INTRODUCTION
Nearly one million patients are diagnosed with colorectal cancers (CRC) annually in the world[1]. The incidence of CRC is highest in the western world where it is the second commonest cause of cancer death and fourth commonest cause of death from cancer worldwide[2]. In the western world there is a life time risk of CRC of 5%. Overall the 5 year survival has improved in the UK (55% in males and 51% in females) but to a lesser extent than in the USA and Europe[3].
Around 30%-40% of colorectal cancer is defined to arise from the rectum which is defined as the distal margin of tumor within 15 cm of the anal verge[4,5].
Colonoscopy and biopsy is considered as the gold standard investigation to confirm the diagnosis of rectal cancer and to exclude synchronous lesions. Patients are then staged to assess the extent of local disease and to identify the distant spread.
Traditional rectal cancer surgery is associated with high rates of local recurrence of 5%-20%[6]. However, with the combination of high quality surgery using total mesorectal excision[7] along with use of neoadjuvant and adjuvant treatment there has been a significant reduction in local recurrence and improved survival[8]. The surgeon aims to achieve a microscopic tumor free (R0) resection. Despite this, there is a risk of local failure. Careful preoperative assessment of the pelvis identifies high risk patients in whom the resection margins are either involved or within 1 mm of the mesorectal fascia. Involvement or threatened CRM (tumors within 1 mm of the mesorectal fascia) have a reduced chance of obtaining complete clearance. Thus, the status of the CRM has become more important than the TNM staging. In Europe and the UK, patients with involved CRM/threatened CRM are considered for long course chemoradiation prior to surgery.
IMPORTANCE OF PREOPERATIVE STAGING IN RECTAL CANCER
Accurate pre-operative staging of rectal cancer is crucial in planning the surgical treatment and is the strongest predictor for recurrence[9]. The staging helps us to formulate a structured multidisciplinary management care plan and assess the prognosis. It is also used to compare the results of hospitals offering rectal cancer treatment and to define the role of different treatment modalities.
Preoperative staging of rectal cancer can be divided into either local or distant staging. Local staging incorporates the assessment of mural wall invasion, circumferential resection margin involvement, and the nodal status for metastasis. Distant staging assesses for evidence of metastatic disease.
Rectal cancer is palpable in 40%-80% of cases[10]. Digital rectal examination helps in documentation of the size, location, distance from the anal verge, and fixity. Lesions felt by digital rectal examination can be visualized using a rigid proctoscope. The procedure allows an accurate localization and assessment of the tumor including fixity. Biopsies can be carried out where necessary. Rectal examination using proctoscopy may be considered as an important tool for newly diagnosed rectal cancers. Painful local perineal and anal conditions such as fissures or abscesses can restrict the use of this excellent tool. A trial comparing the use of CT virtual proctoscopy with rectal ultrasound examination in determining the stage of rectal cancer is being conducted in the USA and its results are awaited (http://clinical trials.gov/ct2/show/NCT00585728).
Currently, several modalities exist for the preoperative staging of rectal cancer. A combination of modalities involving use of computed tomography (CT), magnetic resonance imaging (MRI), and/or endorectal ultrasonography (EUS) is used to precisely assess the extent of spread of rectal cancer. The choice of investigations performed, however, is influenced by local expertise, guidelines and availability. Imaging in rectal cancer plays a crucial role in optimizing radiotherapy target definition to avoid adjacent vital structures[11]. EUS and MRI of the pelvis are used to assess the local spread while CT is the main modality to assess systemic spread. PET is indicated when there is clinical, biochemical or radiological suspicion of local recurrence or systemic disease.
Computerized tomography and computerized tomography colonography or virtual colonoscopy
CT scan of the entire chest, abdomen and pelvis is used for the detection of metastatic disease. CT is widely available and has faster acquisition times. However, it is not considered as the investigation of choice when it comes to assessing the layers of the rectal wall; hence it is not useful for local staging in rectal cancer and certainly is poor at evaluating superficial rectal cancers. The accuracy of CT to assess the tumor has been reported to be between 80%-95% in patients with advanced local disease[12]. The accuracy, however, decreased to around 63% when a broader spectrum of tumor sizes was analyzed. Sensitivity to pick up nodal disease has been found to be between 55%-70%[13]. In a meta-analysis involving 5000 patients, CT showed an accuracy for T staging of 73% and for nodal staging of 22%-73%[14].
The use of contrast enhanced multidetector CT colonography has improved the staging accuracy[15], by achieving superior spatial resolution and visualizing pictures in a variety of planes. However, its role in staging remains to be determined and currently it is used mainly to assess the distant metastatic disease (Figure 1A and B).
