Published online Feb 7, 2011. doi: 10.3748/wjg.v17.i5.578
Revised: June 29, 2010
Accepted: July 6, 2010
Published online: February 7, 2011
Inflammatory bowel diseases (IBD) are a complex group of diseases involving alterations in mucosal immunity and gastrointestinal physiology during both initiation and progressive phases of the disease. At the core of these alterations are endothelial cells, whose continual adjustments in structure and function coordinate vascular supply, immune cell emigration, and regulation of the tissue environment. Expansion of the endothelium in IBD (angiogenesis), mediated by inflammatory growth factors, cytokines and chemokines, is a hallmark of active gut disease and is closely related to disease severity. The endothelium in newly formed or inflamed vessels differs from that in normal vessels in the production of and response to inflammatory cytokines, growth factors, and adhesion molecules, altering coagulant capacity, barrier function and blood cell recruitment in injury. This review examines the roles of the endothelium in the initiation and propagation of IBD pathology and distinctive features of the intestinal endothelium contributing to these conditions.
- Citation: Cromer WE, Mathis JM, Granger DN, Chaitanya GV, Alexander JS. Role of the endothelium in inflammatory bowel diseases. World J Gastroenterol 2011; 17(5): 578-593
- URL: https://www.wjgnet.com/1007-9327/full/v17/i5/578.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i5.578
Inflammatory bowel diseases (IBD) include Crohn’s disease (CD), ulcerative colitis (UC) (and indeterminate colitis), which share several inflammatory characteristics with other chronic immune disturbances including immune activation, leukocyte infiltration into tissues and increased vascular density[1]. In UC, the colon shows a continuous, superficial inflammation, while CD occurs as patchy transmural inflammation which may affect any region of the gastrointestinal tract. Genetic susceptibilities may play an important role in the development of IBD[2-6] with polymorphisms in CARD15/NOD2 haplotypes (especially in Caucasians) and HLA-DR haplotypes (especially in Asian IBD) and possible defects in interleukin (IL)-23, IL-2, and IL-10 signaling[2,7-10]. IBD is more prevalent in developed nations[11], with several mechanisms being considered to explain disease pathology including environment, hygiene and altered gut flora[11-13]. These different contributing causes may underlie divergent forms and patterns of IBD, which ultimately may lead to a redefinition of different sub forms of UC and CD.
While the mechanisms initiating and sustaining IBD may differ, both UC and CD may reflect dysfunction within antigen-presenting cells (e.g. dendritic cells) or excess activation of CD4+ T-cells (resembling T-cell disturbances in psoriasis). Reduced activation of T-cells in some forms of CD appear to allow gut microbiota that have breached the gut epithelium to trigger microvascular inflammation[1,5,9,14-16]. The activation of immune responses in IBD release inflammatory cytokines [e.g. tumor necrosis factor (TNF)-α] and growth factors [e.g. vascular endothelial growth factor (VEGF)-A] into gut tissues provoking gut inflammation and injury[5,17]. Antibodies produced against “normal” gut antigens (e.g. anti-colon, anti-mucin, anti-tropomyosin) have been found in IBD and are suggested to activate cytotoxic T lymphocytes, further increasing inflammation[7]. As IBD progresses, cytokine-mediated inflammation and epithelial apoptosis disturb the intestinal barrier, to allow penetration of gut flora beyond the lamina propria causing intense inflammatory responses[18] while also provoking endothelial microvascular permeability[19].
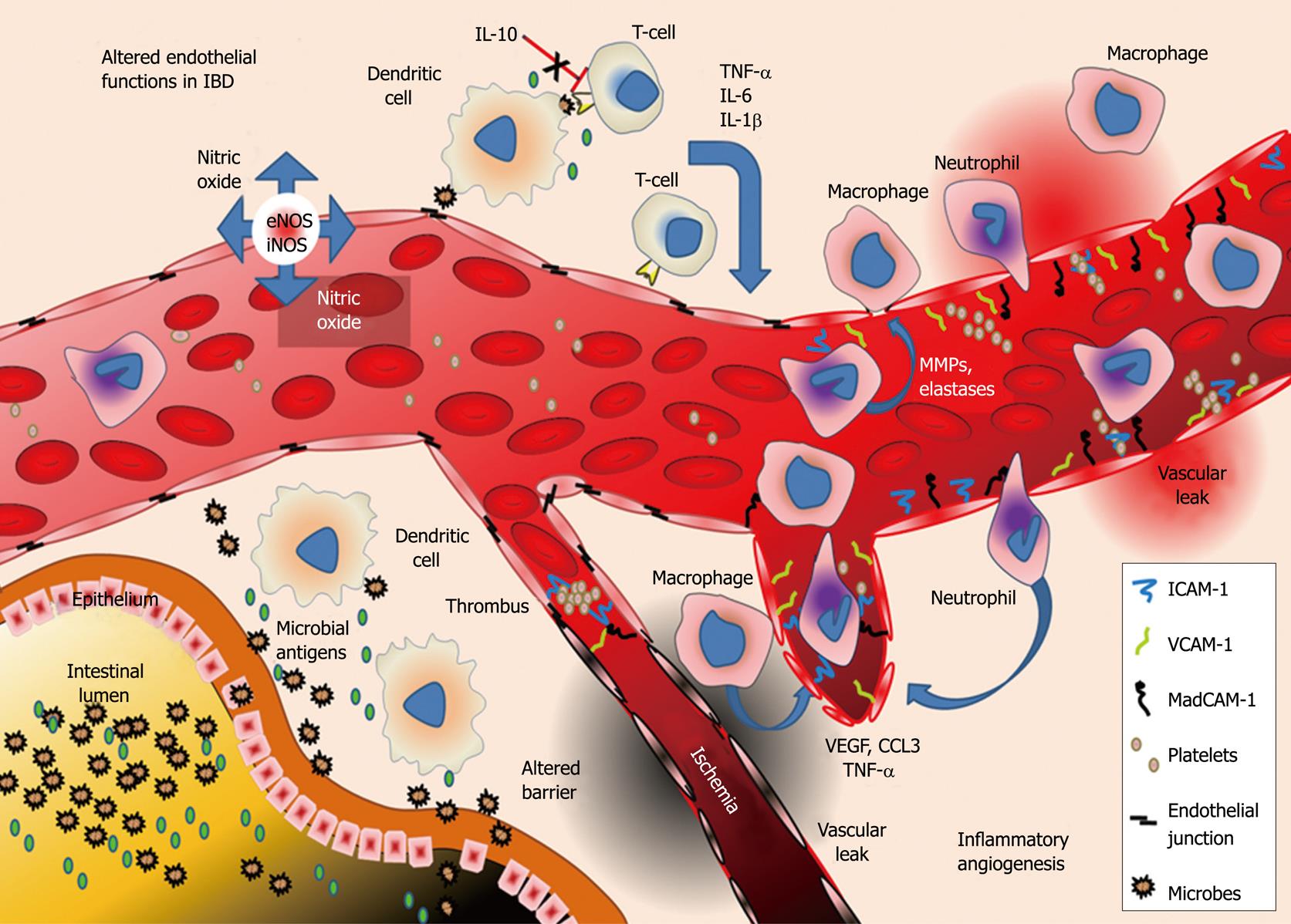
Another key event in IBD progression is the expansion of the intestinal microvasculature. Angiogenesis in IBD sustains inflammation through alterations in the endothelial lining of these vessels. The endothelium regulates recruitment of inflammatory cells, tissue damage (e.g. vasogenic edema), and production of inflammatory mediators[19-22]. In this review we describe the key roles of the endothelium in mediating and aggravating inflammation in IBD (Figure 1).
Endothelial cells (ECs) are the major constituent of the microvasculature that line blood and lymphatic vessels. ECs during IBD undergo rapid and remarkable changes in response to elevated levels of cytokines and growth factors often producing injury to gut tissues. Normally ECs provide an anti-adhesive and selectively permeable exchange barrier[23]. Even though ECs have long been recognized as participants in inflammation their roles in intestinal inflammation during IBD are not yet clear. The unique physiological and molecular characteristics of gut microvessels may help explain several characteristics of IBD. The close relationships between gut metabolism, tissue perfusion, microvascular expansion and immune cell infiltration are unclear but suggest that microvascular alterations may be maladaptive in IBD. Intestinal vascular ECs basally exhibit unique properties which may contribute to IBD. Haraldsen et al[24] first described characteristics of human intestinal ECs (HIMECs) in long-term cultures and differences from ECs of different origin. For example lipopolysaccharide (LPS) only transiently increases HIMEC adhesion molecule expression, while causing long-lasting increases in human umbilical vein ECs (HUVECs)[25]. Nilson et al[26] found that HIMEC cultures produce different cytokines (IL-1β, IL-3 and IL-6) upon stimulation with inflammatory cytokines (e.g. TNF-α, IL-1) compared to HUVECs. Binion et al[27,28] have shown distinctive HIMEC properties such as constitutive inducible nitric oxide (NO) synthase (iNOS) as well as unique adhesive determinants, and that these properties were altered in IBD and may underlie endothelial dysfunction in IBD development.
Endothelial-derived NO reduces leukocyte and platelet adhesion to the endothelium[29,30], mediates flow-dependent and agonist-dependent vasodilatation, and couples VEGF-A signaling with NO-dependent permeability[31,32]. NO-mediated endothelial permeability involves 2 separate mechanisms: (1) increased guanylate cyclase and phospholipase C activity which increases intracellular Ca2+; and (2) permeability mediated by Erk1/2 via Ras/Raf/PKC causing increased actin contractility[29,33,34]. Increased p38 mitogen-activated protein kinase (MAPK) signaling, Rho-GTPase activity and increased Ca2+ release mediated by upregulated cytokines and growth factors may also represent possible mechanisms for increased endothelial permeability[35-37].
Endothelial nitric oxide synthase (eNOS)-derived NO is a radical scavenger not only absorbing O2- but also generating the potent oxidant ONOO-. eNOS expression is reduced in IBD; eNOS deficiency in IBD is exacerbated by arginase-mediated depletion of substrate as well as eNOS uncoupling[38-40]. Decreased eNOS activity in IBD reduces endothelium-dependent vasodilation, leading to ungoverned oxidant formation, prominent in IBD[41]. Deletion of eNOS (eNOS-/-) increases severity of experimental IBD[42,43] consistent with protective roles of NO against inflammation. NO may prevent development of endothelial inflammatory and hyper-adhesive phenotype in IBD by suppressing cytokine-induced EC adhesion molecules (ECAMs) and matrix metalloproteinases (MMPs)[44]. Increased endothelial oxidant stress (e.g. in IBD) also disturbs tight junctional organization via p38, p42/44 MAPK[45-47].
