Published online Feb 21, 2011. doi: 10.3748/wjg.v17.i7.906
Revised: September 29, 2010
Accepted: October 6, 2010
Published online: February 21, 2011
AIM: To evaluate outcome of patients with Budd-Chiari syndrome after balloon angioplasty ± stenting or transjugular intrahepatic portosystemic shunt (TIPS).
METHODS: Twenty five patients with Budd-Chiari syndrome admitted to Ain Shams University Hospitals, Tropical Medicine Department were included. Twelve patients (48%) with short segment occlusion were candidates for angioplasty; with stenting in ten cases and without stenting in two. Thirteen patients (52%) had Transjugular Intrahepatic Portosystemic Shunt. Patients were followed up for 12-32 mo.
RESULTS: Patency rate in patients who underwent angioplasty ± stenting was 83.3% at one year and at end of follow up. The need of revision was 41.6% with one year survival of 100%, dropped to 91.6% at end of follow up. In patients who had Transjugular Intrahepatic Portosystemic Shunt, patency rate was 92.3% at one year, dropped to 84.6% at end of follow up. The need of revision was 38.4% with one year and end of follow up survival of 100%. Patients with patent shunts showed marked improvement compared to those with occluded shunts.
CONCLUSION: Morbidity and mortality following angioplasty ± stenting and TIPS are low with satisfactory outcome. Proper patient selection and management of shunt dysfunction are crucial in improvement.
- Citation: Eldorry A, Barakat E, Abdella H, Abdelhakam S, Shaker M, Hamed A, Sakr M. Outcome of non surgical hepatic decompression procedures in Egyptian patients with Budd-Chiari. World J Gastroenterol 2011; 17(7): 906-913
- URL: https://www.wjgnet.com/1007-9327/full/v17/i7/906.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i7.906
Budd-Chiari syndrome (BCS) results from hepatic venous outflow obstruction at any level, from hepatic venules to the right atrium[1]. If obstruction is due to endoluminal venous lesion like thrombosis, primary BCS is considered. In secondary BCS, the cause originates from neighboring structures like extrinsic compression or tumor invasion[2].
Imaging studies combined with clinical information are often essential for reaching a definitive diagnosis[3].
The goals of treatment are to prevent extension of thrombosis in hepatic veins (HVs) and to alleviate venous obstruction in order to decrease hepatic congestion. Few patients respond to medical treatment (anticoagulation ± thrombolytic therapy, diuretics). However, most patients need intervention to restore the hepatic blood flow[4].
If there is a possibility of restoring hepatic venous outflow in one of the major HVs by balloon dilatation, recanalization, or stent insertion, then this is the procedure of choice as it is the most physiological method. However, in cases where blood flow cannot be restored or when the approach fails, transjugular intrahepatic portosystemic shunt (TIPS) is used as a decompressing non-surgical procedure[5].
Study Design & Sampling: This prospective follow-up study was conducted on twenty five patients with confirmed diagnosis of primary BCS and eligible criteria for radiological intervention, who were presented to the Budd-Chiari Study Group and admitted to the Tropical Medicine Department, Ain Shams University Hospitals.
Patients were subjected to: (1) Complete Clinical Evaluation; and (2) Radiological Assessment, with special stress on the patency of HVs, portal vein and inferior vena cava (IVC) by abdominal Duplex/US. Abdominal MRI, MR venography or multislice CT scan were done to confirm diagnosis and to delineate vascular anatomy before intervention.
They were divided into two groups: (1) Patients with short segment occlusion of any of HVs who were candidates for angioplasty ± stenting; and (2) Patients with complete occlusion of all HVs who were candidates for TIPS.
Exclusion criteria: (1) Secondary BCS; (2) Retro or suprahepatic IVC obstruction; (3) Complete portal vein thrombosis; (4) Presence of comorbid etiology for liver disease in addition to BCS (e.g.: viral hepatitis); (5) Hepatocellular carcinoma; (6) Cardiac contraindications to TIPS (congestive heart failure and severe pulmonary hypertension); (7) Marked coagulopathy (INR > 5) and Thrombocytopenia (platelets < 20 000)[6]; (8) Biliary obstruction; and (9) Uncontrolled sepsis.
Details of the study and interventions were explained to recruited patients who signed a written consent form.
Routine laboratory investigations and thrombophilia workup were done aiming at identification of etiology of BCS, in addition to assessment of liver disease severity.
Patients’ general health was assessed according to WHO performance status scale[7]: 0: patient is fully active, able to carry on all pre-disease performance without restriction; 1: patient is restricted in physically strenuous activity but ambulatory and able to carry out work of a light or sedentary nature, e.g. light house work; 2: patient is ambulatory and capable of all self care but unable to carry out any work activities. Up and about more than 50% of waking hours; 3: patient is capable of only limited self care, confined to bed or chair more than 50% of waking hours; 4: patient cannot carry on any self care and totally confined to bed or chair.
