Published online Feb 28, 2011. doi: 10.3748/wjg.v17.i8.1051
Revised: November 26, 2010
Accepted: December 3, 2010
Published online: February 28, 2011
AIM: To evaluate the difference in diagnostic performance of hydro-stomach computed tomography (CT) to detect early gastric cancer (EGC) between blinded and unblinded analysis and to assess independent factors affecting visibility of cancer foci.
METHODS: Two radiologists initially blinded and then unblinded to gastroscopic and surgical-histological findings independently reviewed hydro-stomach CT images of 110 patients with single EGC. They graded the visibility of cancer foci for each of three gastric segments (upper, middle and lower thirds) using a 4-point scale (1: definitely absent, 2: probably absent, 3: probably present, and 4: definitely present). The sensitivity and specificity for detecting an EGC were calculated. Intraobserver and interobserver agreements were analyzed. The visibility of an EGC was evaluated with regard to tumor size, invasion depth, gastric segments, histological type and gross morphology using univariate and multivariate analysis.
RESULTS: The respective sensitivities and specificities [reviewer 1: blinded, 20% (22/110) and 98% (215/220); unblinded, 27% (30/110) and 100% (219/220)/reviewer 2: blinded, 19% (21/110) and 98% (216/220); unblinded, 25% (27/110) and 98% (215/220)] were not significantly different. Although intraobserver agreements were good (weighted κ = 0.677 and 0.666), interobserver agreements were fair (blinded, 0.371) or moderate (unblinded, 0.558). For both univariate and multivariate analyses, the tumor size and invasion depth were statistically significant factors affecting visibility.
CONCLUSION: The diagnostic performance of hydro-stomach CT to detect an EGC was not significantly different between blinded and unblinded analysis. The tumor size and invasion depth were independent factors for visibility.
- Citation: Park KJ, Lee MW, Koo JH, Park Y, Kim H, Choi D, Lee SJ. Detection of early gastric cancer using hydro-stomach CT: Blinded vs unblinded analysis. World J Gastroenterol 2011; 17(8): 1051-1057
- URL: https://www.wjgnet.com/1007-9327/full/v17/i8/1051.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i8.1051
With the introduction of a screening test for stomach cancer, the incidence of early gastric cancer (EGC) has increased more than 40% in South Korea[1,2]. Since the prognosis of EGC after curative resection is highly favorable (5-year survival rate > 90%), early detection of stomach cancer is essential to improve prognosis[3-5].
Due to the limited role of computed tomography (CT) for the detection of EGC, preoperative stomach CT imaging has been principally used for N and M staging[6-8]. However, considering that an EGC lesion sometimes lacks a tactile sensation on the operative field, especially for laparoscopic surgery, the exact detection and localization of a cancer focus on preoperative stomach CT imaging is of great help to omit preoperative gastroscopic clipping for localization of a cancer focus, as well as for surgical planning. Therefore, preoperative CT imaging is important for not only N and M staging, but also for detection and localization of EGC.
Traditionally, both air and tap water have been used as oral contrast agents to achieve adequate gastric distension. As compared to the use of CT gastrography with air-distension, stomach CT with water-distension (hydro-stomach CT) is less hindered by artifacts caused by air in the lumen. Thus, detailed mucosal enhancement of cancer foci is well demonstrated on hydro-stomach CT[9,10]. However, despite the use of multiplanar reconstruction (MPR) images, the detection rate of EGC on hydro-stomach CT is still low, ranging from 36% to 48%[9,11,12]. Therefore, we have postulated that hydro-stomach CT is intrinsically limited for the detection of EGC. If this assumption is correct, the detection rate of EGC on an unblinded analysis will not improve as compared to that of a blinded analysis.
The purpose of this study was to (1) evaluate the difference in diagnostic performance of hydro-stomach CT to detect EGC between a blinded analysis and unblinded analysis with reference to gastroscopic and surgical-histological findings and to (2) assess factors affecting visibility of cancer foci on hydro-stomach CT imaging.