Sera from patients with CD reduce, while UC sera increase eNOS in HUVECs; both UC and CD sera increase iNOS[48]. This may reflect differences in anatomic origins of the endothelium i.e. venous vs intestinal. HIMEC iNOS expression appears to be a unique feature of gut microvessels. In HIMECs, iNOS appears at least as important a source of NO as eNOS. Binion et al[30], have shown that HIMECs persistently express iNOS, and that iNOS-derived NO limits leukocyte adhesion in normal HIMECs. Paradoxically iNOS inhibition increases binding of leukocytes. Thus, while leukocyte-derived iNOS may drive inflammation, HIMEC expression of iNOS limits the inflammatory responses (leukocyte adhesion, permeability, vasodilatation) in the gut, and decreased endothelial iNOS abundance and activity in IBD may represent an underrated basis of IBD pathology[27,30]. HIMECs derived from CD patients also show a persistent loss of iNOS expression[27]. Interestingly, iNOS can be decreased by injury to normal HIMECs (opposite to most tissues which mobilize iNOS in response to injury[27]) suggesting that during injury, reduced iNOS might trigger inflammatory responses. Even with the loss of endothelial iNOS, there is often increased NO in tissues surrounding the area of inflammation. Despite decreased endothelial iNOS derived-NO, IBD frequently exhibits increased leukocyte recruitment and activation of gut epithelial cells to increase overall NO production[44]. Krieglstein et al[49] found that tissue-derived iNOS, and to some extent leukocyte iNOS, mediate colitis injury, but could not specifically distinguish between tissue and endothelial contributions of iNOS in colitis. Aoi et al[50] have suggested that iNOS-derived NO plays an important role in gut healing after injury through induction of VEGF, necessary for angiogenesis in wound healing. We have previously shown that excess NO may play an important role in IBD exacerbation. Using STAT-6-/- mice (which have high iNOS levels) in dextran sulfate sodium (DSS) colitis, we found more severe IBD in STAT-6-/- mice correlate with extraordinary NO flux suggesting that excess NO may also drive gut injury[51]. Despite elevated NO abundance, downstream guanylate cyclase signaling appears to be depressed in DSS colitis leading to decreased cGMP in the inflamed intestine[52]. Under these conditions, cGMP dependent protective NO effects may be masked by pro-oxidant effects of NO metabolites. Conner et al[53] and Grisham et al[54] revealed an important role of the 26S proteasome in the regulation of endothelial nuclear factor-κB (NF-κB) and cumulative iNOS NO production and adhesion molecule expression. Cumulatively, these studies suggest that intestinal homeostasis is controlled by distinctive and compartmentalized NO sources, and that excess NO formation may support the pathophysiology of IBD.
The gut is an organ supporting a high bacterial load; despite physical and chemical barriers, some bacterial antigens will ultimately penetrate the gut wall to activate gut microvascular ECs through Toll-like receptor (TLR) signaling[18,55]. The intestinal microvascular endothelium also differs from ECs of other origins in TLR responses. For example, repeated exposure and activation of TLR4 in HIMECs leads to development of lipopolysaccharide tolerance; however HUVECs lack such a mechanism, indicating the importance in controlling endothelial-dependent inflammation and host commensal interactions[25,56,57]. Protease activated receptors activate transforming growth factor (TGF)-β to induce TLR4 and lead to increased disease severity in IBD[58,59]. TLR5 is constitutively expressed in all ECs, and is of particular interest in gut pathophysiology. TLR5, a receptor for flagellin[60], is constitutively expressed on the basolateral surface of the gut endothelial (an epithelial) layers[61]. TLR5 signaling induces endothelial intercellular adhesion molecule-1, TNF-α production and leukocyte binding and emigration[61]. Loss of TLR5 activity in murine models leads to the development of infectious as a result of deficient and improper responses to normal flora and pathological microorganisms[61,62]. Conversely, endothelial TLR3 has been shown to be protective in the DSS model of acute colitis. This process is mediated by interferon (IFN) type 1 induction of IL-10, a potent anti-inflammatory cytokine[63]. However, Heidemann et al[64] in 2007 found that IL-12 expression and its associated gene products were also induced by TLR3 signaling in addition to increased adhesion and transmigration of leukocytes and TLR functions in the gut remain complex, and requires further study.
During inflammation there is an increase in plasma levels of inflammatory cytokines, including IL-6, IL-23, IL-12 and TNF-α, in both human IBD and animal IBD models[1,2,15]. Kawachi et al[65,66] examined cytokine alterations in the adoptive T-cell transfer and the IL-10-/- IBD models and found IL-1, IL-6, IL-18 and TNF-α were upregulated in both models. Many of the inflammatory cytokines that are upregulated in IBD are pro-angiogenic, the best examples being IL-17 (produced by invasive Th17 cells) and TNF-α produced by several tissue types, including infiltrating immune cells (macrophages and monocytes)[67,68] and the endothelium[69]. EC produce inflammatory mediators in response to activation by immune cells and alterations in the tissue microenvironment[64,70].
TNF-α is one example of a cytokine with pleiotropic effects on the endothelium in IBD, ranging from adhesion molecule induction [vascular cellular adhesion molecule (VCAM)-1 and mucosal addressin cellular adhesion molecule (MAdCAM)-1], promoting interaction of platelets with ECs and inducing expression of pro-angiogenic growth factors such as VEGF-A[25,44,71-73]. Defects in the activity of the anti-inflammatory cytokines such as IL-10 may play a role in the establishment of some IBD, and IL-10 deficient mice (IL-10-/-) develop IBD spontaneously, while other animal models of colitis show reduced injury when treated with exogenous IL-10[2,74-76]. Interestingly Oshima et al[19] observed that pretreatment of ECs with IL-10 prevented IFN-γ mediated endothelial barrier disruption, indicating that an important role of IL-10 may be to prevent cytokine mediated EC barrier disturbances which initiate and exacerbate disease. This is supported by the finding that several EC adhesion molecules such as intercellular adhesion molecule (ICAM)-1, VCAM-1 and MAdCAM-1 are increased in IL-10-/- mouse colitis and may mediate leukocyte recruitment in this model[66].
Over 40 chemokines in 4 separate families interact with as many as 19 receptors to regulate trafficking of leukocytes. Of these, several chemokines may mediate leukocyte trafficking to the gut and colon dysfunction in IBD. Papadakis et al[77] showed that CCL2 and CCL5-/- mice are protected from colitis. Interestingly, Barcelos et al[78] and Wu et al[79] showed that CCL5 and CCL3 can induce inflammatory angiogenesis in a murine sponge model and promote angiogenesis in murine tumors. Eyman et al[80] have also shown that CCL5 upregulates pro-angiogenic genes. CCL25 interacting with its receptor on CCR9+ leukocytes plays a major role in the early stages of experimental IBD pathogenesis[81]. CXCL8 (IL-8) another pro-angiogenic chemokine, is known to be stored in EC Weibel-Palade bodies, can be rapidly secreted, and induces HIMEC proliferation in culture via binding to CXCR2[82,83]. Although angiogenesis may support injury IBD, IL-8 may be dysregulated in some forms of IBD. IL-8 seems to be downregulated in leukocytes and in the endothelium of patients with CD. There appears to be no upregulation in the endothelium of UC patients, suggesting a possible link to TGF-β1 over expression in IBD[84-86]. In contrast, Scaldaferri et al[87,88] found that intestinal fibroblasts treated with TNF-α produce IL-8 and monocyte chemoattractant protein-1 via p38/p42/44 mitogen-activated protein kinase.
CX3CL1/fractalkine is a chemokine expressed by EC, can be upregulated by TNF-α, IL-1, LPS and IFN-γ, and is highly upregulated in IBD[89,90]. CX3CL1 can function as an endothelial adhesive determinant to recruit a sub-population of dendritic cells and macrophages that have high CX3CR1 expression. CXCL1 can be shed from the surface of the ECs (in response to increased IL-1β in IBD). This form of CX3CL1 acts as a chemoattractant for CD4+ and CD8+ T-cells[90]. Sans et al[91] reported that in fact there is enhanced recruitment of CX3CR1 expressing T-cell to the gut via interactions with CX3CL1. CXCR4/SDF-1α and its ligand CXCL12 is an important chemokine/receptor pair in angiogenesis, but have received very little attention in IBD. Heidemann et al[92] reported that blocking this CXCR4/CXCL12 interaction is sufficient to inhibit migration and proliferation of HIMECs in response to VEGF-A. CXCR4/SDF-1α plays an important role in the recruitment of EC precursors to sites of angiogenesis, and may be impaired in IBD, leading to the conclusion that this pathway may be interrupted[93-95]. Midkine, another chemokine of great interest is increased in serum and is associated with tumor drug resistance and poor cancer prognosis[96-98]. Midkine is also upregulated in IBD serum, and has prognostic value like VEGF, TNF-α, sVCAM and VCAM[20,99-102]. Midkine has a pronounced angiogenic effect, like some other inflammatory factors, and also increases the levels of surface glycosaminoglycans on ECs to favor recruitment of circulating leukocytes in IBD[103].
Inflammation in IBD is characterized by increases in both blood and lymphatic vessels in the intestine. This increase in endothelial surface area provides a powerful means of increasing leukocyte recruitment with the mobilization of ECAMs including selectins[28]. Animal models of IBD (IL-10-/-, IL-2-/-, SAMP1/Yit and T-bet-/-), like human IBD, all show ECAM upregulation is linked to disease severity[66,104-106], allowing use of adhesion antagonists in IBD therapy[55,102,107]. Endogenous endothelial-derived inhibitors of leukocyte binding (e.g. sVCAM-1) may also be downregulated in IBD[21,108-111] and could provide new diagnostic or anti-adhesive strategies.
P and E-selectins, glycoproteins expressed on the surface of platelets and other leukocytes, are also expressed on the surface of activated or inflamed endothelium in IBD. P-selectin can interact with ECAMs such as VCAM-1/ICAM-1, as well as with O-glycans collectively referred to as peripheral lymph node addressins (PNAds) containing sialyl Lewis X moieties[112,113]. P-selectin at least partially mediates rolling and recruitment of gut-infiltrating leukocytes in IBD, with approximately 50% increase in gut P-selectin in UC vs control groups; serum levels of soluble P-selectin, an inhibitor of selectin binding, are decreased in IBD patients[109,114,115]. Increased platelet P-selectin, with the enhanced prothrombotic surface of the gut EC in IBD increases thrombus formation and tissue damage by ischemic injury[115]. E-selectin, a relative of P-selectin is expressed solely on the surface of activated ECs during inflammation and is a major contributor to leukocyte rolling injury. E-selectin is not stored in Weibel-Palade bodies and must be produced in response to inflammatory stimuli such as IL-1, TNF-α and VEGF-A[116,117]. In contrast to sP-selectin, sE-selectin is not downregulated in IBD and in CD, and actually increases in comparison to controls[109].
