perfringens (6.54 ± 0.65 vs 6.94 ± 0.57, P = 0.001) and Bacteroides (9.81 ± 0.58 vs 10.06 ± 0.39, P = 0.012) than their normal-weight counterparts. No major differences were observed in Pro12Ala genotype distribution between the two groups; however, obese individuals with a Pro/Ala genotype had a significantly lower level of Bacteroides (9.45 ± 0.62 vs 9.93 ± 0.51, P = 0.027) than those with a Pro/Pro genotype. In addition, the obese group demonstrated a higher stool frequency (U = 975, P < 0.001) and a looser stool (U = 1062, P = 0.015) than the normal-weight group.
CONCLUSION: Our results indicated interactions among cultivable gut flora, host genetic factors and obese phenotype and this might be helpful for obesity prevention.
- Citation: Zuo HJ, Xie ZM, Zhang WW, Li YR, Wang W, Ding XB, Pei XF. Gut bacteria alteration in obese people and its relationship with gene polymorphism. World J Gastroenterol 2011; 17(8): 1076-1081
- URL: https://www.wjgnet.com/1007-9327/full/v17/i8/1076.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i8.1076
Obesity and its concomitant consequences are a major cause of metabolic disease in both developed and developing countries[1]. Its occurrence is attributed to a variety of factors. Recent studies have revealed that gut flora, as an environmental factor, might play an important role in the development of obesity[2-6]. However, the majority of these results are based on animal experiments or on studies that involved a small number of human samples. Although molecular methods can detect the majority of uncultivable gut flora, microorganisms present in low numbers but with critical functions are usually omitted; thus, molecular methods provide only a rough profile of gut microbial ecology[7]. This prompted us to consider using culture methods to reveal differences in cultivable gut bacteria. Moreover, culture methods can demonstrate differences in predominantly cultivable bacteria and provide isolates for further characterization and potential application in animal models, which is important for uncovering the relationship between obesity and gut flora.
Along with gut flora, genetic factors are thought to be important in initiating obesity[8]. Previous studies have suggested that the nuclear hormone receptor peroxisome proliferator-activated receptor γ2 (PPAR-γ2) modulates cellular differentiation and lipid accumulation during adipogenesis[9-12], which is essential for the development of obesity. Although it is widely accepted that obesity is caused by various environmental and genetic factors, as well as the complex interactions between them, how environmental and genetic factors interact with each other in obesity remains to be elucidated.
Thus, the aim of this study was to analyze the composition of cultivable bacteria in obese individuals and their normal-weight counterparts to obtain cultivable, obesity-related gut bacteria for further study, and to investigate the potential relationship between the PPAR-γ2 gene Pro12Ala polymorphism and cultivable gut bacteria.
Participants were randomly recruited from Chengdu, a city located in southwest China. All subjects were Chengdu residents. Participants who were taking antibiotics or microecological modulators, and those who had had a gastrointestinal disease during the preceding month, were excluded from the study. Pregnant or lactating women and individuals with major systemic disorders or a history of malignant tumors were also excluded from the study.
The body height and weight of study participants were measured to determine their body mass index (BMI; kg/m2). According to the definition of obesity and normal-weight recommended by the Chinese guidelines for prevention and management of overweight and obesity in Chinese adults, subjects were divided into obese (BMI ≥ 28 kg/m2) and normal-weight (BMI 18.5-24 kg/m2) groups[13]. Routine blood, hepatic and renal function tests and electrocardiogram and B-mode ultrasonic abdominal examinations were performed to assess the health status of each participant. Ultimately, 104 volunteers were included in the study: 52 obese and 52 normal-weight subjects. The study was approved by the Ethics Committee of Sichuan University and informed consent was obtained from all participants before data collection.
A comprehensive questionnaire was used to gather basic information as well as information on dietary intake, physical activity and defecation conditions for each participant. In the questionnaire, a 72-h diet recall for each participant was recorded, which was used to assess macronutrient intake from food composition tables for Chinese diets[14]. For each food, participants selected their serving size, which was represented by standard cups and spoons. Questions about stool form scales and bowel frequency were also included. According to the Bristol stool scale[15], stool forms are classified into three categories: type 1, emerging stools exhibiting fluffy pieces with ragged edges or soft blobs (transit fast, defecate easily); type 2, emerging stools exhibiting a smooth snake or sausage shape with cracks in the surface (transit normally, defecate normally); and type 3, stools that appear as separate, hard or even nut-like lumps (transit slowly, defecate with difficulty).