The institutional review board approved this retrospective study and informed consent of patients was waived. Between July 2008 and August 2008, 159 consecutive patients with a pathologically proven single EGC in our institution, a tertiary care hospital, were enrolled in the study. Surgical or endoscopic submucosal dissection was performed within 1 mo after CT image acquisition. Of the 159 patients, 49 patients were excluded from the analysis for one of the following reasons: (1) CT scanning obtained at other hospital (n = 31); (2) post-endoscopic clipping state (n = 10); (3) post-endoscopic submucosal dissection state (n = 1); (4) no available MPR images (n = 2); (5) the presence of a synchronous advanced gastric cancer at another site (n = 1); (6) the presence of a synchronous gastric polyp at another site (n = 1); and (7) improper gastric distension (n = 3). Ultimately, 110 patients comprised the study population. The patients consisted of 74 men and 36 women (age range, 22-86 years; mean age, 57 years).
Hydro-stomach CT was performed with a 64-detector row CT scanner (LightSpeed VCT; GE Healthcare, Milwaukee, WI, USA) with the patient in the prone position on the CT table. With this protocol, non-dependent side of the stomach (i.e. the fundus for a patient in the prone position) was frequently distended by air rather than by water. In order to avoid air distension, if the cancer was located in the gastric fundus, a CT scan was performed with the patient in the supine position. Before CT scanning, each patient had fasted for over 6 h. A total of 500-1000 mL tap water was administered orally to obtain gastric distension just prior to scanning. A CT scan was obtained 70 s after the injection of a dose of 2 mg/kg of nonionic contrast material iopromide (Ultravist 300; Schering, Berlin, Germany) at a rate of 4 mL/s using an automated power injector. The scanning ranged from the diaphragm to the lower end of the symphysis pubis. The CT parameters used were as follows: 40 mm beam collimation (0.625 mm × 64); pitch, 0.984; kVp/mA, 120/300 with automatic exposure control using both Auto mA and Smart mA; gantry rotation time, 0.6 s. Axial images (slice thickness/interval, 5 mm/5 mm) and sagittal and coronal MPR images (slice thickness/interval, 3 mm/3 mm) were obtained with the use of isotropic raw data (slice thickness/interval, 0.625 mm/0.625 mm).
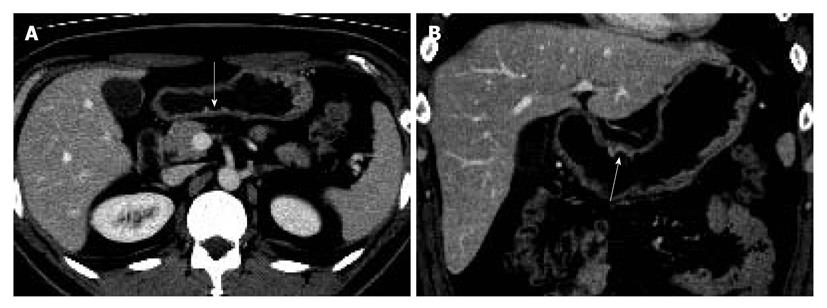
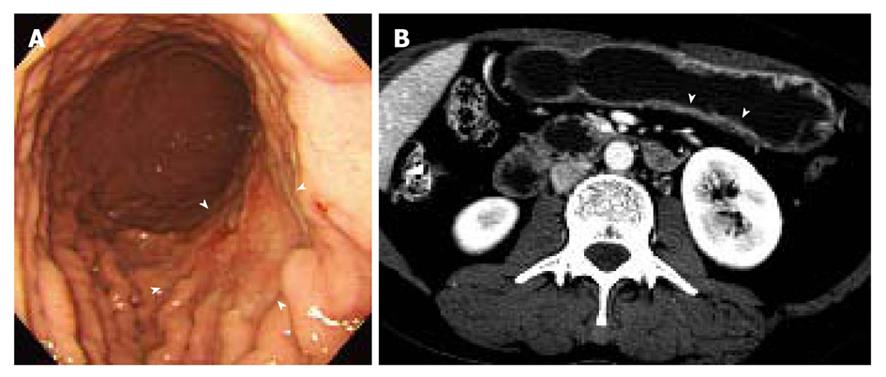
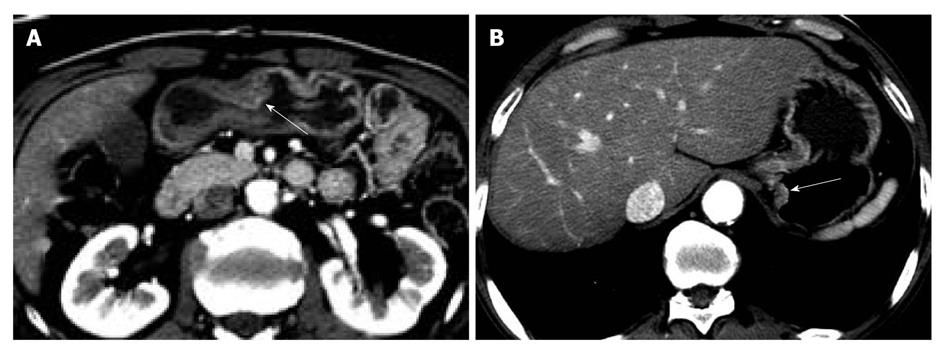
Two gastrointestinal radiologists (D.C., 11 years experience; M.W.L., 6 years experience) evaluated the visibility of EGC by interpretation of both axial and MPR images on a 2K × 2K picture archiving and communication system (PACS, GE Medical Systems Integrated Imaging Solutions, Mt Prospect, IL, USA) monitor (MDL9DLB020; Totoku, Tokyo, Japan), independently. At the first interpretation session, although the radiologists were aware that each patient was confirmed to have an EGC, the radiologists were blinded to the gastroscopic and surgical-histological findings. The stomach was divided into three segments along the longitudinal axis (from gastroesophageal junction to pyloric canal): upper, middle and lower thirds. For 330 gastric segments, both reviewers graded