High endothelial venules (HEV) are specialized post-capillary venules that allow trafficking of leukocytes between immune (e.g. Peyer’s patches) and vascular compartments, and are increased in IBD[113]. L-selectin expressed on leukocytes (after activation) binds PNAd on HEV and recruits leukocytes expressing L-selectin in IBD. The gut and brain selective adhesion determinant, MAdCAM-1 is also expressed on HEV, and in UC MAdCAM-1 O-glycosylation increases, allowing greater L-selectin binding[118]. MAdCAM-1 interacts with α4β7 integrins on the surface of a subset of naive CD4+ T-cells[119,120]. MAdCAM-1 induction is found only in chronically inflamed gut endothelium and suggests that in IBD there is a fundamental alteration in the phenotype and gene expression pattern in the inflamed intestinal EC[28]. Mizushima et al[121] demonstrated that inhibition of angiotensin-II type 1 receptors reduced TNF-α dependent MAdCAM-1 expression and reduce the severity of DSS-induced colitis, possibly linking vasoregulation and inflammation. In HIMEC MAdCAM-1 is also expressed inversely with cell density, with proportionally greater levels of MAdCAM-1 found at low densities. This indicates that in newly formed vessels, larger amounts of MAdCAM-1 may be available to recruit leukocytes to these “leaky and permissive” vessels[122,123].
ICAM-1, another important ECAM in IBD binds LFA-1 (aLb2), Mac-1 (aMb2) and α4β2 integrins, and is expressed by inflamed ECs to mediate the firm adhesion of leukocytes to activated ECs[124,125]. ICAM-1 has a unique relationship with VEGF-A; Goebel et al[125] reported that HIMECs constitutively express ICAM-1, which is significantly upregulated following treatment with 50 ng/mL VEGF-A, linking inflammation and angiogenesis. In addition to direct activation and upregulation of ICAM-1 by VEGF, Zitterman et al [117] found that VEGF treatment also sensitizes cells to TNF-α induced ICAM-1 mobilization. Normally ICAM-1 concentrates at EC junctions, but is redistributed to apical surfaces of ECs under inflammatory conditions where it supports firm adhesion of leukocytes[25]. In the adoptive T-cell transfer model of murine IBD, Ostanin et al[126] found that T-cells that lack LFA-1, (a T-cell ICAM-1 ligand), fail to induce disease, revealing a critical role for EC modulated immune responses. ICAM-1 was the one of the first clinical targets in IBD, but an antisense IBD therapy showed limited success[21].
VCAM-1, an ECAM highly expressed on the luminal surface of activated ECs in IBD, mediates the adhesion of α4β1 expressing lymphocytes. In HIMECs, the expression of VCAM-1 is regulated by the PI3K/NF-κB signaling pathway and its stimulation by mediators can be inhibited by curcumin[127]. Like ICAM-1, VCAM-1 can also up regulated by VEGF-A via NF-κB[117,128]. Studies in the picrylsulfonic acid model of UC using radiolabeled anti-VCAM-1 antibodies show that leukocyte infiltration and histological damage are proportionate to VCAM-1 expression in the gut microvasculature[129]. In addition, in the DSS model of UC, there is an upregulation of VCAM-1 which if blocked (by specific antibodies) attenuates disease activity, while ICAM-1 and MAdCAM-1 blockade do not protect in this manner[129,130].
CD31/PECAM-1 expressed by ECs and leukocytes mediates homophilic binding between activated ECs and leukocytes especially during extravasation. CD31 is found on the endothelial surface and in endothelial junctions. Work by Romer et al[72] found that CD31 is not upregulated in response to inflammatory cytokines but is redistributed from cellular junctions. CD31 blockade inhibits leukocyte transmigration, and CD31 inhibition in IBD reduced leukocyte rolling and firm adhesion suggesting a unique role for CD31 in IBD or in the function of the gut microvasculature[131].
Originally considered a mesenchymal stem cell marker[132-134], CD146 is now described as a novel immunoglobulin super family adhesion molecule which is increased in gut tissue of IBD patients[108]. The function of CD146 in IBD is not completely understood, but has potential roles in inflammation since it supports rolling and invasion of natural killer T-cells[135]. The upregulation of CD146 in IBD, like ICAM-1 and VCAM-1, may be driven by VEGF-A overexpression during IBD[100]. Additionally, the soluble form of CD146, regulates endothelial and leukocyte CD146 interactions with their ligands, and is reduced in IBD, enhancing leukocyte extravasation[100,108,135]. Interestingly Tsiolakidou et al[100] determined that new vessels formed in IBD are disproportionately CD146+. Inflamed ECs from CD and UC patients show an increased ability to recruit naïve T-cells and macrophages to the intestinal immune compartment after stimulation with several inflammatory cytokines, but not with LPS[28,120]. These data are consistent with IBD not being initially driven by immune cells, but rather by the endothelial response to an increased inflammatory mediatory load.
Platelet and leukocyte aggregation as well as activation of the coagulation cascade increase during IBD, reflecting loss of the non-thrombogenic EC phenotype in IBD. Thrombi aggravate inflammation by binding of micro infarcts to the endothelial surface often leading to ischemic inflammation in the intestinal microvasculature[136]. Mesenteric venous thrombosis has been observed in a fraction of IBD cases, and thrombotic processes are being recognized in altered perfusion, inflammation and tissue injury in IBD[137]. Indeed, subclinical thrombosis is common in IBD, and is a major source of morbidity in approximately 25% of IBD deaths[136]. Increased markers of coagulation include thrombin anti-thrombin complex, tissue factor and fibrinopeptide B[55], and can be described early in IBD. Factor XIIIa, a fibrin-stabilizing coagulation factor (and agonist for VEGFR-2), is increased in IBD, while factor XIII TT has an increased number of mutations in IBD patients compared to controls suggesting links between thrombosis, angiogenesis and inflammation. However, Bernstein et al[138], Dardik et al[139] and Vrij et al[140] reported that factor XIII activity is reduced in IBD patients.
In addition to increased levels of coagulation cascade proteins in IBD, CD40, CD40L and soluble CD40L are increased in IBD. CD40, expressed on several cell types (including ECs) is involved in inflammatory and immune activation, and interacts with CD40L on T-cells. Danese et al[141] suggested that the primary source of sCD40L was from activated platelets. CD40 signaling increases production of pro-inflammatory cytokines and chemokines by ECs and surrounding tissue[142]. CD40L release also leads to binding of platelets and immune cells to ECs by increasing tissue factor, ECAM expression and pro-thrombotic phenotype in HIMECs[141-143]. Danese et al[71] suggested that a possible therapeutic benefit of TNF-α blockade was downregulation of CD40/CD40L signaling in IBD. A still unanswered question is whether coagulation is a secondary or initiating event in inflammation. It is worth mentioning that individuals with coagulation cascade disorders (e.g. hemophilia, factor V deficiency and von Willebrand disease) rarely develop IBD[55]. The opposite of the previous observation is also true; patients with IBD have an increased likelihood of having genetic pro-thrombotic disease Factor V Lieden[144]. This evidence strongly links thrombus formation as a possible trigger of IBD and suggests prognostic factors which may increase risk of IBD development.
The maintenance of normal vascular barrier supports nutrient and O2 exchange, osmotic balance and leukocyte abundance in the extracellular compartment. In IBD, increased vascular permeability leads to tissue edema and damage in both human IBD and animal models of IBD[19]. This alteration in solute permeability of the vasculature is not restricted to the gut microcirculation but is widespread affecting the vasculature of other organs including the brain[145]. Several classes of mediators in IBD alter both solute permeability and angiogenic balance, including angiogenin (an angiogenic peptide with ribonuclease activity), chemokines (e.g. IL-8, IL-10), coagulation factors (thrombin), cytokines (IFN-γ, IL-13), and growth factors, most notably VEGF, the most potent and important blood vascular angiogenic growth factor and an important inflammatory mediator[19,36,37,47,146-148]. Tolstanova et al[149] found that VEGF-A inhibition by neutralizing antibodies reduced vessel permeability in the iodoacetamide model of colitis. Downregulation of anti-inflammatory cytokines e.g. IL-10 may play an equally important role in increasing endothelial permeability. Oshima et al[19] have shown increased vascular permeability in the IL-10-/- colitis model due to loss of IL-10 inhibition of IFN-γ induced junctional degradation; also IL-10 protects against IFN-γ mediated loss of human microvascular barrier.
Leukocytes, e.g. neutrophils and monocytes, can degrade endothelial junctions through protease secretion and upregulation. Cytokines and growth factors also induce MMP-9, MMMP-3 and MMP-1[150,151], resulting in degradation of junctional and matrix targets[152]. Neutrophil elastase is elevated in IBD and can degrade vascular endothelial cadherin, important in maintaining junctional apposition, adhesion and barrier function[153-156]. Endothelial junctional adhesion molecule-A is also dysregulated in IBD, and is closely linked to disease activity in DSS colitis[37,157].
Angiogenesis (increased blood vessel density) in IBD increases the area of endothelium available for exchange, but also for extravasation of blood constituents into surrounding tissue to increase disease severity in IBD[158]. Increased vessel formation in IBD may represent recruitment of endothelial progenitor cells, vascular intussusception (splitting) and extension from existing vessels[159]. Increased angiogenesis is observed in animal (2,4,6-trinitrobenzene sulphonic acid (TNBS), DSS and iodoacetamide) colitis models and in human colitis. However, inflammatory angiogenesis in IBD does not simply match increased tissue mass. Vessels formed during inflammation are different from those formed during normal development. These vessels are immature, lacking investment with pericytes. They express ECAMs, leak, are hypoperfused, often stenose and are hyperthrombotic, with an elevated ability to respond to growth factors[160-163] actively supporting IBD progression[149,164-168]. Spalinger et al[158] and Maconi et al[169] concluded that there is an increased blood vessel density in the intestines of CD and UC patients and that increased vascular density in IBD was directly correlated with increased IBD disease severity. This is also true in animal models of IBD like TNBS- and DSS-induced colitis models[166,170].
Growth factors, especially VEGF-A, dramatically alter several aspects of the colon microvascular endothelial phenotype, resembling a de-differentiation (loss of maturity) of the vessels which can reflect changes in vascular support cells, e.g. pericytes/smooth muscle, that surrounds the capillaries. Inflamed tissues display increased vascular density resulting from the formation of new vessels during angiogenesis. These changes result in decreased perfusion, increased solute permeability (via cytokines and VEGF-A induced junction remodeling) and contractility, as well as increased leukocyte and platelet adhesiveness[161,171,172]. Ganta et al[163] have demonstrated that in angiotensin-2 knockout mice (using the DSS model of UC), loss of the pericytes around vessels resulted in diminished angiopoietin-1 signaling that destabilized the endothelial layer, increased leukocyte recruitment to the tissue, increased vessel permeability and induced vessel hyper-proliferation. Blood and lymphatic vessels are hyperstabilized by angiopoietin-2 deficiency, and show diminished inflammatory remodeling as well as decreased capacity to recruit leukocytes suggesting a link between maturity and inflammatory capacity[163].