Fecal samples were obtained in the morning and quantitatively cultured for aerobic, facultative and anaerobic bacteria using the methods defined by the Chinese Ministry of Health[16]. Briefly, freshly voided feces were collected in a sterile box. Fecal samples (10 g) were homogenized and serially diluted in sterile anaerobic solution. Appropriate dilutions were incubated aerobically or anaerobically at 37°C in duplicate using selective media within 2 h after collection. The culture times and conditions are shown in Table 1. The target bacterial colonies on each medium at the corresponding dilution were counted, and the bacteria were subsequently characterized by Gram staining and analytical profile index (API) fermentation tests (bioMérieux, France). Colony counts were expressed as the log of colony forming units per gram of wet feces.
DNA extraction and PPAR-γ2 genotyping
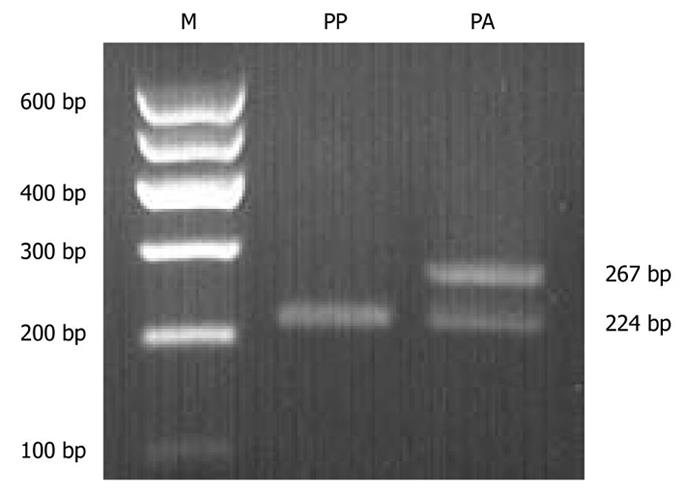
The Pro12Ala polymorphism of the PPAR-γ2 gene was characterized using the polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) assay according to the previously published methods with minor modifications[17]. Briefly, peripheral blood was collected into EDTA-coated tubes. Genomic DNA was isolated using a genomic DNA purification kit (SBS Genetech, China). PCR was performed in a total volume of 50 μL, containing 30 ng of DNA, 0.4 μmol/L of each primer (forward: 5′-GCCAATTCAAGCCCAGTC-3′; reverse: 5′-GATATGTTTGCAGACAGTGTATCAGTGAAGGAA-3′), 1 × PCR buffer, 2 mmol/L of MgCl2, 0.2 mmol/L of dNTP, and 4 U of Taq DNA polymerase (SBS Genetech, China). The PCR cycle conditions consisted of an initial denaturation at 94°C for 2 min followed by 35 cycles of denaturation at 95°C for 15 s, annealing at 58°C for 30 s and extension at 72°C for 30 s, with a final extension at 72°C for 10 min. Then, the PCR product (267 bp) was incubated with 1 U of Hpa II (Fermentas Life Sciences, Canada) for 4 h and digested fragments were separated by electrophoresis in a 3.5% agarose gel and visualized by staining with GoldView I (Solarbio, China). To improve the genotyping quality and validation, all samples were re-genotyped and the results were reproduced with no discrepancies.
The populations of six different types of bacteria were log-transformed for further analysis. Quantitative data were expressed as the mean ± SD. The Student’s t test was used to compare the amounts of cultivable bacteria in obese and normal-weight groups. Chi-squared tests were performed to analyze differences in qualitative data between the two groups. Abnormal distribution data were analyzed using nonparametric tests. The Hardy-Weinberg equilibrium was tested using the χ2 test with one degree of freedom. Two-tailed P values less than 0.05 were considered statistically significant. The Statistical Program for Social Sciences 13.0 software (SPSS Inc., Chicago, IL) was used for all statistical analyses.