the likelihood of the presence of an EGC focus for each segment based on the use of a 4-point scale as follows: (1) definitely absent; (2) probably absent; (3) probably present; and (4) definitely present. The presence of an EGC was defined as focal plaque-like wall thickening compared to adjacent gastric wall with or without prominent enhancement of the gastric inner layer[12]. In addition, the radiologists marked the lesion with an arrow during image analysis. A study coordinator (K.J.P.) captured and stored the marked images into a JPEG file whenever one reviewer finished the interpretation of the CT images. Then the study coordinator erased the mark on the CT images before the initiation of the next interpretation session.
Two weeks after the first blinded interpretation session, a second interpretation was performed. For the second session, the reviewers were not blinded to the clinical information of patients and were provided with all data including the gastroscopic, surgical and pathological findings.
The two reviewers and a study coordinator assessed whether the indicated lesions on CT images were in accord or not in consensus based on the gastroscopic, surgical, and pathological findings. If at least one of the two reviewers correctly indicated an EGC lesion, it was regarded as a visible EGC. If both reviewers missed an EGC lesion, it was regarded as an invisible EGC.
The scores 1 and 2 for the likelihood of the presence of an EGC focus for each segment were regarded as an absence of an EGC focus. The scores 3 and 4 were regarded as a presence of an EGC focus. The sensitivity and specificity for the detection of an EGC focus on CT images were calculated and compared between the blinded analysis and unblinded analysis. Intraobserver and interobserver agreements were analyzed with the use of weighted κ statistics.
Reasons of visibility for the subjective assessment by the radiologists were analyzed. Visible EGCs were compared with invisible EGCs for tumor size, depth of invasion, involved gastric segments, type of histology and type of gross morphology by use of univariate analysis and multivariate analysis. The size of an EGC was based on the maximal diameter as measured on a gross specimen. The univariate association between individual variables for visibility was tested using the χ2 test for categorical variables (depth of invasion, involved gastric segments, type of histology and type of gross morphology). The size of a tumor between visible and invisible EGC was compared using the unpaired t test. In order to assess independent factors that affected the visibility of an EGC on hydro-stomach CT images, multiple logistic regression analysis was used to test the significance of adjusted factors. Variables with P < 0.05 determined by univariate analysis were chosen as variables for multiple logistic regression analysis. For both univariate analysis and multiple logistic regression analysis, P < 0.05 was considered to indicate a statistically significant difference.
No significant technical failure, such as a severe artifact that interfered with image interpretation of hydro-stomach CT, was noted for all patients. Thus, 330 segments (110 segments with EGC and 220 segments without EGC) were included and were analyzed.