VEGF-A is the first described and best known VEGF, which controls developmental angiogenesis, wound healing and pathology[173,174]. Bousvaros et al[175], Kapsoritakis et al[101] and Ozawa et al[176] all found elevated VEGF-A levels in plasma and tissue during active human and animal IBD, often twice normal[101,109,166,175,176]. However, Chidlow et al[166] have reported that DSS diminishes levels of VEGF-A as well as VEGF-C and VEGF-D, suggesting complex, concentration-dependent and inhibitor-regulated effects of VEGF in different animal models of IBD. Danese et al[177] and Scaldaferri et al[167] have shown that inhibition of VEGF signaling can attenuate disease activity in the DSS model of UC while overexpression of VEGF-A increases disease severity in the same model[167,177]. VEGF-A is released by several cell sources (e.g. neutrophils, platelets, macrophages, pericytes, fibroblasts, ECs, and colonic epithelial cells) and is transcriptionally activated by hypoxia through hypoxia inducible factor 1α, and message stabilization via eukaryotic translation initiation factor 4e[70,178-183]. Interestingly Birmingham et al[184] have shown that activated colonic epithelium represents an important source of VEGF-A, and injury or inflammation of the colon epithelium may provide a local stimulus for blood vessel growth. Invasive leukocytes, specifically neutrophils, granulocytes, macrophages and platelets, are increased in tissue during active IBD, and are also important sources of VEGF-A in inflamed tissues[178,179,185-187]. Salivary secretions also contain high levels of VEGF-A and VEGF-C, which have been suggested as important sources of these growth factors in IBD[188] released site-specifically during denudation. Apart from VEGFs, other angiogenic growth factors, e.g. basic fibroblast growth factor (bFGF), TGF-β and platelet-derived growth factor (PDGF) are upregulated in IBD and may be of clinical relevance[86,189].
TGF-β is an important regulator of the cell cycle and apoptosis, especially in mucosal immune cells. The expression of TGF-β and its 2 receptors (TGFR1 and TGFR2) are increased in IBD, specifically UC; however, it appears that the levels are decreased in CD[190]. In IBD either tachyphylaxis develops for TGF-β (UC), or the lack of TGF-β (CD) allows mucosal immune cells to proliferate when they would have undergone apoptosis[190,191]. Early studies on the role of TGF-β in IBD indicated a protective role; more recent studies may point to a pathological role of TGF-β signaling in IBD[191,192]. In fact, TGF-β is important in the formation of fibrosis in the colon of IBD patients by stimulating the transition of many cell types to fibroblasts[193]. Over one-third of the fibroblasts responsible for inflammatory fibrotic injury may actually originate from the transformation of ECs to fibroblasts (not counting contributions of pericytes to fibroblast formation). Therefore the vasculature may provide a significant proportion (if not the majority of fibroblasts) and associated fibrosis in IBD[194,195]. bFGF, a potent mitogen for the cells of mesodermal origin, stimulates EC proliferation, activates MMPs resulting in proteolysis of extracellular matrix, and increases cellular motility[191]. Even though levels of bFGF are elevated in IBD there is no correlation with the stage or severity of the disease. However, the contribution of bFGF in the initiation or maintenance of IBD should not be discounted[196]. PDGF is a close relative to VEGF and is upregulated in IBD. PDGF is predictive of both oxidative stress and angiogenesis in the intestine[189]. PDGF is released in response to inflammatory and thrombotic stimuli. PDGF increases P-selectin expression on ECs and induces histamine secretion which induces other effects such as increased vascular leakage[197,198]
Recruitment of endothelial progenitor cells (EPCs) may contribute to angiogenesis in IBD, although reduced numbers of VEGFR2+, CD34+, CD133+ cells (endothelial, bone marrow, and stem cell markers) have been reported in IBD[199], and EPCs from IBD have reduced antigenic activity[95]. These findings suggest that recruitment of EPCs is unlikely to be a source of increased vessels, however, these findings are from patients with established disease as initial angiogenesis in early stages of IBD may rely on EPCs. Apart from EPC recruitment, angiogenic sprouting is active in IBD; sprouting ECs referred to as “tip” cells, are highly motile with distinct gene expression compared to that in quiescent ECs[200]. VEGF-A induces the tip cell phenotype and also guides vessel sprouting, indicating that in IBD, VEGF might induce new vessel formation in this way[201]. Normally, not all sprouts survive, many undergoing apoptosis, (vessel “pruning”) suggesting that high levels of VEGF prevent endothelial apoptosis resulting in increased numbers of surviving sprouts in IBD[201].
While increased pro-angiogenic growth factors increase angiogenesis, reductions in anti-angiogenic factors (seen in the DSS model of colitis) may be as important for permitting expansion of the vascular endothelium[166,167]. Angiopoietin-1 a competitive inhibitor of Ang-2, binds to the Tie-2 receptor and inhibit vascular remodeling. Angiopoietin-2 is upregulated during inflammation and angiogenesis[163,202] and competes with angiopoietin-1, to allow ECs to maximally respond to cytokines and growth factors. Work by Ganta et al[163] found that angiopoietin-2 signaling also appears to be necessary for neutrophil infiltration, and blood and lymphatic vessel proliferation in DSS colitis. Interestingly angiopoietin-2 can be upregulated by both bFGF and VEGF, potent pro-angiogenic growth factors also upregulated in IBD[203-205].
Angiostatin, a fragment of plasminogen generated by MMPs has anti-angiogenic and anti-proliferative effects on ECs and blocks vessel maturation[206]. During IBD, levels of MMPs are elevated and generate angiostatin[153]. In fact 2 models of experimental colitis (iodoacetamide, TNBS) show increased angiostatin, and may represent a feedback control for angiogenesis[207]. Interestingly, the effect of angiostatin hinges less on inhibition of EC proliferation, but more on inhibiting final vessel maturity[208]. Much like angiostatin, endostatin results from the cleavage of collagen type XVIII yielding an anti-angiogenic fragment that is upregulated in experimental colitis[207,209]. Endostatin reduces EC migration and proliferation; however like angiostatin, endostatin fails to block angiogenesis in the TNBS model, but may play a role in disease progression and maintenance by impairing vessel maturity and tissue healing by antagonizing VEGF-A induced tissue repair[207]. Interestingly Deng et al[210] showed mesalamine treatment of iodoacetamide colitis restored levels of endogenous angiogenesis inhibitors, endostatin and angiostatin helping reduce disease severity.
Soluble VEGF receptors (sVEGFRs) are truncated forms of VEGFR1 or VEGFR2 genes[211] that under normal physiological conditions maintain tissue avascularity (e.g. in the cornea) and might be dysregulated in IBD. During inflammation, sVEGFR1 inhibition seems to be lost (e.g. in the case of an alkali burn)[211,212]. sVEGFR2 seems to plays an important role in the inhibition of lymphangiogenesis compared to sVEGFR1, but sVEGFR2 blocks transplant rejection which points out its greater immunomodulatory effect[213]. Additionally, Scaldaferri et al[167] found that over expression of sVEGFR1 reduced disease severity in the DSS model of colitis, suggesting that loss of this molecule in IBD would be detrimental. Interestingly the anti-angiogenic VEGFs, alternate splice variants of VEGFs, are downregulated in several inflammatory diseases, and are linked to the alteration of the cytokine milieu in the tissues[214-217]. These inhibitory VEGFs make up a majority of the VEGF load in the normal intestinal micro-environment with approximately 20 times greater levels in the healthy gut[217]. Currently, the levels of these inhibitory VEGFs are unknown in IBD, but may provide a new avenue for anti-angiogenic therapies, we are pursuing this possibility which is currently showing great promise (unpublished data).
It is increasingly clear that IBD therapies affect the microvasculature, and that the microvasculature is a central target in IBD, coordinating cell infiltration, solute permeability, cytokine/chemokine production and gut immunological responses. An increasing number of drugs that show efficacy in treating IBD have now been found to affect the endothelium. Accumulating evidence suggests inhibition of angiogenesis as a secondary mechanism of action for many IBD therapies including anti-TNF-α antibodies, and some immunosuppressive agents (cyclosporine A)[218-220]. Scaldaferri et al[87] found TNF-α mediated lymphocyte adhesion and chemotaxis across intestinal microvascular ECs depends on expression of ICAM-1, VCAM-1 and fractalkine in the affected ECs mediated by p38 MAPK, p42/44 MAPK and JNK. Danese et al[71] found that anti-TNF-α therapies can reduce thrombus formation and adhesion to the endothelium by interfering with CD40/CD40L signaling. Integrin-blocking antibodies have been used in the treatment of IBD, but not without a controversial side effect. Natalizumab (Tysabri), an α4-integrin blocking monoclonal antibody originally developed for use in the treatment of multiple sclerosis, but has recently been approved for the treatment CD[21,221]. AJM300, a peptide blocker for α4 integrins, successfully blocked α4 -VCAM-1 and MAdCAM-1 adhesion and prevented exacerbation in IBD models[20,21]. However, recent preclinical trials using AJM300 failed to inhibit disease progression[20,21]. Rafiee et al[222] found that 2 drugs used in IBD, thalidomide and cyclosporine-A, are anti-angiogenic; thalidomide targets TNF-α and VEGF-A, while cyclosporin-A targets VEGF-A alone[222,223]. Studies by Ogawa et al[224] determined that HIMEC expression of the inflammatory mediators IL-6 and cyclooxygenase (COX)-2 by LPS were inhibited by butyrate, and that butyrate also inhibited HIMEC angiogenesis[224-226]. Despite its anti-inflammatory properties[223,227,228], cyclosporin-A increases leukocyte binding, unlike thalidomide which reduces leukocyte binding to HIMECs[227,228].