Figure 1 Computerized tomography.
A: Computerized tomography (CT) abdomen showing a patient with rectal cancer having liver metastasis and ascites; B: CT Chest showing a patient with rectal cancer having lung metastasis.
Virtual colonoscopy or CT colonogram (CTC) has been reported to be safer than colonoscopy[16] while being more sensitive than barium enema, and appears to be more acceptable to patients than either of the other tests[17]. The procedure can be performed by technicians thus saving clinicians time. In principle the data could be analysed by computer-assistance thus accelerating diagnosis time[18]. The results of the SIGGAR trial evaluating CTC versus colonoscopy or barium enema in symptomatic elderly patients are awaited[19]. CTC is the best radiological imaging for assessing the colon and rectum and at the same time identifies nodal disease and distant metastasis. The diagnosis of rectal cancer still needs to be confirmed by colonoscopy and biopsy.
Magnetic resonance imaging
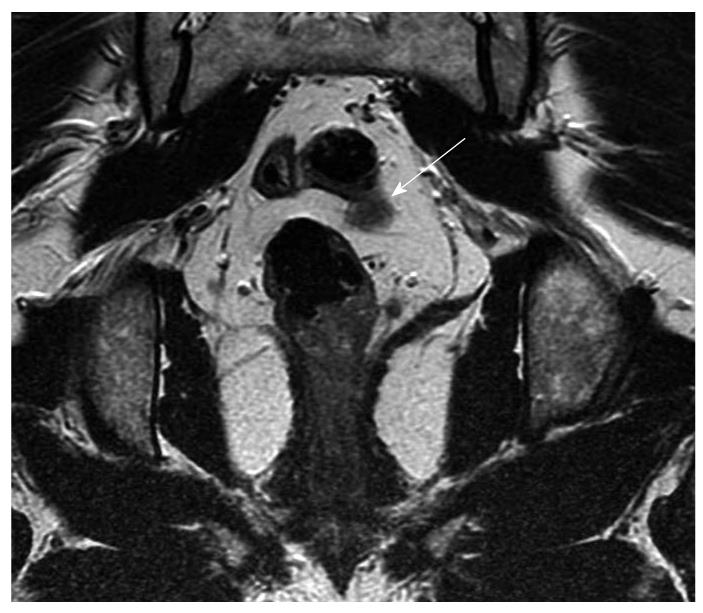
Magnetic resonance imaging (MRI) is routinely used for preoperative staging of rectal cancer as it provides an accurate assessment of the tumor and the surrounding mesorectal fascia. It identifies patients at risk of local recurrence and those likely to benefit from neoadjuvant therapy. When compared with CT and ultrasound, MRI is more reliable for the evaluation of the extent of locoregional disease, planning radiation therapy, assessing postoperative changes and pelvic recurrence. The evaluation of nodal metastases remains a challenge with MRI (Figure 2).
Figure 2 Coronal magnetic resonance imaging (arrow) showing possible lymph node or early vascular involvement.
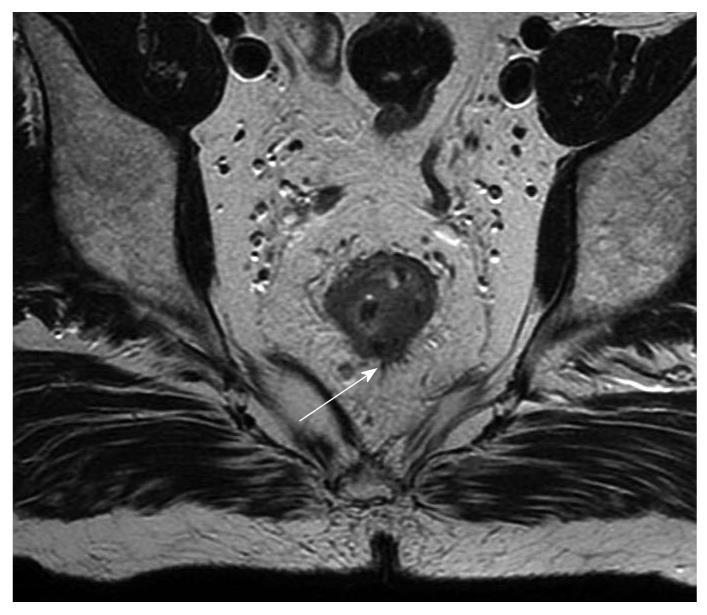
Earlier MRI studies used body coils which lacked the resolution to differentiate the different layers of the rectal wall and added no advantage to conventional CT[20]. Subsequent use of phased-array coils permitted reliable identification of the mesorectal fascia which is crucial in the management of rectal cancer[21]. Initial studies suggested a histological clearance of at least 10 mm could be accurately predicted when the radiological clearance from the mesorectal fascia and critical structure was at least 5 mm[22]. Subsequent single centre study showed 92% accuracy in prediction of CRM involvement when the CRM cutoff of 1 mm was used and this is now confirmed from the multicentre European MERCURY study[21,23]. In Europe, MRI is now routinely used in the preoperative investigation for rectal cancer. Techniques for obtaining optimal MRI images are described in the literature[24]. An axial picture enables identification of the distance of the CRM to the tumor. Coronal sections are useful in low rectal tumors to identify the relation to anal sphincter complex, pelvic floor, and pelvic side wall[25]. High signal intensity of the tumor on T2 w images suggest the presence of mucinous carcinoma which has poor prognosis compared to non-mucinous carcinoma[26]. The standard phased array MRI produces good quality