Patients were classified as follows: According to Rotterdam prognostic classification[8] into 3 classes with scores according to the equation: 1.27 × encephalopathy + 1.04 × ascites + 0.72 × prothrombin time + 0.004 × bilirubin [Ascites and hepatic encephalopathy were scored as present (1) or absent (0) and prothrombin time as higher (1) or lower (0) than 2.3 INR. Bilirubin was included as a continuous variable]. Where Class I (0-1.1): good prognosis; Class II (1.1-1.5): intermediate prognosis and Class III (> 1.5): poor prognosis.
According to Child-Pugh score into 3 classes (A, B and C)[9].
All patients started anticoagulation therapy when diagnosis of BCS was evident; in the form of low molecular weight heparin (LMWH) or unfractionated heparin. Then oral warfarin was added till INR reached its target (2-3), then continued on oral therapy alone after withdrawal of LMWH or unfractionated heparin.
Five days before procedure, oral anticoagulation therapy was stopped with administration of LMWH or unfractionated heparin only; to be stopped (6-12 h) before intervention in case of unfractionated heparin and (12-24 h) in case of LMWH to avoid intra or postoperative bleeding[10].
Antibiotic prophylaxis was administered for all patients (1-2 h) before intervention in the form of combination of ampicillin- sulbactam 1.5 gm IV and cefotaxime 1 gm IV[11].
All procedures were performed in an angiographic interventional room with high resolution C-arm fluoroscopy, and digital subtraction angiography.
Interventions were done under general anesthesia.
All cases of TIPS or angioplasty with stenting had self expandable non covered metallic stents.
Patients were admitted to hospital for 1 wk after procedure for early detection and management of any procedure-related complications and adjustment of anticoagulation.
Antibiotics regimen taken before procedure was continued for 5 d after.
Oral warfarin was introduced together with parental anticoagulation (LMWH after 24 h or unfractionated Heparin after 6 h) till INR reaches (2-3) then oral therapy was continued alone for life[12].
Duplex U/S was performed to detect shunt patency at days 1, 3, and 7 after the procedure.
Patients were followed up clinically, by laboratory investigations (mainly liver profile and PT and PTT for monitoring of anticoagulation) and radiologically by duplex U/S.
Follow up after intervention was every three mo or when indicated (e.g.: clinical manifestations suggestive of angioplasty or TIPS dysfunction). Follow up was intended to be at least one year (Minimum: 12 mo, Maximum: 32 mo).
(1) Assessment of patients’ survival and shunt survival (i.e.; shunt patency and function) (at one year interval and at the end of follow up); (2) Description of procedures related complications and their management; and (3) Assessment of patients’ improvement after intervention by comparison of clinical, laboratory and performance status criteria before intervention and one year after.
Descriptive statistics: (1) Quantitative data: mean, standard deviation (± SD); and (2) Qualitative data: frequency and percentage.
Analytical statistics: (1) Quantitative data: Wilcoxon Signed Ranks Test; and (2) Qualitative data: McNemar Test.
Levels of significance: (1) P > 0.05 = non significant (NS); (2) P < 0.05 = significant (S); (3) P < 0.01 = highly significant (HS); and (4) P < 0.001 = very highly significant (VHS).
Survival: (1) Patient Survival was defined as the duration between diagnosis of BCS, and patient death or loss to follow up. Survival rates were Kaplan-Meier estimates; (2) Shunt Survival was defined as the duration between shunt application, and shunt occlusion or loss to follow up. Survival rates were Kaplan-Meier estimates.
This study was conducted on twenty five patients with BCS who underwent non surgical hepatic decompression procedures in the form of either angioplasty ± stenting or TIPS. They were 16 females (64%) and 9 males (36%) with a mean age of 28.28 ± 8.93 years (range 14-57 years). BCS was chronic form in 21 patients (84%), acute in three patients (12%), and fulminant in 1 patient (4%). When tested for underlying thrombophilia, 8 were negative (idiopathic), 4 primary antiphospholipid antibody syndrome (APS), 4 protein C deficiency, 3 Antithrombin III deficiency, 1 myeloproliferative disorder, 1 combined protein C, S deficiency, 1 combined protein C, S, Antithrombin III deficiency, 1 combined Antithrombin III deficiency + factor V Leiden mutation (FVLM), 1 combined protein S deficiency + FVLM and 1 was primary APS + FVLM.
According to Child Classification, 5 patients (20%) were Child A, 16 (64%) were Child B and 4 (16%) were Child C. According to Rotterdam Classification, 7 patients (28%) were Class I, 15 (60%) were Class II and 3 (12%) were Class III. The Performance status score was “0” in none of the patients, “1” in 4 patients (16%), “2” in 5 patients (20%), “3” in 11 patients (44%) and “4” in 5 patients (20%).