The pathological findings of 110 EGCs are summarized in Table 1. Seventy-one EGCs were confined to the mucosa and the other 39 EGCs invaded the submucosal layer. There were 18 elevated (type I and IIa), 24 flat (type IIb) and 68 depressed (type IIc and III) EGCs.
| Characteristic | Visible EGC(n = 39) | Invisible ECC (n = 71) | P value at univariate analysis | Multiple logistic regression analysis | ||
| P value | Odds ratio | 95% CI | ||||
| Size (cm) | 3.59 ± 1.91 | 2.20 ± 1.37 | < 0.001 | 0.002 | 1.573 | 1.185-2.088 |
| Depth of invasion | 0.001 | 0.018 | 2.923 | 1.201-7.116 | ||
| Mucosa | 17 | 54 | ||||
| Submucosa | 22 | 17 | ||||
| Involved segment | 0.378 | |||||
| Upper 1/3 | 6 | |||||
| Middle 1/3 | 14 | 28 | ||||
| Lower 1/3 | 19 | 38 | ||||
| Type of histology | 0.862 | |||||
| Tubular adenocarcinoma, well differentiated | 10 | 15 | ||||
| Tubular adenocarcinoma, moderately differentiated | 14 | 21 | ||||
| Tubular adenocarcinoma, poorly differentiated | 8 | 17 | ||||
| Poorly differentiated carcinoma | 1 | 3 | ||||
| Signet ring cell carcinoma | 6 | 15 | ||||
| Gross morphology of tumor | 0.541 | |||||
| I | 1 | 2 | ||||
| IIa | 8 | 7 | ||||
| IIb | 7 | 17 | ||||
| IIc | 23 | 44 | ||||
| III | 0 | 1 | ||||
The sensitivity and specificity of the blinded analysis and unblinded analysis are summarized in Table 2. Based on a consensus review, 39 EGCs were indicated by at least one reviewer and were regarded as visible EGCs. The other 71 EGCs were regarded as invisible. There were frequent false positives at blinded analysis for both reviewers (five for reviewer 1 and four for reviewer 2). The sensitivity and specificity of both reviewers were not significantly different between the blinded analysis and unblinded analysis (Figures 1-3). Despite an unblinded analysis, the detection rates were not improved for both reviewers. For mucosal cancer (n = 71), the sensitivity and specificity for reviewer 1 blinded to the findings were 17% (12/71) and 98% (139/142), respectively, and were 21% (15/71) and 100% (142/142), respectively, for reviewer 1 unblinded to the findings. The sensitivity and specificity for reviewer 2 blinded to the findings were 10% (7/71) and 98% (139/142), respectively, and were 14% (10/71) and 100% (142/142), respectively, for reviewer 2 unblinded to the findings. For both reviewers, differences were not significant (McNemar test, each P > 0.05). The intraobserver agreements were good for both reviewers (weighted κ = 0.677 for reviewer 1 and 0.666 for reviewer 2). However, interobserver agreements were fair (blinded, 0.371) or moderate (unblinded, 0.558).
| Reviewer 1 | Reviewer 2 | |||||
| Blinded | Unblinded | P value | Blinded | Unblinded | P value | |
| Sensitivity | 22/110 (20) | 30/110 (27) | > 0.05 | 21/110 (19) | 27/110 (25) | > 0.05 |
| Specificity | 215/220 (98) | 219/220 (100) | > 0.05 | 216/220 (98) | 215/220 (98) | > 0.05 |
The baseline characteristics of 110 EGCs are summarized in Table 1. For both univariate analysis and multivariate analysis, the size and depth of invasion of the tumor were statistically significant factors affecting visibility (Table 1).
Our study demonstrated that EGCs are poorly visible on hydro-stomach CT images even though both axial and MPR (sagittal and coronal) images were evaluated. The detection rate was not significantly different between blinded and unblinded analysis for both reviewers, which indicated that EGC detection on hydro-stomach CT imaging is intrinsically limited due to poor detection performance. These findings correspond well with results of an earlier study by Yu et al[13]. In that study, almost all primary gastric cancers (43/44, 98%) not visualized on preoperative hydro-stomach CT images were identified as EGCs.