images with good contrast resolution and a relatively large field of view. Routine use of intravenous contrast does not appear to improve the accuracy[27]. MRI cannot differentiate between T2 and early T3 lesions; a nodular or rounded advancing margin at the interface between muscularis propria and perirectal fat is suggestive of T3 (Figure 3). Sometimes spiculations in the perirectal fat are considered as T3 when in fact they are T2 with desmoplastic reaction[22,28]. MRI certainly cannot differentiate between a T1 and T2 cancer (Figure 4). Another area of drawback is restaging following long course chemoradiotherapy. Studies by Chen and Hoffmann found T staging accuracy was 52% and 54% when compared to histology[29]. This is due to the inability to distinguish fibrosis from tumor with MRI similar to EUS. In low anterior tumors where the mesorectal fascia is close to the muscularis propria early T3 can still infiltrate the mesorectal fascia[24]. Extramural vascular invasion is known to be an independent predictor of local recurrence[30,31]. The presence of a tubular structure in proximity to a T3 tumor or nodules with an irregular margin probably represents vascular invasion[21,32]. Recently there has been interest in the use of functional imaging such as diffusion weighted MRI imaging (DWI) and CT/PET to distinguish fibrosis from tumor[33].
Figure 3 Magnetic resonance imaging (arrow) showing possible extension beyond the muscularis propria, radiologically staged as early T3.
Figure 4 Coronal T2 W magnetic resonance imaging (arrow) showing the intact muscularis propria in a patient with rectal cancer.
Radiologically staged as T1 or T2.
MRI has been found to be useful in more advanced disease by providing clearer definition of the mesorectum and mesorectal fascia and seems to be a promising tool in assessing the locally advanced disease. With the advent of endorectal coils, the T staging accuracy has been reported to be between 70%-90%[34]. However, this technique has its limitations specially when evaluating the surrounding tissue, owing to signal attenuation at a short distance from the coil. Patient’s compliance, limited availability and cost also contribute to its less wide application. Obstructing or nearly obstructing lesions can be difficult to negotiate as are high rectal cancers leading to failed/improper coil insertion in approximately 40% of patients[34].
Nodal accuracy has also been found to be variable although use of superparamagnetic iron oxide particles appears to be promising[35] as evidenced by studies in head, neck and urological cancers.
Ultrasound
Abdominal ultrasound (USS) is used to evaluate liver for metastasis, ascites, adenopathy, and for omental cake. The false negative rate is reported to be around 8%[36]. The technique, although inexpensive and widely available, is operator dependent. Intraoperative USS is rarely used apart from when synchronous rectal and liver resections are planned. Rapid advancement in imaging modalities has made USS a less favoured imaging modality in rectal cancer staging[37].