A total of 104 participants were recruited for the study: 52 obese (18 females) and 52 normal-weight (26 females) individuals. A homogeneity test indicated that the two groups were comparable with respect to age and sex (Table 2). With regard to the results of the clinical laboratory examination, total cholesterol, triglycerides and fasting glucose in the obese group were considerably higher than those in the normal-weight group (P < 0.01). Leukocyte counts, granulocytes and intermediate cell counts in the obese group were significantly lower than those of normal-weight subjects (P < 0.05). No obvious differences between the obese and normal-weight groups were detected in other parameters (data not shown).
| Obese (n = 52) | Normal-weight (n = 52) | |
| Age (yr) | 34.65 ± 11.91 | 33.02 ± 10.37 |
| Sex | ||
| Male | 34 | 26 |
| Female | 18 | 26 |
| Body mass index (kg/m2) | 30.79 ± 2.80b | 20.26 ± 1.50 |
| Total cholesterol (mmol/L) | 4.91 ± 0.80b | 4.26 ± 0.75 |
| Triglycerides (mmol/L) | 2.33 ± 1.22b | 0.95 ± 0.37 |
| Fasting glucose (mmol/L) | 5.42 ± 0.88b | 4.90 ± 0.45 |
| Leukocyte counts (× 109/L) | 6.96 ± 1.71b | 5.85 ± 1.49 |
| Intermediate cells (× 109/L) | 0.48 ± 0.12a | 0.43 ± 0.10 |
| Granulocytes (× 109/L) | 4.44 ± 1.24b | 3.59 ± 1.14 |
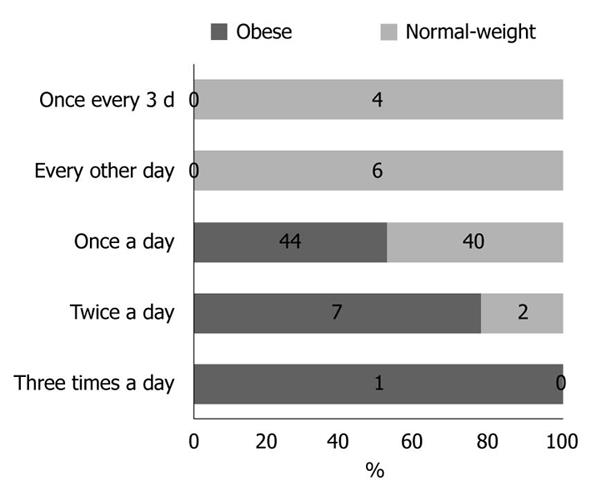
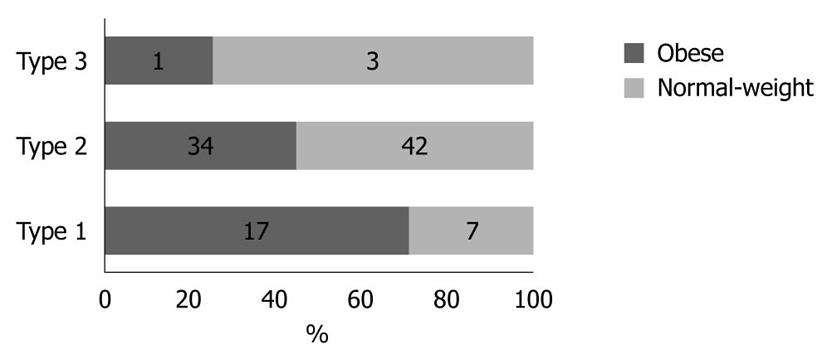
Physical activity and dietary intake of energy and macronutrients demonstrated no statistical differences between the two groups (data not shown). The results obtained for stool frequency and stool form scales are shown in Figures 1 and 2, respectively. For most people in the obese (44 of 52) and normal-weight (40 of 52) groups, stool frequency was once a day. However, stool frequency was higher in the obese group than in the normal-weight group (Figure 1; U = 975, P < 0.001). Furthermore, a notable difference in stool form scales was observed between the two groups (Figure 2; U = 1062, P = 0.015). In general, obese participants produced looser stools and defecated more easily than normal-weight controls.