Clinical manifestations and baseline radiological criteria of studied patients using duplex U/S, MRV and/or Multislice CT scan are shown in Table 1.
| Findings | Patients, n (%) |
| Clinical manifestations | |
| Abdominal pain | 23 (92) |
| Jaundice | 9 (36) |
| Lower limb edema | 10 (40) |
| Dilated veins over abdomen and trunk | 5 (20) |
| Tender hepatomegaly | 16 (64) |
| Ascites | 24 (96) |
| Radiological criteria | |
| Hepatomegaly | 24 (96) |
| Splenomegaly | 22 (88) |
| Ascites | |
| Absent | 1 (4) |
| Present | 24 (96) |
| Liver mottling appearance | 17 (68) |
| Intra hepatic collaterals | 16 (64) |
| Caudate lobe hypertrophy | 12 (48) |
| Hepatic Veins:Short segment occlusion | |
| RHV | 2 (8) |
| MHV | 7 (28) |
| LHV | 5 (20) |
| Total occlusion | |
| RHV | 23 (92) |
| MHV | 18 (72) |
| LHV | 20 (80) |
Intervention details: The main indications for intervention in the studied patients were ascites associated with large esophageal varices; uncontrollable ascites only; large esophageal varices only and fulminant hepatic failure in 56%; 36%; 4% and 4% of patients respectively.
Twelve patients (48%) were candidates for angioplasty; of those; 10 patients (40%) had stenting (5; 20% in MHV, 4; 16% in LHV and 1; 4% in RHV) and 2 patients (8%) had angioplasty without stenting (1 patient in both LHV and MHV and the other patient in both RHV and MHV, where they shared a common short stenotic segment at their entrance into IVC).
Thirteen patients (52%) were candidates for TIPS.
The need of revision was 41.6% (5 out of 12 patients) in cases of angioplasty ± stenting and 38.4% (5 out of 13 patients) in cases of TIPS as shown in Table 2.
| Patient | Intervention | Time of dysfunction | Action taken | No of revisions | 1 yr patency | End of FUP patency |
| 23 yr F | Angioplasty without stenting | Day 7 and Day 10 | TIPS was done, occluded at day 10; then re-angioplasty was done1 | 2 | Patent | Patent at 20th mo |
| 27 yr M | Angioplasty and stenting | Day 7 and 2nd yr | Angioplasty was done-then angioplasty + thrombectomy | 2 | Patent | Patent at 24th mo |
| 28 yr F | Angioplasty and stenting | 4th mo | Angioplasty + local thrombolytic therapy | 1 | Patent | Patent at 12th mo |
| 30 yr F | Angioplasty and stenting | 1st, 4th, 6th and 9th mo | TIPS was done-then angioplasty (3 times) | 4 | Occluded at 9th mo | Occluded at 24th mo |
| 28 yr M | Angioplasty and stenting | 3rd mo and 14th mo | Angioplasty + stent was done-then mesoatrial shunt | 1 | Occluded at 1 yr | Dead2 at 17th mo |
| 27 yr F | TIPS | Day 1 | (stent occlusion and migration to portal vein) - Re (TIPS) | 1 | Patent | Patent at 20th m |
| 33 yr F | TIPS | Day 3 | Angioplasty + thrombectomy + systemic thrombolytic therapy | 1 | Patent | Patent at 32nd mo |
| 37 yr F | TIPS | Day 7 and 1st mo | Angioplasty (2 times) | 2 | Patent | Patent at 12th mo |
| 27 yr M | TIPS | Day 7, 3rd and 8th mo | Angioplasty (3 times) | 3 | Patent | Occluded at 20th mo |
| 17 yr M | TIPS | 1st mo | Patient refused intervention | 0 | Occluded | Occluded at 12th mo |
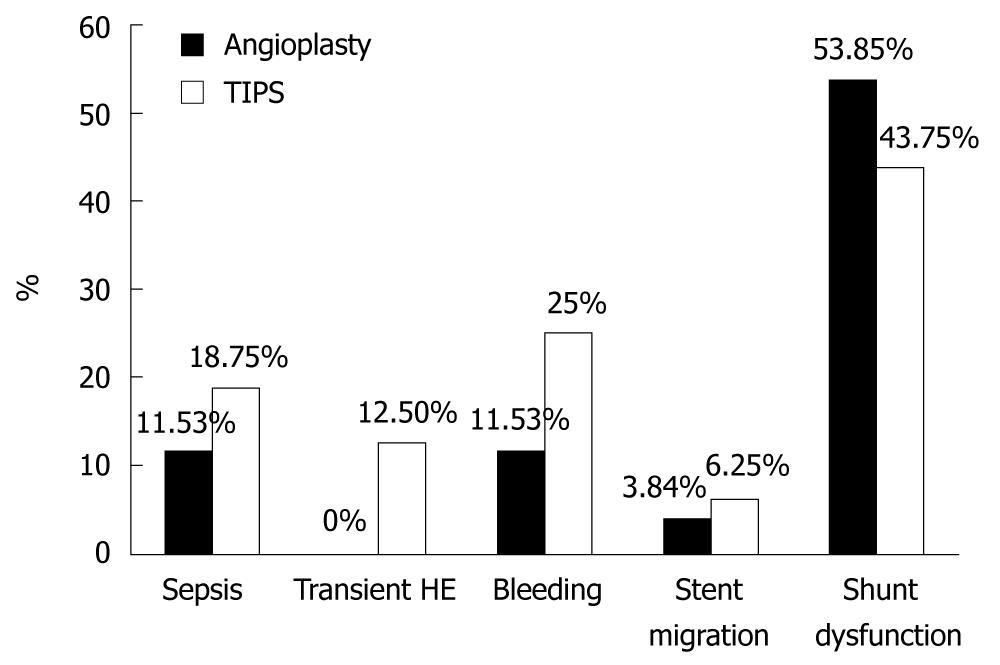
Figure 1 shows frequency of all complications in total procedures done [Twenty six angioplasty ± stenting procedures (12 as primary intervention and 14 as a trial for maintenance of previously occluded angioplasty or TIPS) and 16 TIPS procedures (13 as primary intervention and 3 in patients with occluded stents following angioplasty in whom redilatation was not possible)].