Endorectal ultrasound
Endorectal ultrasound (EUS) is sensitive for early rectal cancers (T1 and T2 lesions) with an accuracy of 69%-97%[38-43] and is useful in the surveillance following post transanal surgery. The standard technique involves a transanal probe enclosed in a water filled balloon introduced into the rectum to allow radial visualization of the rectum. High resolution allows the assessment of the rectal wall but the assessment of the mesorectal fascia is not possible and the assessment of the lymph nodes can be an issue and overstating has been a concern. Peritumor inflammation and artifacts due to faeces may lead to an ultrasound appearance which can be misinterpreted as tumor. These drawbacks can be exaggerated between the muscle layer and the surrounding fat which makes T2 and T3 lesions difficult to distinguish[44]. The accuracy of the T stage evaluation varies from 62%-92%[45]. In a meta-analysis of 11 studies it has been shown that sensitivities for superficial tumors are better than advanced lesions[46]. A 20 year (1984-2004) systematic review looking at studies with a minimum of 50 patients, evaluating the use of endorectal ultrasound and magnetic resonance imaging (MRI) in the local staging of rectal cancer, have found a complementary role for these imaging modalities in the assessment of tumor depth. Ultrasound was found to be highly accurate in early lesions (T1, 2, 40%-100%; T3, 4, 25%-100%, overall 82%). The review also found a similar accuracy in the assessment of nodal metastases[47]. Two meta-analyses in literature have shown that the sensitivity is affected by T stage[48]. A meta-analysis including 84 studies found EUS to be slightly superior in assessing the local involvement such as lymph nodes, however, no significant differences were noted when compared to other imaging modalities such as MRI. The results suggest that none of the current imaging modalities enable reliable detection of metastatic nodal disease[49].
EUS however, has its limitations as it cannot reliably distinguish an irregular outer rectal wall due to peritumoral inflammation or transmural tumor extension. Obstructing lesions may be difficult to scan especially with rigid probes leading to suboptimal staging. The scanning, although less expensive and portable, is operator dependent and has a steep learning curve. Bulky, high, stenotic, advanced (T3) lesions or post-neoadjuvant therapy downstaged tumors can be a challenge[50-52].
EUS nodal staging accuracy is around 75%[53]. Morphologic characteristics suggestive of malignant involvement include hypoechoic appearance, round shape, peritumoral location, and size > 5 mm[45,46,51-53]. The loco-regional tumor assessment using three-dimensional EUS consists of transverse, coronal and sagital scan and has been found to be superior to CT and two-dimensional EUS. The 3D-reconstructed image shows tumor protrusion infiltrating into adjacent structures, thus, allowing for improved T and N staging[54]. Further, EUS-guided fine-needle aspiration can be carried out at the same time from the lesion or suspiciously looking lymph nodes.
Positron emission tomography
The principle of positron emission tomography (PET) is based on the differential metabolic profile of tumors compared to normal tissue. Fluoro-deoxy-glucose (FDG) is the most common PET tracer used. Due to increased metabolic activity, and change in the tumor biology, tumors preferentially show an increased uptake which results in radiolabelling[55]. Although selective, FDG accumulates in areas of infection, inflammation, in organs of increased metabolic activity such as brain, myocardium, liver or kidneys leading to false positive results[55]. FDG uptake is also influenced by the presence of mucin. PET is useful in identifying non-mucinous tumors compared to mucinous tumors. FDG/PET is mainly useful in the assessment of local recurrence and metastatic disease when conventional imaging is not helpful[56,57]. Currently it is not used as a primary staging modality in rectal cancers. Interpretation of PET without anatomic correlation poses difficulties hence PET-CT fusion scans where the pictures of both investigations are fused using software is used. This offers a detailed anatomical and functional imaging and is gaining rapid popularity and acceptance. The combination provides additional value to localize the hot spots. There are some technical limitations with this combination imaging and with the false positive rates due to other disease and physiological processes. The role of PET CT fusion scan has not changed compared to PET scans.
However, a recent study has found preoperative PET changed the management in 17% of patients[58] with improved staging accuracy in combination with CT[56]. Another study carried by Gearhart in 37 patients reported an altered management plan for 27% of patients using FDG-PET/CT imaging modality for low rectal cancer[59].
Staging accuracy post-neoadjuvant therapy
With the increasing use of pre-operative neoadjuvant therapy, rectal tumor re-staging is increasingly performed prior to curative resection.
A reduction in staging accuracy has been noted which may be as a result of effects of neoadjuvant treatment due to post-radiation edema, inflammation, fibrosis, and necrosis[60].
A recent study of 29 patients undergoing neoadjuvant therapy and pretreatment and post-treatment staging with CT, MRI, and PET showed that PET was 100% sensitive in predicting response to therapy (compared with 54% for CT and 71% for MRI). Corresponding specificity for predicting tumor response to treatment was 60%, 80%, and 67% for PET, CT, and MRI, respectively[61], thus suggesting a further possible role of PET in predicting response to neoadjuvant therapy.
Tumor re-staging following post-neoadjuvant therapy remains problematic and it is hoped that a combination of imaging technique (CT, MRI, and EUS) and functional (PET) imaging may improve staging accuracy.