Quantitative bacterial studies (Table 3) demonstrated that the amount of Bacteroides and Clostridium perfringens (C. perfringens) in feces was significantly lower (Bacteroides, 9.81 ± 0.58 vs 10.06 ± 0.39, P = 0.012; C. perfringens, 6.54 ± 0.65 vs 6.94 ± 0.57, P = 0.001) in the obese group than in the normal-weight participants. No differences in the concentrations of Escherichia coli, Enterococci, Lactobacilli or Bifidobacteria were observed. However, there was a tendency for the amount of Enterococci to be higher in the obese group, despite the five other target bacteria demonstrating the reverse trend (Table 3).
| Bacterial groups | Obese (n = 52) | Normal-weight (n = 52) |
| Bacteroidesa | 9.81 ± 0.58 | 10.06 ± 0.39 |
| Escherichia coli | 7.76 ± 0.92 | 8.09 ± 0.81 |
| Enterococci | 7.53 ± 1.05 | 7.27 ± 2.07 |
| Lactobacilli | 7.98 ± 1.38 | 8.26 ± 0.70 |
| Bifidobacteria | 8.75 ± 1.50 | 9.17 ± 0.80 |
| Clostridium perfringensb | 6.54 ± 0.65 | 6.94 ± 0.57 |
Pro12Ala polymorphism in PPAR-γ2 gene
The PPAR-γ2 gene polymorphism was analyzed in 88 subjects. The genotype frequencies were in Hardy-Weinberg equilibrium (χ2= 0.764, P = 0.382). Pro/Pro and Pro/Ala were found in both obese and normal-weight groups; however, Ala/Ala was not detected in either group. No differences were observed in the distribution of Pro12Ala genotypes (χ2 = 0.107, P = 0.743) or allele frequencies (χ2 = 0.300, P = 0.584) between the groups (Table 4). A typical electrophoresis of the PPAR-γ2 Pro12Ala polymorphism PCR product digested by Hpa II is presented in Figure 3.
| Group | Genotype | Allele | |||
| Pro/Pro | Pro/Ala | Ala/Ala | Pro | Ala | |
| Obese group (n = 41) | 33 (80.5) | 8 (19.5) | 0 (0) | 74 (90.2) | 8 (9.8) |
| Normal-weight group (n = 47) | 40 (85.1) | 7 (14.9) | 0 (0) | 87 (92.6) | 7 (7.4) |
Relationship between PPAR-γ2 gene polymorphism and cultivable gut bacteria
The genetic effects of the Pro12Ala variant on the six gut bacteria were analyzed in the obese and normal-weight groups. No significant differences between the genotype groups, with respect to the six types of gut bacteria, were observed (data not shown) except in the obese group. Obese individuals with a Pro/Ala genotype had a lower amount of Bacteroides than those with a Pro/Pro genotype (9.45 ± 0.62 vs 9.93 ± 0.51, P = 0.027). Moreover, in our study, obese Ala allele carriers had a slightly higher BMI value than obese participants without the Ala allele (31.88 ± 3.26 vs 31.01 ± 2.37, P = 0.393); however, the difference was not statistically significant.
Since the first publication on obesity and gut bacteria in 2004[3], molecular analysis assays have been used to reveal differences in gut bacteria between obese and non-obese individuals as well as in animal models. Although 6 years have passed, data from large-scale human studies using culture methods are lacking, possibly because it is a time-consuming and labor-intensive process. However, culture methods are indispensable for studying the relationship between obesity and gut bacteria.
Our study is the first to use culture methods to analyze differences in fecal bacteria in more than 100 participants (52 obese and 52 normal-weight individuals). Our results demonstrated that the obese people had fewer cultivable Bacteroides than normal-weight individuals, which is consistent with previous studies that detected fewer Bacteroides in obese rodent models and humans using different molecular detection methods[4,6,18,19]. Other studies indicated that the abundance of Bacteroides could promote the generation of propionate, which limits lipid synthesis from acetate and may contribute to a lean phenotype[20,21]. Our study supports this by showing that a larger amount of cultivable Bacteroides occurs in normal-weight people; thus, altering the amount of this cultivable bacterium in obese individuals may help them lose weight.