In total procedures done (whether primary or revision procedures), the frequency of angioplasty dysfunction was 53.85% (14 out of 26 procedures) and the frequency of TIPS dysfunction was 43.75% (7 out of 16 procedures).
The mean duration of follow up was 20.04 ± 7.817 mo (ranging from 12-32 mo). One year survival rate was 100% for all patients and at the end of follow up survival rate was 96% due to death of one patient at the 17th mo of follow up as shown in Table 3.
| Angioplasty | TIPS | Total | |
| One year | |||
| Alive | 12 (100) | 13 (100) | 25 (100) |
| Dead | 0 (0) | 0 (0) | 0 (0) |
| End of follow up | |||
| Alive | 11 (91.6) | 13 (100) | 24 (96) |
| Dead | 1 (8.4) | 0 (0) | 1 (4) |
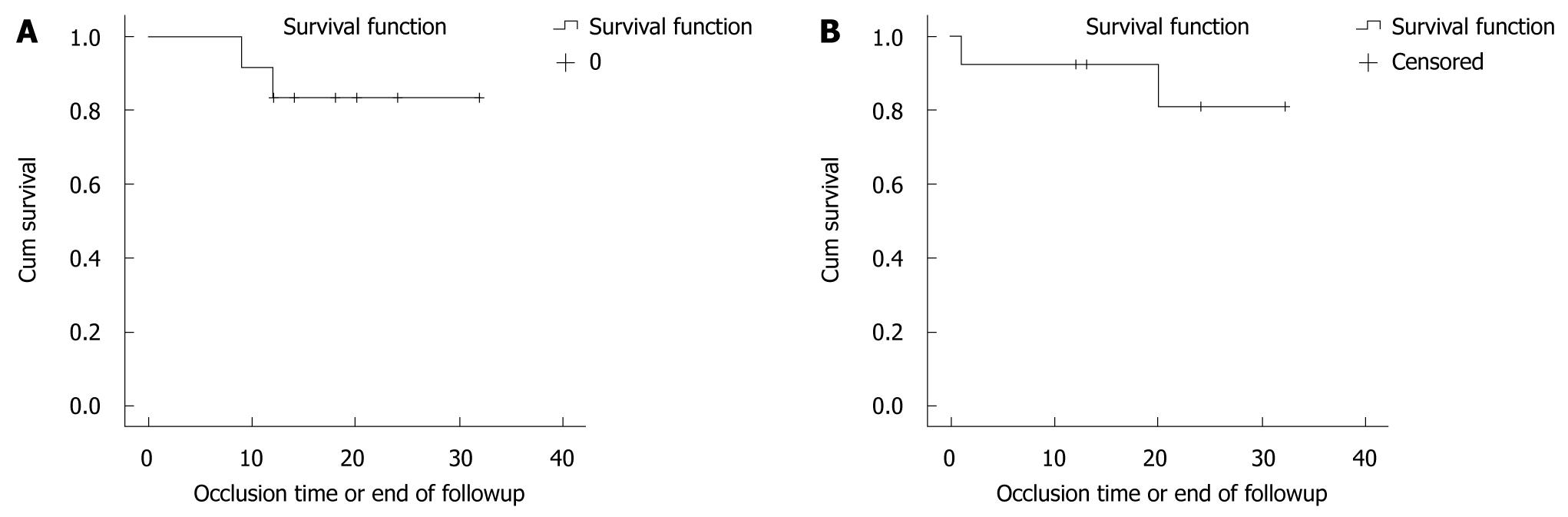
Figure 2A shows patency rate in patients who underwent angioplasty ± stenting procedures; it was 11/12 (91.7%) at 9 mo (due to persistent shunt occlusion in one patient). Patency rate dropped to 10/12 (83.3%) at one year and continued till the end of follow up at 32 mo. (There was persistent shunt occlusion in 2 patients in spite of repeated revisions and optimal anticoagulation therapy).