In comparison, CT gastrography with air-distension has enabled the ability to obtain various three-dimensional (3D) rendering images such as virtual gastroscopy, surface shaded display, volume rendering and transparent rendering images[8,14-17]. On CT gastrography, combined interpretation of axial and various 3D reconstructed images have shown an improved detection rate of EGC in many previous studies[8,15-17]. The detection rate of EGC by the use of CT gastrography has been reported to range from 73% to 96%[8,15-17], which seems to be superior to that of hydro-stomach CT (36% to 48%)[9,11,12]. Using both two-dimensional (2D) and various 3D rendering images of CT gastrography, mucosal nodularity, a malignant fold change and the presence of a depressed or elevated lesion could be demonstrated[15,16]. Furthermore, virtual gastroscopy can be used as a complimentary study to conventional gastroscopy, as the modality provides endoluminal images with a wider field of view, resulting in no blind spots unlike those which occur with the use of conventional gastroscopy[16]. However, as compared to conventional gastroscopy, virtual gastroscopy is limited in the detection of superficial flat lesions, as the method cannot provide discoloration of a flat EGC lesion.
Although hydro-stomach CT has been known to provide improved visualization of the gastric wall and gastric tumors without image degradation by artifacts derived from intraluminal air[6,18], only 2D-based images (axial and MPR images) are used for the interpretation of preoperative evaluation of stomach cancer with the use of hydro-stomach CT. Although the addition of MPR images to conventional axial images has offered improved detection and localization of EGC[11], detection of EGC in the absence of thickened wall and enhancement is still difficult with the use of 2D images only. In addition, partial volume averaging artifacts can be problematic for the evaluation of the gastric wall, especially in a case located on the antrum or angle where the gastric wall is tangent to an axial scan[11]. Nevertheless, hydro-stomach CT has been widely utilized for the preoperative staging work-up of stomach cancer due to increased workload of radiologists and time-consuming postprocessing of CT gastrography.
In our study, the detection of EGC was highly related to the depth of invasion. Mucosal cancer is less visible as compared to submucosal cancer, a finding similar to the results of previous studies[11,12]. As compared to the EGC detection rate (48%, 34/71) determined by Kim et al[9], although the exact proportion of mucosal and submucosal cancer in that study was not known, the detection rate of EGC based on the blinded analysis (20%, 22/110, reviewer 1; 19%, 21/110, reviewer 2) in our study was inferior. This finding could be explained by the fact that the proportion of mucosal cancer that is known as difficult to detect on hydro-stomach CT was as high as 65% (71/110) in our study. The sensitivity for mucosal cancer as determined by the two reviewers was only 17% and 10% in our study. These results are in close agreement with sensitivity values of 17% (3/18) reported by Shimizu et al[11] and 16% (11/69) reported by Woo et al[12].
In addition to the depth of invasion, the size of a tumor was another cause of invisibility in our study (Table 1). The size (3.59 ± 1.91 cm) of a visible EGC was larger as compared to an invisible EGC (2.20 ± 1.37 cm). However, this result is in disagreement with that (2.80 ± 1.35 cm vs 2.18 ± 1.85 cm) reported in a study by Woo et al[12] in which the size was not significantly different between invisible and visible EGCs. This discrepancy between two studies could have been influenced by differences in the study population.
Gross morphology was not a factor that affected EGC visibility in our study. If lesions were grouped into two categories (depressed vs non-depressed lesions), visibility was not significantly different between the two categories (Table 1), which coincides well with a previous study[12]. We believe that the perception of a shallow depressed lesion on 2D images is more difficult as compared to various 3D reconstructed images provided by CT gastrography, as the 3D images intuitively show malignant fold changes, mucosal nodularity and elevated or depressed lesions[15,16].