Bacteroides are the most dominant group of bacteria in the gut and comprise at least four key species (B. thetaiotaomicron, B. vulgatus, B. distasonis and B. fragilis)[22,23]. B. thetaiotaomicron salvages energy by breaking down numerous types of otherwise indigestible polysaccharides and helps shape the metabolic milieu of the intestinal ecosystem[24-26]; however, symbiotic roles for other members of Bacteroides remain unclear. In vitro and in vivo culture methods, such as the inoculation of target species of Bacteroides into animal models, could facilitate further studies on the role of the different Bacteroides. Culture methods can also help isolate and characterize specific cultivable Bacteroides, and strains with desirable traits may be applied in animal models to elucidate how their mechanisms of action may be associated with obesity.
In our study, the obese group also demonstrated a lower amount of C. perfringens than the normal-weight group. This ubiquitous bacterium is a normal inhabitant of the mammalian colon[27]. A previous study demonstrated that Clostridia produces medium-length fatty acids that increase water absorption, dry up feces, weaken stool mobility and eventually result in constipation[28]. Another study found that lower numbers of C. perfringens might enhance fecal moisture content[29]. These results may help interpret our findings that the obese group had looser feces and higher stool frequencies (Figures 1 and 2), along with lower levels of C. perfringens. However, no study has previously shown that C. perfringens interferes with host energy balance, and the role of this bacteria in the development of obesity requires further studies.
Our study also found that the amount of Enterococci in the obese group was higher than that in the normal-weight group. Enterococci are the most controversial group of gut bacteria because of their beneficial and virulent characteristics[30], and their role in the development of obesity remains unknown. The abundance of Enterococci in the obese group in our study indicates that they may play a role in the development of obesity; however, this has yet to be conclusively demonstrated.
Genetic variations and gut flora could both affect obesity status. Recent evidence[31] suggests that factors related to host genotype have an important effect on determining the bacterial composition of the gastrointestinal tract. Our study evaluated the possible relationship between polymorphism of the PPAR-γ2 gene and gut bacteria, and found that the obese Ala allele carriers had a lower amount of Bacteroides than their obese counterparts without the Ala allele. This may indicate an association between reduced Bacteroides in the gut and the obese Ala allele carriers (Pro/Ala, BMI ≥ 28 kg/m2).
Of the potential thrifty genes, PPAR-γ2 plays a key role in modulating adipogenic differentiation[11,12]. During the past decade, the relationship between Pro12Ala variants of the PPAR-γ2 gene and the obese phenotype has been investigated. However, conclusions from the different studies have been inconsistent. To resolve the apparent discrepancies, a meta-analysis was carried out to evaluate data from 19 136 subjects in 30 independent studies. This analysis demonstrated that Ala allele carriers in a recessive model with a BMI above 27 kg/m2 had a significantly higher BMI than noncarriers[32]. As described above, a lower level of Bacteroides might be associated with obesity. Thus, the Pro12Ala variant of the PPAR-γ2 gene may only have an effect on individuals with a higher BMI because of the collective actions of decreased Bacteroides levels in the gut, the Pro12Ala polymorphism and their complex interactions on obesity status.
In this study, we tested a relatively larger sample (52 participants for each group) and decreased numbers of Bacteroides and C. perfringens were found in the obese group. However, further studies are needed to make a clear conclusion on the relationship between gut bacteria and obesity. Our study also found that obese individuals with a Pro/Ala genotype had a statistically lower level of Bacteroides than obese participants with a Pro/Pro genotype. However, the relationship between genotype and gut bacteria requires further elucidation. Furthermore, as this study was only confined to a Chinese population, caution should be taken when extrapolating these results to other races.
In conclusion, this study provides for the first time the data on the differences between six types of gut bacteria in obese and normal-weight Chinese individuals in a large sample (104 participants) using culture methods. We found that the obese group exhibited a lower amount of Bacteroides and C. perfringens, and a slightly higher amount of Enterococci than the normal-weight group. This suggests that obesity may be combated through alteration of specific cultivable gut bacteria. Moreover, our results found that gut bacteria (Bacteroides) might be affected by variations in human genetic factors. These results indicate that interactions between gut flora, host genetic factors and the obese phenotype might lead to development of new measures for the prevention, intervention and treatment of obesity.