Figure 2B shows patency rate in patients who had TIPS procedures; it was 12/13 (92.3%) at one year (due to persistent shunt occlusion in one patient despite repeated revisions). Patency rate dropped to 11/13 (84.6%) at 20 mo and this continued till the end of follow up at 32 mo (due to persistent shunt occlusion in another patient).
At one year of follow up, only three patients of 25 (12%) had occluded shunts. Patients with occluded shunts showed no improvement regarding their clinical manifestations, laboratory profile and performance status. On the contrary, patients with patent shunts (22 of 25; 88%) showed marked improvement as shown in Tables 4 and 5.
| Before intervention | One year after intervention | P value | Sig | |||
| +VE | -VE | +VE | -VE | |||
| Patients with occluded shunts (n = 3) | ||||||
| Abdominal pain | 3 | 0 | 2 | 1 | > 0.05 | NS |
| Jaundice | 1 | 2 | 0 | 3 | > 0.05 | NS |
| Lower limb edema | 2 | 1 | 1 | 2 | > 0.05 | NS |
| Dilated veins | 1 | 2 | 0 | 3 | > 0.05 | NS |
| Ascites | 3 | 0 | 3 | 0 | > 0.05 | NS |
| Patients with patent shunts (n = 22) | ||||||
| Abdominal pain | 20 | 2 | 1 | 21 | < 0.001 | VHS |
| Jaundice | 8 | 14 | 0 | 22 | < 0.01 | HS |
| Lower limb edema | 8 | 14 | 1 | 21 | < 0.05 | S |
| Dilated veins | 4 | 18 | 0 | 22 | > 0.05 | NS |
| Ascites | 21 | 1 | 1 | 21 | < 0.001 | VHS |
| Before intervention | One year after intervention | P value | Sig | |||
| mean | SD | mean | SD | |||
| Patients with occluded shunts (n = 3) | ||||||
| ALT (N = 7-40 IU/L) | 70.33 | 75.070 | 29.66 | 24.66 | > 0.05 | NS |
| AST (N = 7-37 IU/L) | 42 | 24.240 | 42.33 | 32.51 | > 0.05 | NS |
| Total bilirubin (N = 0.2-1.2 mg/dL) | 2.9 | 2.940 | 1.26 | 0.832 | > 0.05 | NS |
| Direct bilirubin (N = 0-0.3 mg/dL) | 1.53 | 1.560 | 0.53 | 0.577 | > 0.05 | NS |
| Albumin (N = 3.5-5.3 g/dL) | 3.7 | 0.800 | 3.56 | 0.901 | > 0.05 | NS |
| Performance status | 3.33 | 0.577 | 2.00 | 1.730 | > 0.05 | NS |
| Patients with patent shunts (n = 22) | ||||||
| ALT (N = 7-40 IU/L) | 66.95 | 117.265 | 26.45 | 8.528 | < 0.05 | S |
| AST (N = 7-37 IU/L) | 53.95 | 33.832 | 32.22 | 9.586 | < 0.01 | HS |
| Total bilirubin (N = 0.2-1.2 mg/dL) | 2.818 | 3.198 | 1.21 | 0.414 | < 0.01 | HS |
| Direct bilirubin (N = 0-0.3 mg/dL) | 1.29 | 2.022 | 0.51 | 0.296 | < 0.01 | HS |
| Albumin (N = 3.5-5.3 g/dL) | 3.5 | 0.475 | 3.93 | 0.576 | < 0.01 | HS |
| Performance status | 2.59 | 1.007 | 0.18 | 0.664 | < 0.001 | VHS |
This is the first study that addresses the short term outcome of interventional radiology procedures in management of Egyptian patients with BCS. In this study, 12 patients (48%) had short segment occlusion that enabled us to perform angioplasty with stenting in ten cases and without stenting in two cases. Thirteen patients (52%) were not suited for angioplasty and had TIPS.
According to Xu et al[13], short-term results of balloon angioplasty alone without stenting were excellent but the sustained patency rate was only 50% at two years after the procedure. In this study, one of the cases that had angioplasty alone was still having patent shunt at 24 mo after the procedure without any need for shunt revision; the other one had occluded shunt on the seventh day that necessitated re-intervention in the form of TIPS which was still patent at 20 mo after procedure.