The risk of developing cancer is elevated by inflammation, and the link between IBD and colorectal cancer (CRC) is convincing[229-231]. Inhibition of angiogenesis in CRC by bevacizumab (anti-VEGF monoclonal antibody) improves clinical outcomes, revealing the importance of angiogenesis in the progression from IBD to CRC[232]. As stated before, IBD in human disease and animal models is associated with an increase in vascular density, and it is possible this vascular endothelial expansion may enable CRC[158,163,169]. CRC incidence may depend on COX expression (seen in adenomatous polyposis coli, pre-cancerous lesions enriched in COX[233-235]. COX-2 is increased in human IBD and IBD models, and may promote CRC through angiogenesis[236,237]. COX-2 promotes EC proliferation by prostaglandin induction of VEGF-A, important for tumor angiogenesis[237,238]. Chan et al[239] reported that the regular use of aspirin, a non-selective COX inhibitor, significantly reduced the risk of COX-2+ CRC, which constitutes approximately 67% of human CRC[239,240]. Additionally COX-2 inhibition reduces tumor growth and increased tumor apoptosis, and is associated with reduced tumor angiogenesis[238,241,242]. Conversely, Ishikawa et al[243] found that COX-deficient animals were not protected from tumor formation in azoxymethane (tumor promoter)-induced colorectal cancer, and concluded that COX expression was not a major determinant of tumor formation in UC. While COX expression may not be not necessary for tumor formation in UC, COX-2 upregulation is only one mechanism for increased angiogenesis in IBD[86,166,189]. VEGF-A and other angiogenic factors are upregulated independent of COX-2 in IBD; therefore, while COX-2 may be important in CRC in the absence of IBD, expansion of the vasculature in IBD through other mechanisms may contribute to the development and growth of CRC[166,244].
A unique combination of genetic and environmental factors may contribute to development of IBD. ECs are now recognized as central and fundamental elements in IBD pathophysiology. ECs are indirectly affected by many IBD medications, which are increasingly targeting ECs directly. As treatments for IBD are developed and refined there will be an increased interest in inhibiting functions of ECs in IBD such as immune cell recruitment and inflammatory angiogenesis, and improving beneficial lymphatic function. Use of endogenous inhibitors of leukocyte binding (sVCAM) and peptides (AJM300) may become novel therapies which supplement or replace current anti-adhesion treatments. Additional studies on the interactions between the gut microvasculature, platelets and their regulation of inflammatory angiogenesis may provide new avenues for treatments that not only reduce thrombosis but also several clinical manifestations of IBD. Inhibition of inflammatory growth factors, cytokines and chemokines that promote angiogenesis by the use of “traps” or decoy receptors, alone or in combination, in addition to current treatments could provide greater anti-inflammatory effects by reducing endothelial expansion in IBD. More importantly, work in our laboratory suggests that endogenous angiogenic inhibitors (VEGF164b) have great potential in the treatment of IBD. Future studies promoting therapeutic intervention by combining anti-angiogenic, anti-immune and anti-inflammatory agents as treatment options focusing on the endothelium as core/vital for IBD pathogenesis will provide greater specificity and efficacy for treating CD and UC patients.
Peer reviewers: Tauseef Ali, MD, Assistant Professor, Section of Digestive Diseases and Nutrition, University of Oklahoma Health Sciences Center, 920 SL Young Blvd, Oklahoma City, OK 73104, United States; John B Schofield, MB, BS, MRCP, FRCP, Department of Cellular Pathology, Preston Hall, Maidstone, Kent, ME20 7NH, United Kingdom
S- Editor Wang JL L- Editor Cant MR E- Editor Ma WH
| 1. | Sands BE, Kaplan GG. The role of TNFalpha in ulcerative colitis. J Clin Pharmacol. 2007;47:930-941. |
| 2. | Parkes M, Satsangi J, Jewell D. Contribution of the IL-2 and IL-10 genes to inflammatory bowel disease (IBD) susceptibility. Clin Exp Immunol. 1998;113:28-32. |
| 3. | Boyko EJ, Perera DR, Koepsell TD, Keane EM, Inui TS. Coffee and alcohol use and the risk of ulcerative colitis. Am J Gastroenterol. 1989;84:530-534. |
| 4. | Langhorst J, Cobelens PM, Kavelaars A, Heijnen CJ, Benson S, Rifaie N, Dobos GJ, Schedlowski M, Elsenbruch S. Stress-related peripheral neuroendocrine-immune interactions in women with ulcerative colitis. Psychoneuroendocrinology. 2007;32:1086-1096. |
| 5. | Langhorst J, Wieder A, Michalsen A, Musial F, Dobos GJ, Rueffer A. Activated innate immune system in irritable bowel syndrome? Gut. 2007;56:1325-1326. |
| 6. | Pousa ID, Gisbert JP. Gastric angiogenesis and Helicobacter pylori infection. Rev Esp Enferm Dig. 2006;98:527-541. |
| 7. | Hibi T, Ogata H. Novel pathophysiological concepts of inflammatory bowel disease. J Gastroenterol. 2006;41:10-16. |
| 8. | Garrett WS, Lord GM, Punit S, Lugo-Villarino G, Mazmanian SK, Ito S, Glickman JN, Glimcher LH. Communicable ulcerative colitis induced by T-bet deficiency in the innate immune system. Cell. 2007;131:33-45. |
| 9. | Sands BE. Inflammatory bowel disease: past, present, and future. J Gastroenterol. 2007;42:16-25. |
| 10. | Mayer L. Evolving paradigms in the pathogenesis of IBD. J Gastroenterol. 2010;45:9-16. |
| 11. | Ruyssers NE, De Winter BY, De Man JG, Loukas A, Herman AG, Pelckmans PA, Moreels TG. Worms and the treatment of inflammatory bowel disease: are molecules the answer? Clin Dev Immunol. 2008;2008:567314. |
| 12. | Fiasse R, Latinne D. Intestinal helminths: a clue explaining the low incidence of inflammatory bowel diseases in Subsaharan Africa? Potential benefits and hazards of helminth therapy. Acta Gastroenterol Belg. 2006;69:418-422. |
| 13. | Moreels TG, Pelckmans PA. Gastrointestinal parasites: potential therapy for refractory inflammatory bowel diseases. Inflamm Bowel Dis. 2005;11:178-184. |
| 14. | Sandborn WJ, Targan SR. Biologic therapy of inflammatory bowel disease. Gastroenterology. 2002;122:1592-1608. |
| 15. | Sawa Y, Oshitani N, Adachi K, Higuchi K, Matsumoto T, Arakawa T. Comprehensive analysis of intestinal cytokine messenger RNA profile by real-time quantitative polymerase chain reaction in patients with inflammatory bowel disease. Int J Mol Med. 2003;11:175-179. |
| 16. | Latinne D, Fiasse R. New insights into the cellular immunology of the intestine in relation to the pathophysiology of inflammatory bowel diseases. Acta Gastroenterol Belg. 2006;69:393-405. |
| 17. | Chidlow JH Jr, Shukla D, Grisham MB, Kevil CG. Pathogenic angiogenesis in IBD and experimental colitis: new ideas and therapeutic avenues. Am J Physiol Gastrointest Liver Physiol. 2007;293:G5-G18. |
| 18. | Jiang HQ, Thurnheer MC, Zuercher AW, Boiko NV, Bos NA, Cebra JJ. Interactions of commensal gut microbes with subsets of B- and T-cells in the murine host. Vaccine. 2004;22:805-811. |
| 19. | Oshima T, Laroux FS, Coe LL, Morise Z, Kawachi S, Bauer P, Grisham MB, Specian RD, Carter P, Jennings S. Interferon-gamma and interleukin-10 reciprocally regulate endothelial junction integrity and barrier function. Microvasc Res. 2001;61:130-143. |
| 20. | Dharmani P, Chadee K. Biologic therapies against inflammatory bowel disease: a dysregulated immune system and the cross talk with gastrointestinal mucosa hold the key. Curr Mol Pharmacol. 2008;1:195-212. |
| 21. | Yacyshyn BR. Adhesion molecule therapeutics in IBD. Inflamm Bowel Dis. 2008;14 Suppl 2:S279-S280. |
| 22. | Danese S, Dejana E, Fiocchi C. Immune regulation by microvascular endothelial cells: directing innate and adaptive immunity, coagulation, and inflammation. J Immunol. 2007;178:6017-6022. |
| 23. | Cines DB, Pollak ES, Buck CA, Loscalzo J, Zimmerman GA, McEver RP, Pober JS, Wick TM, Konkle BA, Schwartz BS. Endothelial cells in physiology and in the pathophysiology of vascular disorders. Blood. 1998;91:3527-3561. |
| 24. | Haraldsen G, Rugtveit J, Kvale D, Scholz T, Muller WA, Hovig T, Brandtzaeg P. Isolation and longterm culture of human intestinal microvascular endothelial cells. Gut. 1995;37:225-234. |
| 25. | Haraldsen G, Kvale D, Lien B, Farstad IN, Brandtzaeg P. Cytokine-regulated expression of E-selectin, intercellular adhesion molecule-1 (ICAM-1), and vascular cell adhesion molecule-1 (VCAM-1) in human microvascular endothelial cells. J Immunol. 1996;156:2558-2565. |
| 26. | Nilsen EM, Johansen FE, Jahnsen FL, Lundin KE, Scholz T, Brandtzaeg P, Haraldsen G. Cytokine profiles of cultured microvascular endothelial cells from the human intestine. Gut. 1998;42:635-642. |
| 27. | Binion DG, Rafiee P, Ramanujam KS, Fu S, Fisher PJ, Rivera MT, Johnson CP, Otterson MF, Telford GL, Wilson KT. Deficient iNOS in inflammatory bowel disease intestinal microvascular endothelial cells results in increased leukocyte adhesion. Free Radic Biol Med. 2000;29:881-888. |