The development of obesity is associated with various factors, but the exact etiologic mechanisms still remain unclear. Previous studies demonstrated that gut bacteria along with genetic factors might be important in obesity development.
The relationship between gut bacteria and obesity has been addressed in several journals like Nature and Science in recent years. But the majority of the results are based on animal experiments or on studies that involved a small number of human samples using molecular analysis. Few reports have focused on the relationship between obesity-related genes and gut flora variation. In this study, the authors demonstrated the gut bacteria alteration in obese people using culture methods and its relationship with peroxisome proliferator-activated receptor γ2 (PPAR-γ2) gene polymorphism.
Recent reports demonstrated less Bacteroidetes in obese rodent models and humans using different molecular detection methods, and it was also confirmed in this research by culture method. This study first noticed that the obese individuals with a Pro/Ala genotype had a lower level of Bacteroides than obese participants with a Pro/Pro genotype. These results indicate that there may be a relationship between gut bacteria, the PPAR-γ2 gene and obesity.
The results of this study indicate that interactions between gut flora, host genetic factors and the obese phenotype might lead to development of new measures for the prevention, intervention and treatment of obesity.
The PPAR-γ2 gene is one of the potential thrifty genes, which encodes the nuclear hormone receptor PPAR-γ2. This hormone receptor is selectively expressed in adipose tissue and is strongly up-regulated during adipogenesis, suggesting that it has a specific role in fat-cell differentiation. Thus, the PPAR-γ2 gene might be related to the development of obesity.
The work was well carried out and the manuscript is well written and includes potentially interesting findings.
Peer reviewer: Sung-Gil Chi, Professor, School of Life Sciences and Biotechnology, Korea University, #301, Nok-Ji Building, Seoul 136-701, South Korea
S- Editor Tian L L- Editor Ma JY E- Editor Zheng XM
| 2. | Cani PD, Delzenne NM. Gut microflora as a target for energy and metabolic homeostasis. Curr Opin Clin Nutr Metab Care. 2007;10:729-734. |
| 3. | Bäckhed F, Ding H, Wang T, Hooper LV, Koh GY, Nagy A, Semenkovich CF, Gordon JI. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci USA. 2004;101:15718-15723. |
| 4. | Ley RE, Bäckhed F, Turnbaugh P, Lozupone CA, Knight RD, Gordon JI. Obesity alters gut microbial ecology. Proc Natl Acad Sci USA. 2005;102:11070-11075. |
| 5. | Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027-1031. |
| 6. | Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: human gut microbes associated with obesity. Nature. 2006;444:1022-1023. |
| 7. | Cole JR, Chai B, Farris RJ, Wang Q, Kulam SA, McGarrell DM, Garrity GM, Tiedje JM. The Ribosomal Database Project (RDP-II): sequences and tools for high-throughput rRNA analysis. Nucleic Acids Res. 2005;33:D294-D296. |
| 8. | Rankinen T, Zuberi A, Chagnon YC, Weisnagel SJ, Argyropoulos G, Walts B, Pérusse L, Bouchard C. The human obesity gene map: the 2005 update. Obesity (Silver Spring). 2006;14:529-644. |
| 9. | Ren D, Collingwood TN, Rebar EJ, Wolffe AP, Camp HS. PPARgamma knockdown by engineered transcription factors: exogenous PPARgamma2 but not PPARgamma1 reactivates adipogenesis. Genes Dev. 2002;16:27-32. |
| 10. | Barak Y, Nelson MC, Ong ES, Jones YZ, Ruiz-Lozano P, Chien KR, Koder A, Evans RM. PPAR gamma is required for placental, cardiac, and adipose tissue development. Mol Cell. 1999;4:585-595. |
| 11. | Lowell BB. PPARgamma: an essential regulator of adipogenesis and modulator of fat cell function. Cell. 1999;99:239-242. |