Patency rate in patients who underwent angioplasty ± stenting procedures was 10/12 (83.3%) at one year and at the end of follow up due to persistent shunt occlusion in 2 patients in spite of repeated revisions and optimal anticoagulation therapy. This is a more or less satisfactory outcome; however it might have been influenced by the relatively short follow up period (ranging from 12 to 32 mo) as well as most of the patients having good or intermediate prognosis according to Rotterdam score. The need of revision in cases with angioplasty ± stenting was 41.6% (5 out of 12 cases). One year survival was 100% and at the end of follow up, survival dropped to 91.6% due to death of one patient who had occluded shunt after one year and was also referred for mesoatrial shunt due to occlusion of IVC.
Although angioplasty is considered a simple procedure; some complications were reported in the current study. Twenty six angioplasty ± stenting procedures have been done (12 procedures as primary intervention and 14 procedures as a trial for maintenance of previously occluded angioplasty or TIPS); of these procedures, angioplasty dysfunction was reported in 53.85%. This is consistent with Senzolo et al[14] who stated that although long-term patency rates can reach 80%-90% in angioplasty ± stenting procedures; angioplasty may later be required in 50% of these cases to overcome angioplasty dysfunction.
Stent migration, which is very rare, occurred in one angioplasty procedure (3.84%) where stent migrated to the heart just after insertion. However, no serious complications occurred and stent was embedded in the wall of right atrium and the patient was quite well.
Post procedure (angioplasty ± stenting) bleeding was encountered in 3 procedures (11.53%), 2 of which were intraperitoneal and one of which was hemobilia. All 3 cases were managed conservatively by temporary stoppage of anticoagulation and blood transfusion when indicated. This complication could be attributed to the application of a transhepatic approach in these procedures. Beckett and Olliff[5] stated that this approach has the merit of simplicity over a transjugular or transfemoral approach, as well as feasibility with major superior vena caval obstruction but with a potentially greater risk of bleeding.
Post procedure sepsis occurred in 3 procedures (11.53%) in spite of antibiotic prophylaxis with cefotaxime in combination with ampicillin-sulbactam. This could be due to infection from resistant organisms. According to McDermott et al[15], pathogens that precipitated infection after angioplasty and stent were Staphylococcus aureus and S. epidermidis, which were sensitive to cefazolin.
In this study, the results of angioplasty ± stenting agreed with Fisher et al[16] who stated that, with appropriate case selection, many patients with BCS caused by short length HV stenosis or occlusion may be managed successfully by angioplasty ± stenting with a good outcome following the procedure, provided that anticoagulation is maintained. According to the authors’ comparative study between percutaneous angioplasty and operative shunt surgery; both groups had the same re-occlusion rate and both were related to suboptimal dose of anticoagulation.
In the current study, 13 patients (52%) were not candidates for angioplasty and underwent TIPS. The need for revision was 38.4% (compared to 41.6% in angioplasty ± stenting). One year and end of follow up survival rates following TIPS were 100%. This could be attributed to the relatively short follow up duration (ranging from 12 to 32 mo) and good selection of cases, as most of our patients had good or intermediate predictable prognosis according to Rotterdam score.
Patency rate in patients who had TIPS procedures was 12/13 (92.3%) at one year due to persistent shunt occlusion in one patient despite repeated revisions. At the end of follow up; patency rate dropped to 11/13 (84.6%) due to persistent shunt occlusion in another patient.
The results of the current study are much better than what had been reported by Valla[17], namely that secondary thrombosis or shunt dysfunction requiring revision occurs in about 70% of cases by 6 mo. However, the results of this study are more or less comparable to those reported by Senzolo et al[14] who stated that 36%-72% of patients needed reintervention after TIPS. The authors also reported a long-term patency rate of about 50% despite of routine anticoagulation therapy.
Comparison between the results of the current study, regarding TIPS, with other studies is shown in Table 6.
| Points of comparison | Mancuso et al[18] | Perelló et al[19] | Rössle et al[20] | Hernández-Guerra et al[21] | Current study |
| No. of patients | 15 | 13 | 35 | 25 (9 covered stents) | 13 |
| Mean age in years (range) | 40 (20-73) | 36 (17-67) | 43 (12-74) | 40 (17-54) | 29 (14-57) |
| Median child score | 11 | 9 | 9 | 9 | 8 |
| Acute, fulminant/chronic presentation | 8/6 | 4/6 | 11/13 | ND | 2/11 |
| Mean follow-up (mo) | 24 | 48 | 37 | 20 | 18 |
| Stent stenosis (%) | 36 | 72 | 47 | 67 (19% covered stents) | 38.4 |
| Anticoagulation (%) | 100 | 95 | 100 | ND | 100 |
| Patients with acute presentation who died | 4 | ND | 2 | ND | 0 |
| Patients with chronic presentation who died | 0 | ND | 1 | ND | 0 |
| Death total (%) | 30 | 10 | 9 | 0 | 0 |
| Liver transplantation | 0 | 1 | 2 | 0 | 0 |
| Surgical portocaval shunt | 0 | 2 | 0 | 0 | 0 |
Sixteen TIPS procedures have been done throughout the current study (13 as primary intervention and 3 in patients with occluded stents following angioplasty in which predilatation was not possible).