This study has some limitations. Firstly, this study was a retrospective, single institution study over a defined period. The ability to detect an EGC lesion on a CT scan may be different, depending on factors such as the experience of the radiologists, CT protocol, patient population and tumor characteristics. In addition, we did not perform CT gastrography with air-distension. Therefore, although we compared our results with findings of previous investigations that used CT gastrography, a direct comparison is limited due to the different patient populations, type of CT equipment and experience of the radiologists. Thus, a further comparison study may be warranted between hydro-stomach CT and CT gastrography to detect EGC lesions. Secondly, we did not evaluate the visibility of EGC according to gastric distension. As a collapsed stomach could obscure a gastric lesion or simulate the pathology, detection of EGC could be influenced by gastric distension[14]. In addition, as with the transverse colon as visualized on CT colonography[19], the lower one-third segment of the stomach is more likely to be compressed than the upper one-third segment for a patient in the prone position. However, as oral administration of more than 500 mL of water has been widely used for optimal gastric distension on hydro-stomach CT[9,12,13], gastric distension was not taken into consideration at the time of analysis. Thirdly, some of the EGC lesions marked by the reviewers were discordant between each other. In addition, there were frequent false positives at blinded analysis by both reviewers. Therefore, visible EGC lesions in our study have a possibility of false positives. However, this problem seems to be intrinsically inevitable for this style of study, since a fully reliable method was not available to confirm the location of EGC on CT images. Fourthly, since patient position at CT acquisition partially gives the information about the location of an EGC focus, this might have influenced the blinded image analysis of reviewers.
In conclusion, hydro-stomach CT imaging was not a reliable tool for the detection of EGC. The poor diagnostic performance of hydro-stomach CT to detect EGC was not significantly different between blinded and unblinded analysis. The size and depth of invasion of an EGC were two independent factors for visibility.
The incidence of early gastric cancer (EGC) is currently rising faster than previously, correlating with the introduction of a screening test for stomach cancer. Considering the poor tactile sensation of an EGC lesion on the operative field, especially for laparoscopic surgery, the exact localization of a cancer focus on preoperative stomach CT imaging is important to omit preoperative gastroscopic clipping for localization of a cancer focus as well as for surgical planning.
Despite introduction of multi-detector row CT techniques and the use of multiplanar reconstruction (MPR) images, the detection rate of EGC on hydro-stomach CT has still been unsatisfactory. In order to see whether the detection rate of EGC on unblinded analysis can be improved as compared to that of blinded analysis with reference to gastroscopic and surgical-histological findings, diagnostic performance of hydro-stomach CT to detect EGC was compared between blinded analysis and unblinded analysis. In this study, the authors demonstrate that the diagnostic performance of hydro-stomach CT to detect EGC was poor and was not significantly different between blinded and unblinded analysis.
Traditionally, both air and tap water have been used as oral contrast agents to achieve adequate gastric distension for preoperative CT imaging in patients with EGC. This study demonstrates that diagnostic performance of hydro-stomach CT for the detection of EGC is poor and unenhanced even when unblinded analysis is performed. The tumor size and invasion depth were independent factors for visibility of EGC on hydro-stomach CT.
Hydro-stomach CT imaging seems not to be a reliable tool for the detection of EGC. In this context, for the preoperative evaluation of patients with EGCs, it is time to consider CT gastrography which can offer not only 2D images but also various 3D reconstructed images showing malignant fold changes, mucosal nodularities and elevated or depressed lesions.
Hydro-stomach CT refers to stomach CT with water-distension. Compared with CT gastrography with air-distension, hydro-stomach CT has been believed to be less affected by artifacts caused by intraluminal air and is likely to demonstrate detailed mucosal enhancement of cancer foci.
This study was designed to evaluate the difference of diagnostic performance of hydro-stomach CT to detect EGC between blinded and unblinded analysis. The English is written well.