| 28. | Binion DG, West GA, Volk EE, Drazba JA, Ziats NP, Petras RE, Fiocchi C. Acquired increase in leucocyte binding by intestinal microvascular endothelium in inflammatory bowel disease. Lancet. 1998;352:1742-1746. |
| 29. | Sessa WC. Molecular control of blood flow and angiogenesis: role of nitric oxide. J Thromb Haemost. 2009;7 Suppl 1:35-37. |
| 30. | Binion DG, Fu S, Ramanujam KS, Chai YC, Dweik RA, Drazba JA, Wade JG, Ziats NP, Erzurum SC, Wilson KT. iNOS expression in human intestinal microvascular endothelial cells inhibits leukocyte adhesion. Am J Physiol. 1998;275:G592-G603. |
| 31. | Petersson J, Schreiber O, Steege A, Patzak A, Hellsten A, Phillipson M, Holm L. eNOS involved in colitis-induced mucosal blood flow increase. Am J Physiol Gastrointest Liver Physiol. 2007;293:G1281-G1287. |
| 32. | Spyridopoulos I, Luedemann C, Chen D, Kearney M, Chen D, Murohara T, Principe N, Isner JM, Losordo DW. Divergence of angiogenic and vascular permeability signaling by VEGF: inhibition of protein kinase C suppresses VEGF-induced angiogenesis, but promotes VEGF-induced, NO-dependent vascular permeability. Arterioscler Thromb Vasc Biol. 2002;22:901-906. |
| 33. | Breslin JW, Pappas PJ, Cerveira JJ, Hobson RW 2nd, Durán WN. VEGF increases endothelial permeability by separate signaling pathways involving ERK-1/2 and nitric oxide. Am J Physiol Heart Circ Physiol. 2003;284:H92-H100. |
| 34. | Rousseau S, Houle F, Kotanides H, Witte L, Waltenberger J, Landry J, Huot J. Vascular endothelial growth factor (VEGF)-driven actin-based motility is mediated by VEGFR2 and requires concerted activation of stress-activated protein kinase 2 (SAPK2/p38) and geldanamycin-sensitive phosphorylation of focal adhesion kinase. J Biol Chem. 2000;275:10661-10672. |
| 35. | Mihaescu A, Santen S, Jeppsson B, Thorlacius H. p38 Mitogen-activated protein kinase signalling regulates vascular inflammation and epithelial barrier dysfunction in an experimental model of radiation-induced colitis. Br J Surg. 2010;97:226-234. |
| 36. | Kumar P, Shen Q, Pivetti CD, Lee ES, Wu MH, Yuan SY. Molecular mechanisms of endothelial hyperpermeability: implications in inflammation. Expert Rev Mol Med. 2009;11:e19. |
| 37. | Vetrano S, Danese S. The role of JAM-A in inflammatory bowel disease: unrevealing the ties that bind. Ann N Y Acad Sci. 2009;1165:308-313. |
| 38. | Horowitz S, Binion DG, Nelson VM, Kanaa Y, Javadi P, Lazarova Z, Andrekopoulos C, Kalyanaraman B, Otterson MF, Rafiee P. Increased arginase activity and endothelial dysfunction in human inflammatory bowel disease. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1323-G1336. |
| 39. | Förstermann U. Janus-faced role of endothelial NO synthase in vascular disease: uncoupling of oxygen reduction from NO synthesis and its pharmacological reversal. Biol Chem. 2006;387:1521-1533. |
| 40. | Förstermann U, Münzel T. Endothelial nitric oxide synthase in vascular disease: from marvel to menace. Circulation. 2006;113:1708-1714. |
| 41. | Hatoum OA, Binion DG, Otterson MF, Gutterman DD. Acquired microvascular dysfunction in inflammatory bowel disease: Loss of nitric oxide-mediated vasodilation. Gastroenterology. 2003;125:58-69. |
| 42. | Palatka K, Serfozo Z, Veréb Z, Hargitay Z, Lontay B, Erdodi F, Bánfalvi G, Nemes Z, Udvardy M, Altorjay I. Changes in the expression and distribution of the inducible and endothelial nitric oxide synthase in mucosal biopsy specimens of inflammatory bowel disease. Scand J Gastroenterol. 2005;40:670-680. |
| 43. | Sasaki M, Bharwani S, Jordan P, Elrod JW, Grisham MB, Jackson TH, Lefer DJ, Alexander JS. Increased disease activity in eNOS-deficient mice in experimental colitis. Free Radic Biol Med. 2003;35:1679-1687. |
| 44. | Oshima T, Jordan P, Grisham MB, Alexander JS, Jennings M, Sasaki M, Manas K. TNF-alpha induced endothelial MAdCAM-1 expression is regulated by exogenous, not endogenous nitric oxide. BMC Gastroenterol. 2001;1:5. |
| 45. | Kevil CG, Ohno N, Gute DC, Okayama N, Robinson SA, Chaney E, Alexander JS. Role of cadherin internalization in hydrogen peroxide-mediated endothelial permeability. Free Radic Biol Med. 1998;24:1015-1022. |
| 46. | Kevil CG, Oshima T, Alexander B, Coe LL, Alexander JS. H(2)O(2)-mediated permeability: role of MAPK and occludin. Am J Physiol Cell Physiol. 2000;279:C21-C30. |
| 47. | Kevil CG, Okayama N, Alexander JS. H(2)O(2)-mediated permeability II: importance of tyrosine phosphatase and kinase activity. Am J Physiol Cell Physiol. 2001;281:C1940-C1947. |
| 48. | Palatka K, Serfozo Z, Veréb Z, Bátori R, Lontay B, Hargitay Z, Nemes Z, Udvardy M, Erdodi F, Altorjay I. Effect of IBD sera on expression of inducible and endothelial nitric oxide synthase in human umbilical vein endothelial cells. World J Gastroenterol. 2006;12:1730-1738. |
| 49. | Krieglstein CF, Anthoni C, Cerwinka WH, Stokes KY, Russell J, Grisham MB, Granger DN. Role of blood- and tissue-associated inducible nitric-oxide synthase in colonic inflammation. Am J Pathol. 2007;170:490-496. |
| 50. | Aoi Y, Terashima S, Ogura M, Nishio H, Kato S, Takeuchi K. Roles of nitric oxide (NO) and NO synthases in healing of dextran sulfate sodium-induced rat colitis. J Physiol Pharmacol. 2008;59:315-336. |
| 51. | Elrod JW, Laroux FS, Houghton J, Carpenter A, Ando T, Jennings MH, Grisham M, Walker N, Alexander JS. DSS-induced colitis is exacerbated in STAT-6 knockout mice. Inflamm Bowel Dis. 2005;11:883-889. |
| 52. | Van Crombruggen K, Van Nassauw L, Demetter P, Cuvelier C, Timmermans JP, Lefebvre RA. Influence of soluble guanylate cyclase inhibition on inflammation and motility disturbances in DSS-induced colitis. Eur J Pharmacol. 2008;579:337-349. |
| 53. | Conner EM, Brand S, Davis JM, Laroux FS, Palombella VJ, Fuseler JW, Kang DY, Wolf RE, Grisham MB. Proteasome inhibition attenuates nitric oxide synthase expression, VCAM-1 transcription and the development of chronic colitis. J Pharmacol Exp Ther. 1997;282:1615-1622. |
| 54. | Grisham MB, Palombella VJ, Elliott PJ, Conner EM, Brand S, Wong HL, Pien C, Mazzola LM, Destree A, Parent L. Inhibition of NF-kappa B activation in vitro and in vivo: role of 26S proteasome. Methods Enzymol. 1999;300:345-363. |
| 55. | Deban L, Correale C, Vetrano S, Malesci A, Danese S. Multiple pathogenic roles of microvasculature in inflammatory bowel disease: a Jack of all trades. Am J Pathol. 2008;172:1457-1466. |
| 56. | Ogawa H, Rafiee P, Heidemann J, Fisher PJ, Johnson NA, Otterson MF, Kalyanaraman B, Pritchard KA Jr, Binion DG. Mechanisms of endotoxin tolerance in human intestinal microvascular endothelial cells. J Immunol. 2003;170:5956-5964. |
| 57. | Gewirtz AT. Flag in the crossroads: flagellin modulates innate and adaptive immunity. Curr Opin Gastroenterol. 2006;22:8-12. |
| 58. | Hokari R, Miura S. Neutrophil elastase in colitis: more than a marker of disease activity? J Gastroenterol. 2006;41:395-396. |
| 59. | Morohoshi Y, Matsuoka K, Chinen H, Kamada N, Sato T, Hisamatsu T, Okamoto S, Inoue N, Takaishi H, Ogata H. Inhibition of neutrophil elastase prevents the development of murine dextran sulfate sodium-induced colitis. J Gastroenterol. 2006;41:318-324. |
| 60. | Maaser C, Heidemann J, von Eiff C, Lugering A, Spahn TW, Binion DG, Domschke W, Lugering N, Kucharzik T. Human intestinal microvascular endothelial cells express Toll-like receptor 5: a binding partner for bacterial flagellin. J Immunol. 2004;172:5056-5062. |
| 61. | Vijay-Kumar M, Aitken JD, Gewirtz AT. Toll like receptor-5: protecting the gut from enteric microbes. Semin Immunopathol. 2008;30:11-21. |
| 62. | Vijay-Kumar M, Sanders CJ, Taylor RT, Kumar A, Aitken JD, Sitaraman SV, Neish AS, Uematsu S, Akira S, Williams IR. Deletion of TLR5 results in spontaneous colitis in mice. J Clin Invest. 2007;117:3909-3921. |
| 63. | Vijay-Kumar M, Wu H, Aitken J, Kolachala VL, Neish AS, Sitaraman SV, Gewirtz AT. Activation of toll-like receptor 3 protects against DSS-induced acute colitis. Inflamm Bowel Dis. 2007;13:856-864. |
| 64. | Heidemann J, Rüther C, Kebschull M, Domschke W, Brüwer M, Koch S, Kucharzik T, Maaser C. Expression of IL-12-related molecules in human intestinal microvascular endothelial cells is regulated by TLR3. Am J Physiol Gastrointest Liver Physiol. 2007;293:G1315-G1324. |
| 65. | Kawachi S, Morise Z, Jennings SR, Conner E, Cockrell A, Laroux FS, Chervenak RP, Wolcott M, van der Heyde H, Gray L. Cytokine and adhesion molecule expression in SCID mice reconstituted with CD4+ T cells. Inflamm Bowel Dis. 2000;6:171-180. |
| 66. | Kawachi S, Jennings S, Panes J, Cockrell A, Laroux FS, Gray L, Perry M, van der Heyde H, Balish E, Granger DN. Cytokine and endothelial cell adhesion molecule expression in interleukin-10-deficient mice. Am J Physiol Gastrointest Liver Physiol. 2000;278:G734-G743. |
| 67. | Polzer K, Baeten D, Soleiman A, Distler J, Gerlag DM, Tak PP, Schett G, Zwerina J. Tumour necrosis factor blockade increases lymphangiogenesis in murine and human arthritic joints. Ann Rheum Dis. 2008;67:1610-1616. |
| 68. | Heidenreich R, Röcken M, Ghoreschi K. Angiogenesis drives psoriasis pathogenesis. Int J Exp Pathol. 2009;90:232-248. |