Post TIPS sepsis occurred in 3 procedures (18.75%), in spite of prophylactic antibiotics. According to Dravid et al[22]; an infection rate of 13% following TIPS was reported.
According to Ryan et al[11], acute infection related to TIPS placement appears to be uncommon. Whether or not prophylactic antibiotics are of value remains undetermined. Options for prophylactic antibiotics for TIPS are: (1) no prophylaxis; (2) 1 g ceftriaxone single dose intravenously before procedure; and (3) 1.5-3 g ampicillin/sulbactam single dose intravenously before procedure. We adopted the third strategy successfully in combination with cefotaxime 1 gm IV and completed the course of antibiotics for five days after intervention.
Hepatic encephalopathy after TIPS occurred in 2 patients (12.5%) and was transient, lasting only for 2-3 d and responded well to anti hepatic encephalopathy measures.
Post procedure bleeding was encountered in 4 procedures (25%), 2 intraperitoneal and 2 hemobilia; all were managed conservatively with temporary stoppage of anticoagulation and blood transfusion if indicated.
In the current study, the overall 1 year shunt patency of all procedures (angioplasty ± stenting and TIPS) was 22/25 (88%) as 3 patients had occluded shunts in spite of repeated trials of dilatation and adherence to anticoagulation therapy. We compared clinical and laboratory characteristics before and after intervention in patients with patent shunts (22 patients) and in those with occluded shunts (3 patients) irrespective of the type of procedure performed. We observed that patients with occluded shunts showed no improvement compared to those with patent shunts even after multiple revisions in terms of clinical manifestations, laboratory profile and performance status.
These observations are consistent with Bachet et al[23] who concluded that, in patients with BCS treated with portosystemic shunting, shunt dysfunction has a major impact on morbidity and mortality and maintenance of shunt patency is of major importance for better long-term outcome.
In conclusion; Budd Chiari syndrome is a potentially life-threatening disorder that requires a multidisciplinary approach with hepatologist, hematologist, interventional radiologist and vascular surgeon. Morbidity and mortality following both angioplasty ± stenting and TIPS are low with satisfactory stent and patient survival. Proper selection of procedure candidates and maintenance of shunt patency by strict adherence to anticoagulation and early management of shunt dysfunction are crucial in clinical, laboratory and radiological improvement of BCS patients.
Budd-Chiari syndrome (BCS) results from hepatic venous outflow obstruction at any level from hepatic venules to the right atrium. Few patients respond to medical treatment (anticoagulation ± thrombolytic therapy, diuretics). However, most patients need intervention to restore the hepatic blood flow. Restoring outflow in one of the major hepatic veins by balloon dilatation ± stenting is the management of choice. When not possible or failed, Transjugular Intrahepatic Portosystemic Shunt is used.
Follow up of patients after radiological intervention is crucial in order to assess patient improvement, shunt patency and function and to manage any procedure related complications. In this study, the authors demonstrate that morbidity and mortality following angioplasty ± stenting and transjugular intrahepatic portosystemic shunt (TIPS) are low with satisfactory outcome.
This is the first Egyptian study that addresses the short term outcome of interventional radiology procedures in management of BCS.
This study may represent a future strategy for good selection of procedure candidates, maintenance of shunt patency by strict adherence to anticoagulation and early management of shunt dysfunction which are all crucial in clinical, laboratory and radiological improvement of BCS patients.
Angioplasty means balloon dilatation of hepatic vein; it may be with or without stent insertion. This procedure is performed in BCS patients with short segment stenosis or occlusion of the hepatic veins with significant patent segments. This approach will re-establish hepatic venous outflow via the physiological route. In cases where blood flow cannot be restored or where the approach fails (usually because the remaining patent veins are too small or have insufficient flow), Transjugular Intrahepatic Portosystemic Shunt is used; in which the shunt connects the hepatic vein to the portal vein to bypass the obstruction.
The authors evaluated the outcome of patients with BCS after non surgical hepatic decompression procedures (either balloon angioplasty ± stenting or TIPS). It revealed that morbidity and mortality following both procedures are low with satisfactory stent and patient survival. Thus, proper selection of procedure candidates and maintenance of shunt patency by strict adherence to anticoagulation and early management of shunt dysfunction are crucial in clinical, laboratory and radiological improvement of those patients. Their results are excellent on managing a very challenging group of patients and their program should be commended for this outcome.