Peer reviewer: Dr. Wei-Dong Tong, MD, MS, BS, Associate Professor, Department of General Surgery, Veterans Affairs Medical Center, Medical college of Wisconsin, Milwaukee, WI 53295, United States
S- Editor Tian L L- Editor Logan S E- Editor Zheng XM
| 1. | Lee HJ, Yang HK, Ahn YO. Gastric cancer in Korea. Gastric Cancer. 2002;5:177-182. |
| 2. | Park CH, Song KY, Kim SN. Treatment results for gastric cancer surgery: 12 years' experience at a single institute in Korea. Eur J Surg Oncol. 2008;34:36-41. |
| 3. | Shimada S, Yagi Y, Shiomori K, Honmyo U, Hayashi N, Matsuo A, Marutsuka T, Ogawa M. Characterization of early gastric cancer and proposal of the optimal therapeutic strategy. Surgery. 2001;129:714-719. |
| 4. | Martin RC 2nd, Jaques DP, Brennan MF, Karpeh M. Extended local resection for advanced gastric cancer: increased survival versus increased morbidity. Ann Surg. 2002;236:159-165. |
| 5. | Nitti D, Marchet A, Mammano E, Ambrosi A, Belluco C, Mencarelli R, Maino M, Marconato G, Farinati F, Lise M. Extended lymphadenectomy (D2) in patients with early gastric cancer. Eur J Surg Oncol. 2005;31:875-881. |
| 6. | Takao M, Fukuda T, Iwanaga S, Hayashi K, Kusano H, Okudaira S. Gastric cancer: evaluation of triphasic spiral CT and radiologic-pathologic correlation. J Comput Assist Tomogr. 1998;22:288-294. |
| 7. | Lee JH, Jeong YK, Kim DH, Go BK, Woo YJ, Ham SY, Yang SO. Two-phase helical CT for detection of early gastric carcinoma: importance of the mucosal phase for analysis of the abnormal mucosal layer. J Comput Assist Tomogr. 2000;24:777-782. |
| 8. | Shin KS, Kim SH, Han JK, Lee JM, Lee HJ, Yang HK, Choi BI. Three-dimensional MDCT gastrography compared with axial CT for the detection of early gastric cancer. J Comput. Assist Tomogr. 2007;31:741-749. |
| 9. | Kim YN, Choi D, Kim SH, Kim MJ, Lee SJ, Lee WJ, Kim S, Kim JJ. Gastric cancer staging at isotropic MDCT including coronal and sagittal MPR images: endoscopically diagnosed early vs. advanced gastric cancer. Abdom Imaging. 2009;34:26-34. |
| 10. | Lim JS, Yun MJ, Kim MJ, Hyung WJ, Park MS, Choi JY, Kim TS, Lee JD, Noh SH, Kim KW. CT and PET in stomach cancer: preoperative staging and monitoring of response to therapy. Radiographics. 2006;26:143-156. |
| 11. | Shimizu K, Ito K, Matsunaga N, Shimizu A, Kawakami Y. Diagnosis of gastric cancer with MDCT using the water-filling method and multiplanar reconstruction: CT-histologic correlation. AJR Am J Roentgenol. 2005;185:1152-1158. |
| 12. | Woo SK, Kim S, Kim TU, Lee JW, Kim GH, Choi KU, Jeon TY. Investigation of the association between CT detection of early gastric cancer and ultimate histology. Clin Radiol. 2008;63:1236-1244. |
| 13. | Yu JS, Choi SH, Choi WH, Chung JJ, Kim JH, Kim KW. Value of nonvisualized primary lesions of gastric cancer on preoperative MDCT. AJR Am J Roentgenol. 2007;189:W315-W319. |
| 14. | Kim AY, Kim HJ, Ha HK. Gastric cancer by multidetector row CT: preoperative staging. Abdom Imaging. 2005;30:465-472. |
| 15. | Kim HJ, Kim AY, Oh ST, Kim JS, Kim KW, Kim PN, Lee MG, Ha HK. Gastric cancer staging at multi-detector row CT gastrography: comparison of transverse and volumetric CT scanning. Radiology. 2005;236:879-885. |
| 16. | Kim JH, Eun HW, Choi JH, Hong SS, Kang W, Auh YH. Diagnostic performance of virtual gastroscopy using MDCT in early gastric cancer compared with 2D axial CT: focusing on interobserver variation. AJR Am J Roentgenol. 2007;189:299-305. |
| 17. | Yang DM, Kim HC, Jin W, Ryu CW, Kang JH, Park CH, Kim HS, Jung DH. 64 multidetector-row computed tomography for preoperative evaluation of gastric cancer: histological correlation. J Comput Assist Tomogr. 2007;31:98-103. |
| 18. | Düx M, Richter GM, Hansmann J, Kuntz C, Kauffmann GW. Helical hydro-CT for diagnosis and staging of gastric carcinoma. J Comput Assist Tomogr. 1999;23:913-922. |
| 19. | Tolan DJ, Armstrong EM, Burling D, Taylor SA. Optimization of CT colonography technique: a practical guide. Clin Radiol. 2007;62:819-827. |