| 69. | Hatoum OA, Heidemann J, Binion DG. The intestinal microvasculature as a therapeutic target in inflammatory bowel disease. Ann N Y Acad Sci. 2006;1072:78-97. |
| 70. | Pufe T, Lemke A, Kurz B, Petersen W, Tillmann B, Grodzinsky AJ, Mentlein R. Mechanical overload induces VEGF in cartilage discs via hypoxia-inducible factor. Am J Pathol. 2004;164:185-192. |
| 71. | Danese S, Sans M, Scaldaferri F, Sgambato A, Rutella S, Cittadini A, Piqué JM, Panes J, Katz JA, Gasbarrini A. TNF-alpha blockade down-regulates the CD40/CD40L pathway in the mucosal microcirculation: a novel anti-inflammatory mechanism of infliximab in Crohn's disease. J Immunol. 2006;176:2617-2624. |
| 72. | Romer LH, McLean NV, Yan HC, Daise M, Sun J, DeLisser HM. IFN-gamma and TNF-alpha induce redistribution of PECAM-1 (CD31) on human endothelial cells. J Immunol. 1995;154:6582-6592. |
| 73. | Kosmadaki MG, Yaar M, Arble BL, Gilchrest BA. UV induces VEGF through a TNF-alpha independent pathway. FASEB J. 2003;17:446-448. |
| 74. | Scheinin T, Butler DM, Salway F, Scallon B, Feldmann M. Validation of the interleukin-10 knockout mouse model of colitis: antitumour necrosis factor-antibodies suppress the progression of colitis. Clin Exp Immunol. 2003;133:38-43. |
| 75. | Lindsay JO, Ciesielski CJ, Scheinin T, Brennan FM, Hodgson HJ. Local delivery of adenoviral vectors encoding murine interleukin 10 induces colonic interleukin 10 production and is therapeutic for murine colitis. Gut. 2003;52:981-987. |
| 76. | Lindsay J, Van Montfrans C, Brennan F, Van Deventer S, Drillenburg P, Hodgson H, Te Velde A, Sol Rodriguez Pena M. IL-10 gene therapy prevents TNBS-induced colitis. Gene Ther. 2002;9:1715-1721. |
| 77. | Papadakis KA. Chemokines in inflammatory bowel disease. Curr Allergy Asthma Rep. 2004;4:83-89. |
| 78. | Barcelos LS, Coelho AM, Russo RC, Guabiraba R, Souza AL, Bruno-Lima G Jr, Proudfoot AE, Andrade SP, Teixeira MM. Role of the chemokines CCL3/MIP-1 alpha and CCL5/RANTES in sponge-induced inflammatory angiogenesis in mice. Microvasc Res. 2009;78:148-154. |
| 79. | Wu Y, Li YY, Matsushima K, Baba T, Mukaida N. CCL3-CCR5 axis regulates intratumoral accumulation of leukocytes and fibroblasts and promotes angiogenesis in murine lung metastasis process. J Immunol. 2008;181:6384-6393. |
| 80. | Eyman D, Damodarasamy M, Plymate SR, Reed MJ. CCL5 secreted by senescent aged fibroblasts induces proliferation of prostate epithelial cells and expression of genes that modulate angiogenesis. J Cell Physiol. 2009;220:376-381. |
| 81. | Rivera-Nieves J, Ho J, Bamias G, Ivashkina N, Ley K, Oppermann M, Cominelli F. Antibody blockade of CCL25/CCR9 ameliorates early but not late chronic murine ileitis. Gastroenterology. 2006;131:1518-1529. |
| 82. | Heidemann J, Ogawa H, Dwinell MB, Rafiee P, Maaser C, Gockel HR, Otterson MF, Ota DM, Lugering N, Domschke W. Angiogenic effects of interleukin 8 (CXCL8) in human intestinal microvascular endothelial cells are mediated by CXCR2. J Biol Chem. 2003;278:8508-8515. |
| 83. | Utgaard JO, Jahnsen FL, Bakka A, Brandtzaeg P, Haraldsen G. Rapid secretion of prestored interleukin 8 from Weibel-Palade bodies of microvascular endothelial cells. J Exp Med. 1998;188:1751-1756. |
| 84. | Gijsbers K, Van Assche G, Joossens S, Struyf S, Proost P, Rutgeerts P, Geboes K, Van Damme J. CXCR1-binding chemokines in inflammatory bowel diseases: down-regulated IL-8/CXCL8 production by leukocytes in Crohn's disease and selective GCP-2/CXCL6 expression in inflamed intestinal tissue. Eur J Immunol. 2004;34:1992-2000. |
| 85. | Lügering N, Kucharzik T, Gockel H, Sorg C, Stoll R, Domschke W. Human intestinal epithelial cells down-regulate IL-8 expression in human intestinal microvascular endothelial cells; role of transforming growth factor-beta 1 (TGF-beta1). Clin Exp Immunol. 1998;114:377-384. |
| 86. | Kanazawa S, Tsunoda T, Onuma E, Majima T, Kagiyama M, Kikuchi K. VEGF, basic-FGF, and TGF-beta in Crohn's disease and ulcerative colitis: a novel mechanism of chronic intestinal inflammation. Am J Gastroenterol. 2001;96:822-828. |
| 87. | Scaldaferri F, Sans M, Vetrano S, Correale C, Arena V, Pagano N, Rando G, Romeo F, Potenza AE, Repici A. The role of MAPK in governing lymphocyte adhesion to and migration across the microvasculature in inflammatory bowel disease. Eur J Immunol. 2009;39:290-300. |
| 88. | Scaldaferri F, Correale C, Gasbarrini A, Danese S. Molecular signaling blockade as a new approach to inhibit leukocyte-endothelial interactions for inflammatory bowel disease treatment. Cell Adh Migr. 2009;3:296-299. |
| 89. | Imaizumi T, Yoshida H, Satoh K. Regulation of CX3CL1/fractalkine expression in endothelial cells. J Atheroscler Thromb. 2004;11:15-21. |
| 90. | Nishimura M, Kuboi Y, Muramoto K, Kawano T, Imai T. Chemokines as novel therapeutic targets for inflammatory bowel disease. Ann N Y Acad Sci. 2009;1173:350-356. |
| 91. | Sans M, Danese S, de la Motte C, de Souza HS, Rivera-Reyes BM, West GA, Phillips M, Katz JA, Fiocchi C. Enhanced recruitment of CX3CR1+ T cells by mucosal endothelial cell-derived fractalkine in inflammatory bowel disease. Gastroenterology. 2007;132:139-153. |
| 92. | Heidemann J, Ogawa H, Rafiee P, Lügering N, Maaser C, Domschke W, Binion DG, Dwinell MB. Mucosal angiogenesis regulation by CXCR4 and its ligand CXCL12 expressed by human intestinal microvascular endothelial cells. Am J Physiol Gastrointest Liver Physiol. 2004;286:G1059-G1068. |
| 93. | Schober A. Chemokines in vascular dysfunction and remodeling. Arterioscler Thromb Vasc Biol. 2008;28:1950-1959. |
| 94. | Hristov M, Zernecke A, Schober A, Weber C. Adult progenitor cells in vascular remodeling during atherosclerosis. Biol Chem. 2008;389:837-844. |
| 95. | Garolla A, D'Incà R, Checchin D, Biagioli A, De Toni L, Nicoletti V, Scarpa M, Bolzonello E, Sturniolo GC, Foresta C. Reduced endothelial progenitor cell number and function in inflammatory bowel disease: a possible link to the pathogenesis. Am J Gastroenterol. 2009;104:2500-2507. |
| 96. | Ikematsu S, Yano A, Aridome K, Kikuchi M, Kumai H, Nagano H, Okamoto K, Oda M, Sakuma S, Aikou T. Serum midkine levels are increased in patients with various types of carcinomas. Br J Cancer. 2000;83:701-706. |
| 97. | Muramatsu T. Midkine and pleiotrophin: two related proteins involved in development, survival, inflammation and tumorigenesis. J Biochem. 2002;132:359-371. |
| 98. | Qi M, Ikematsu S, Ichihara-Tanaka K, Sakuma S, Muramatsu T, Kadomatsu K. Midkine rescues Wilms' tumor cells from cisplatin-induced apoptosis: regulation of Bcl-2 expression by Midkine. J Biochem. 2000;127:269-277. |
| 99. | Krzystek-Korpacka M, Neubauer K, Matusiewicz M. Clinical relevance of circulating midkine in ulcerative colitis. Clin Chem Lab Med. 2009;47:1085-1090. |
| 100. | Tsiolakidou G, Koutroubakis IE, Tzardi M, Kouroumalis EA. Increased expression of VEGF and CD146 in patients with inflammatory bowel disease. Dig Liver Dis. 2008;40:673-679. |
| 101. | Kapsoritakis A, Sfiridaki A, Maltezos E, Simopoulos K, Giatromanolaki A, Sivridis E, Koukourakis MI. Vascular endothelial growth factor in inflammatory bowel disease. Int J Colorectal Dis. 2003;18:418-422. |
| 102. | Koizumi M, King N, Lobb R, Benjamin C, Podolsky DK. Expression of vascular adhesion molecules in inflammatory bowel disease. Gastroenterology. 1992;103:840-847. |
| 103. | Sumi Y, Muramatsu H, Takei Y, Hata K, Ueda M, Muramatsu T. Midkine, a heparin-binding growth factor, promotes growth and glycosaminoglycan synthesis of endothelial cells through its action on smooth muscle cells in an artificial blood vessel model. J Cell Sci. 2002;115:2659-2667. |
| 104. | McDonald SA, Palmen MJ, Van Rees EP, MacDonald TT. Characterization of the mucosal cell-mediated immune response in IL-2 knockout mice before and after the onset of colitis. Immunology. 1997;91:73-80. |
| 105. | Burns RC, Rivera-Nieves J, Moskaluk CA, Matsumoto S, Cominelli F, Ley K. Antibody blockade of ICAM-1 and VCAM-1 ameliorates inflammation in the SAMP-1/Yit adoptive transfer model of Crohn's disease in mice. Gastroenterology. 2001;121:1428-1436. |
| 106. | Iijima H, Neurath MF, Nagaishi T, Glickman JN, Nieuwenhuis EE, Nakajima A, Chen D, Fuss IJ, Utku N, Lewicki DN. Specific regulation of T helper cell 1-mediated murine colitis by CEACAM1. J Exp Med. 2004;199:471-482. |
| 107. | Oshima T, Pavlick KP, Laroux FS, Verma SK, Jordan P, Grisham MB, Williams L, Alexander JS. Regulation and distribution of MAdCAM-1 in endothelial cells in vitro. Am J Physiol Cell Physiol. 2001;281:C1096-C1105. |
| 108. | Bardin N, Reumaux D, Geboes K, Colombel JF, Blot-Chabaud M, Sampol J, Duthilleul P, Dignat-George F. Increased expression of CD146, a new marker of the endothelial junction in active inflammatory bowel disease. Inflamm Bowel Dis. 2006;12:16-21. |
| 109. | Magro F, Araujo F, Pereira P, Meireles E, Diniz-Ribeiro M, Velosom FT. Soluble selectins, sICAM, sVCAM, and angiogenic proteins in different activity groups of patients with inflammatory bowel disease. Dig Dis Sci. 2004;49:1265-1274. |