| 12. | Rosen ED, Sarraf P, Troy AE, Bradwin G, Moore K, Milstone DS, Spiegelman BM, Mortensen RM. PPAR gamma is required for the differentiation of adipose tissue in vivo and in vitro. Mol Cell. 1999;4:611-617. |
| 13. | Chen C, Lu FC. The guidelines for prevention and control of overweight and obesity in Chinese adults. Biomed Environ Sci. 2004;17 Suppl:1-36. |
| 14. | Yang YX, Wang GY, Pan XC. China Food Composition 2002. Beijing: Peking University Medical Press 2002; 21-338. |
| 15. | Lewis SJ, Heaton KW. Stool form scale as a useful guide to intestinal transit time. Scand J Gastroenterol. 1997;32:920-924. |
| 16. | Ministry of Health PR, China. Method for the Assessment of Regulating Gastrointestinal Tract Flora Function. Ministry of Health PR, China. Technical Standards for Testing & Assessment of Health Food. Beijing: People’s Medical Publishing House 2003; 148-153. |
| 17. | Franks PW, Luan J, Browne PO, Harding AH, O'Rahilly S, Chatterjee VK, Wareham NJ. Does peroxisome proliferator-activated receptor gamma genotype (Pro12ala) modify the association of physical activity and dietary fat with fasting insulin level? Metabolism. 2004;53:11-16. |
| 18. | Turnbaugh PJ, Hamady M, Yatsunenko T, Cantarel BL, Duncan A, Ley RE, Sogin ML, Jones WJ, Roe BA, Affourtit JP. A core gut microbiome in obese and lean twins. Nature. 2009;457:480-484. |
| 19. | Santacruz A, Marcos A, Wärnberg J, Martí A, Martin-Matillas M, Campoy C, Moreno LA, Veiga O, Redondo-Figuero C, Garagorri JM. Interplay between weight loss and gut microbiota composition in overweight adolescents. Obesity (Silver Spring). 2009;17:1906-1915. |
| 20. | Pouteau E, Nguyen P, Ballèvre O, Krempf M. Production rates and metabolism of short-chain fatty acids in the colon and whole body using stable isotopes. Proc Nutr Soc. 2003;62:87-93. |
| 21. | Walker AW, Duncan SH, McWilliam Leitch EC, Child MW, Flint HJ. pH and peptide supply can radically alter bacterial populations and short-chain fatty acid ratios within microbial communities from the human colon. Appl Environ Microbiol. 2005;71:3692-3700. |
| 22. | Pang X, Ding D, Wei G, Zhang M, Wang L, Zhao L. Molecular profiling of Bacteroides spp. in human feces by PCR-temperature gradient gel electrophoresis. J Microbiol Methods. 2005;61:413-417. |
| 24. | Xu J, Bjursell MK, Himrod J, Deng S, Carmichael LK, Chiang HC, Hooper LV, Gordon JI. A genomic view of the human-Bacteroides thetaiotaomicron symbiosis. Science. 2003;299:2074-2076. |
| 25. | Hooper LV, Midtvedt T, Gordon JI. How host-microbial interactions shape the nutrient environment of the mammalian intestine. Annu Rev Nutr. 2002;22:283-307. |
| 26. | Stappenbeck TS, Hooper LV, Gordon JI. Developmental regulation of intestinal angiogenesis by indigenous microbes via Paneth cells. Proc Natl Acad Sci USA. 2002;99:15451-15455. |
| 27. | Hatheway CL. Toxigenic clostridia. Clin Microbiol Rev. 1990;3:66-98. |
| 28. | Johnson S. Clostridial constipation's broad pathology. Med Hypotheses. 2001;56:532-536. |
| 29. | Kim YM, Lim MY, Lee HS. Evaluation of potato varieties (Solanum tuberosum L.) on fecal microflora of human volunteers. Food Sci Biotechnol. 2005;14:420-423. |
| 30. | Foulquié Moreno MR, Sarantinopoulos P, Tsakalidou E, De Vuyst L. The role and application of enterococci in food and health. Int J Food Microbiol. 2006;106:1-24. |
| 31. | Zoetendal EG, Akkermans ADL, Akkermans-van Vliet WM, de Visser JAGM, de Vos WM. The host genotype affects the bacterial community in the human gastronintestinal tract. Microb Ecol Health Dis. 2001;13:129-134. |
| 32. | Masud S, Ye S. Effect of the peroxisome proliferator activated receptor-gamma gene Pro12Ala variant on body mass index: a meta-analysis. J Med Genet. 2003;40:773-780. |