Peer reviewer: Bijan Eghtesad, Dr, Associate Professor, Department of General Surgery, Cleveland Clinic Foundation, 9500 Euclid Avenue, Cleveland, OH 44195, United States
S- Editor Cheng JX L- Editor Rutheford A E- Editor Ma WH
| 1. | Zimmerman MA, Cameron AM, Ghobrial RM. Budd-Chiari syndrome. Clin Liver Dis. 2006;10:259-273, viii. |
| 2. | Aydinli M, Bayraktar Y. Budd-Chiari syndrome: etiology, pathogenesis and diagnosis. World J Gastroenterol. 2007;13:2693-2696. |
| 3. | Brancatelli G, Vilgrain V, Federle MP, Hakime A, Lagalla R, Iannaccone R, Valla D. Budd-Chiari syndrome: spectrum of imaging findings. AJR Am J Roentgenol. 2007;188:W168-W176. |
| 4. | Slakey DP, Klein AS, Venbrux AC, Cameron JL. Budd-Chiari syndrome: current management options. Ann Surg. 2001;233:522-527. |
| 5. | Beckett D, Olliff S. Interventional radiology in the management of Budd Chiari syndrome. Cardiovasc Intervent Radiol. 2008;31:839-847. |
| 6. | Boyer TD, Haskal ZJ. The role of transjugular intrahepatic portosystemic shunt in the management of portal hypertension. Hepatology. 2005;41:386-400. |
| 7. | Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, Carbone PP. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649-655. |
| 8. | Darwish Murad S, Valla DC, de Groen PC, Zeitoun G, Hopmans JA, Haagsma EB, van Hoek B, Hansen BE, Rosendaal FR, Janssen HL. Determinants of survival and the effect of portosystemic shunting in patients with Budd-Chiari syndrome. Hepatology. 2004;39:500-508. |
| 9. | Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60:646-649. |
| 10. | Madura JA, Rookstool M, Wease G. The management of patients on chronic Coumadin therapy undergoing subsequent surgical procedures. Am Surg. 1994;60:542-546; discussion 546-547. |
| 11. | Ryan JM, Ryan BM, Smith TP. Antibiotic prophylaxis in interventional radiology. J Vasc Interv Radiol. 2004;15:547-556. |
| 12. | Kraai EP, Lopes RD, Alexander JH, Garcia D. Perioperative management of anticoagulation: guidelines translated for the clinician. J Thromb Thrombolysis. 2009;28:16-22. |
| 13. | Xu K, He FX, Zhang HG, Zhang XT, Han MJ, Wang CR, Kaneko M, Takahashi M, Okawada T. Budd-Chiari syndrome caused by obstruction of the hepatic inferior vena cava: immediate and 2-year treatment results of transluminal angioplasty and metallic stent placement. Cardiovasc Intervent Radiol. 1996;19:32-36. |
| 14. | Senzolo M, Cholongitas EC, Patch D, Burroughs AK. Update on the classification, assessment of prognosis and therapy of Budd-Chiari syndrome. Nat Clin Pract Gastroenterol Hepatol. 2005;2:182-190. |
| 15. | McDermott VG, Schuster MG, Smith TP. Antibiotic prophylaxis in vascular and interventional radiology. AJR Am J Roentgenol. 1997;169:31-38. |
| 16. | Fisher NC, McCafferty I, Dolapci M, Wali M, Buckels JA, Olliff SP, Elias E. Managing Budd-Chiari syndrome: a retrospective review of percutaneous hepatic vein angioplasty and surgical shunting. Gut. 1999;44:568-574. |
| 17. | Valla DC. The diagnosis and management of the Budd-Chiari syndrome: consensus and controversies. Hepatology. 2003;38:793-803. |
| 18. | Mancuso A, Fung K, Mela M, Tibballs J, Watkinson A, Burroughs AK, Patch D. TIPS for acute and chronic Budd-Chiari syndrome: a single-centre experience. J Hepatol. 2003;38:751-754. |
| 19. | Perelló A, García-Pagán JC, Gilabert R, Suárez Y, Moitinho E, Cervantes F, Reverter JC, Escorsell A, Bosch J, Rodés J. TIPS is a useful long-term derivative therapy for patients with Budd-Chiari syndrome uncontrolled by medical therapy. Hepatology. 2002;35:132-139. |
| 20. | Rössle M, Olschewski M, Siegerstetter V, Berger E, Kurz K, Grandt D. The Budd-Chiari syndrome: outcome after treatment with the transjugular intrahepatic portosystemic shunt. Surgery. 2004;135:394-403. |
| 21. | Hernández-Guerra M, Turnes J, Rubinstein P, Olliff S, Elias E, Bosch J, García-Pagán JC. PTFE-covered stents improve TIPS patency in Budd-Chiari syndrome. Hepatology. 2004;40:1197-1202. |
| 22. | Dravid VS, Gupta A, Zegel HG, Morales AV, Rabinowitz B, Freiman DB. Investigation of antibiotic prophylaxis usage for vascular and nonvascular interventional procedures. J Vasc Interv Radiol. 1998;9:401-406. |