Suggested investigations for tumor staging of rectal cancer
On review of the literature, phased array MRI and EUS should be considered as the initial modalities to stage the local tumor. A fixed, locally advanced rectal cancer may be imaged better by MRI (Figure 5), whereas EUS is more appropriate for an early mobile rectal tumor (T1-T2 lesions). MRI has been shown to be highly accurate in predicting a clear circumferential resection margin in patients undergoing TME. Although both MRI and EUS provide a comparable overall T- and N-staging, use of these modalities is limited by issues such as availability, costs and technical expertise. CT scanning, although still the current standard for distant staging, may not be an effective tool to stage the local disease. A combination of CT and PET offering a detailed anatomical and functional imaging, however, seem to be promising and gaining popularity and acceptance for recurrent rectal cancers.
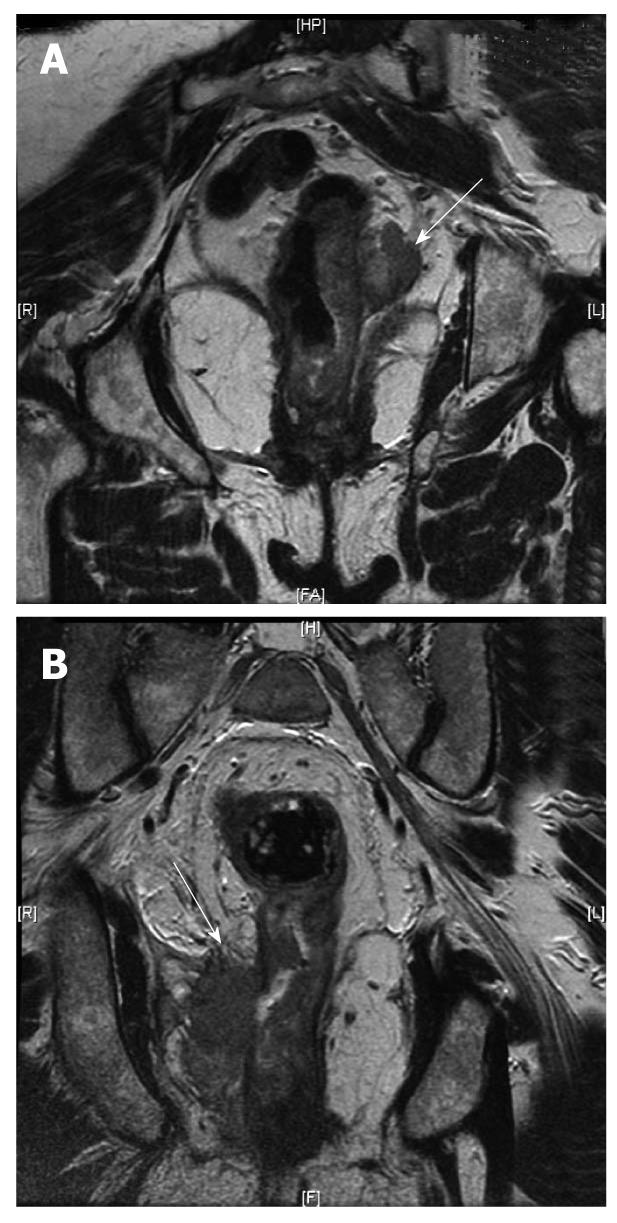
Figure 5 Magnetic resonance imaging.
A: Magnetic resonance imaging (MRI) (arrow) showing the rectal cancer involving the circumferential resection margin; B: MRI (arrow) showing the rectal cancer invading the ischiorectal fat on the right (T4).
Suggested investigation for nodal staging of rectal cancer
The accuracy of MRI, CT and EUS for identifying malignant nodes is poor. Current criteria are based on size, shape and morphology. Any node of 1 cm and over is taken as significant[62]. The enlarged lymph node can be as a result of the inflammatory process but normal size nodes can have micrometastases. Brown et al[54] found 58% of positive malignant nodes were less than 5 mm. Morphological characteristics such as round shape, irregular borders and heterogenous signal intensity suggest nodal involvement[63].
Nodal accuracy has also been found to be variable, although use of superparamagnetic iron oxide particles (SPIO) seem to be promising as evidenced by studies in head, neck and urological cancers. The technique involves use of a contrast media containing SPIO which accumulates in normal lymph nodes, whereas due to defective phagocytosis, the uptake is poor or absent in malignant nodes. Hence by using T2 weighted imaging, these nodes can be identified. Initial studies are promising but further research is needed[35].