| 110. | Newman W, Mirabelli CK. The link between inflammatory disease and patterns of leukocyte recruitment. Expert Opin Investig Drugs. 1998;7:19-25. |
| 111. | Sasaki M, Jordan P, Welbourne T, Minagar A, Joh T, Itoh M, Elrod JW, Alexander JS. Troglitazone, a PPAR-gamma activator prevents endothelial cell adhesion molecule expression and lymphocyte adhesion mediated by TNF-alpha. BMC Physiol. 2005;5:3. |
| 112. | Davenpeck KL, Gauthier TW, Albertine KH, Lefer AM. Role of P-selectin in microvascular leukocyte-endothelial interaction in splanchnic ischemia-reperfusion. Am J Physiol. 1994;267:H622-H630. |
| 113. | Kobayashi M, Fukuda M, Nakayama J. Role of sulfated O-glycans expressed by high endothelial venule-like vessels in pathogenesis of chronic inflammatory gastrointestinal diseases. Biol Pharm Bull. 2009;32:774-779. |
| 114. | Rivera-Nieves J, Burcin TL, Olson TS, Morris MA, McDuffie M, Cominelli F, Ley K. Critical role of endothelial P-selectin glycoprotein ligand 1 in chronic murine ileitis. J Exp Med. 2006;203:907-917. |
| 115. | Fägerstam JP, Whiss PA. Higher platelet P-selectin in male patients with inflammatory bowel disease compared to healthy males. World J Gastroenterol. 2006;12:1270-1272. |
| 116. | Rho YH, Chung CP, Oeser A, Solus J, Asanuma Y, Sokka T, Pincus T, Raggi P, Gebretsadik T, Shintani A. Inflammatory mediators and premature coronary atherosclerosis in rheumatoid arthritis. Arthritis Rheum. 2009;61:1580-1585. |
| 117. | Zittermann SI, Issekutz AC. Endothelial growth factors VEGF and bFGF differentially enhance monocyte and neutrophil recruitment to inflammation. J Leukoc Biol. 2006;80:247-257. |
| 118. | Kobayashi M, Hoshino H, Masumoto J, Fukushima M, Suzawa K, Kageyama S, Suzuki M, Ohtani H, Fukuda M, Nakayama J. GlcNAc6ST-1-mediated decoration of MAdCAM-1 protein with L-selectin ligand carbohydrates directs disease activity of ulcerative colitis. Inflamm Bowel Dis. 2009;15:697-706. |
| 119. | Briskin M, Winsor-Hines D, Shyjan A, Cochran N, Bloom S, Wilson J, McEvoy LM, Butcher EC, Kassam N, Mackay CR. Human mucosal addressin cell adhesion molecule-1 is preferentially expressed in intestinal tract and associated lymphoid tissue. Am J Pathol. 1997;151:97-110. |
| 120. | Burgio VL, Fais S, Boirivant M, Perrone A, Pallone F. Peripheral monocyte and naive T-cell recruitment and activation in Crohn's disease. Gastroenterology. 1995;109:1029-1038. |
| 121. | Mizushima T, Sasaki M, Ando T, Wada T, Tanaka M, Okamoto Y, Ebi M, Hirata Y, Murakami K, Mizoshita T. Blockage of angiotensin II type 1 receptor regulates TNF-alpha-induced MAdCAM-1 expression via inhibition of NF-kappaB translocation to the nucleus and ameliorates colitis. Am J Physiol Gastrointest Liver Physiol. 2010;298:G255-G266. |
| 122. | Alexander JS, Ando T. Density-dependent control of MAdCAM-1 and chronic inflammation. Focus on "Mechanisms of MAdCAM-1 gene expression in human intestinal microvascular endothelial cells". Am J Physiol Cell Physiol. 2005;288:C243-C244. |
| 123. | Ando T, Jordan P, Wang Y, Itoh M, Joh T, Sasaki M, Elrod JW, Carpenter A, Jennings MH, Minagar A. MAdCAM-1 expression and regulation in murine colonic endothelial cells in vitro. Inflamm Bowel Dis. 2005;11:258-264. |
| 124. | Lu M, Perez VL, Ma N, Miyamoto K, Peng HB, Liao JK, Adamis AP. VEGF increases retinal vascular ICAM-1 expression in vivo. Invest Ophthalmol Vis Sci. 1999;40:1808-1812. |
| 125. | Goebel S, Huang M, Davis WC, Jennings M, Siahaan TJ, Alexander JS, Kevil CG. VEGF-A stimulation of leukocyte adhesion to colonic microvascular endothelium: implications for inflammatory bowel disease. Am J Physiol Gastrointest Liver Physiol. 2006;290:G648-G654. |
| 126. | Ostanin DV, Bao J, Koboziev I, Gray L, Robinson-Jackson SA, Kosloski-Davidson M, Price VH, Grisham MB. T cell transfer model of chronic colitis: concepts, considerations, and tricks of the trade. Am J Physiol Gastrointest Liver Physiol. 2009;296:G135-G146. |
| 127. | Binion DG, Heidemann J, Li MS, Nelson VM, Otterson MF, Rafiee P. Vascular cell adhesion molecule-1 expression in human intestinal microvascular endothelial cells is regulated by PI 3-kinase/Akt/MAPK/NF-kappaB: inhibitory role of curcumin. Am J Physiol Gastrointest Liver Physiol. 2009;297:G259-G268. |
| 128. | Kim I, Moon SO, Kim SH, Kim HJ, Koh YS, Koh GY. Vascular endothelial growth factor expression of intercellular adhesion molecule 1 (ICAM-1), vascular cell adhesion molecule 1 (VCAM-1), and E-selectin through nuclear factor-kappa B activation in endothelial cells. J Biol Chem. 2001;276:7614-7620. |
| 129. | Sans M, Fuster D, Vázquez A, Setoain FJ, Piera C, Piqué JM, Panés J. 123Iodine-labelled anti-VCAM-1 antibody scintigraphy in the assessment of experimental colitis. Eur J Gastroenterol Hepatol. 2001;13:31-38. |
| 130. | Soriano A, Salas A, Salas A, Sans M, Gironella M, Elena M, Anderson DC, Piqué JM, Panés J. VCAM-1, but not ICAM-1 or MAdCAM-1, immunoblockade ameliorates DSS-induced colitis in mice. Lab Invest. 2000;80:1541-1551. |
| 131. | Rijcken E, Mennigen RB, Schaefer SD, Laukoetter MG, Anthoni C, Spiegel HU, Bruewer M, Senninger N, Krieglstein CF. PECAM-1 (CD 31) mediates transendothelial leukocyte migration in experimental colitis. Am J Physiol Gastrointest Liver Physiol. 2007;293:G446-G452. |
| 132. | Elshal MF, Khan SS, Raghavachari N, Takahashi Y, Barb J, Bailey JJ, Munson PJ, Solomon MA, Danner RL, McCoy JP Jr. A unique population of effector memory lymphocytes identified by CD146 having a distinct immunophenotypic and genomic profile. BMC Immunol. 2007;8:29. |
| 133. | Elshal MF, Khan SS, Takahashi Y, Solomon MA, McCoy JP Jr. CD146 (Mel-CAM), an adhesion marker of endothelial cells, is a novel marker of lymphocyte subset activation in normal peripheral blood. Blood. 2005;106:2923-2924. |
| 134. | Covas DT, Panepucci RA, Fontes AM, Silva WA Jr, Orellana MD, Freitas MC, Neder L, Santos AR, Peres LC, Jamur MC. Multipotent mesenchymal stromal cells obtained from diverse human tissues share functional properties and gene-expression profile with CD146+ perivascular cells and fibroblasts. Exp Hematol. 2008;36:642-654. |
| 135. | Bardin N, Blot-Chabaud M, Despoix N, Kebir A, Harhouri K, Arsanto JP, Espinosa L, Perrin P, Robert S, Vely F. CD146 and its soluble form regulate monocyte transendothelial migration. Arterioscler Thromb Vasc Biol. 2009;29:746-753. |
| 136. | Tabibian JH, Roth BE. Local thrombolysis: a newer approach to treating inflammatory bowel disease-related thromboembolism. J Clin Gastroenterol. 2009;43:391-398. |
| 137. | Hatoum OA, Spinelli KS, Abu-Hajir M, Attila T, Franco J, Otterson MF, Telford GL, Binion DG. Mesenteric venous thrombosis in inflammatory bowel disease. J Clin Gastroenterol. 2005;39:27-31. |
| 138. | Bernstein CN, Sargent M, Vos HL, Rosendaal FR. Mutations in clotting factors and inflammatory bowel disease. Am J Gastroenterol. 2007;102:338-343. |
| 139. | Dardik R, Loscalzo J, Eskaraev R, Inbal A. Molecular mechanisms underlying the proangiogenic effect of factor XIII. Arterioscler Thromb Vasc Biol. 2005;25:526-532. |
| 140. | Vrij AA, Rijken J, van Wersch JW, Stockbrügger RW. Differential behavior of coagulation factor XIII in patients with inflammatory bowel disease and in patients with giant cell arteritis. Haemostasis. 1999;29:326-335. |
| 141. | Danese S, Katz JA, Saibeni S, Papa A, Gasbarrini A, Vecchi M, Fiocchi C. Activated platelets are the source of elevated levels of soluble CD40 ligand in the circulation of inflammatory bowel disease patients. Gut. 2003;52:1435-1441. |
| 142. | Danese S, de la Motte C, Sturm A, Vogel JD, West GA, Strong SA, Katz JA, Fiocchi C. Platelets trigger a CD40-dependent inflammatory response in the microvasculature of inflammatory bowel disease patients. Gastroenterology. 2003;124:1249-1264. |
| 143. | Vogel JD, West GA, Danese S, De La Motte C, Phillips MH, Strong SA, Willis J, Fiocchi C. CD40-mediated immune-nonimmune cell interactions induce mucosal fibroblast chemokines leading to T-cell transmigration. Gastroenterology. 2004;126:63-80. |
| 144. | Srirajaskanthan R, Winter M, Muller AF. Venous thrombosis in inflammatory bowel disease. Eur J Gastroenterol Hepatol. 2005;17:697-700. |
| 145. | Hathaway CA, Appleyard CB, Percy WH, Williams JL. Experimental colitis increases blood-brain barrier permeability in rabbits. Am J Physiol. 1999;276:G1174-G1180. |
| 146. | Koutroubakis IE, Xidakis C, Karmiris K, Sfiridaki A, Kandidaki E, Kouroumalis EA. Serum angiogenin in inflammatory bowel disease. Dig Dis Sci. 2004;49:1758-1762. |
| 147. | Im E, Choi YJ, Kim CH, Fiocchi C, Pothoulakis C, Rhee SH. The angiogenic effect of probiotic Bacillus polyfermenticus on human intestinal microvascular endothelial cells is mediated by IL-8. Am J Physiol Gastrointest Liver Physiol. 2009;297:G999-G1008. |
| 148. | Roessner A, Kuester D, Malfertheiner P, Schneider-Stock R. Oxidative stress in ulcerative colitis-associated carcinogenesis. Pathol Res Pract. 2008;204:511-524. |
| 149. | Tolstanova G, Khomenko T, Deng X, Chen L, Tarnawski A, Ahluwalia A, Szabo S, Sandor Z. Neutralizing anti-vascular endothelial growth factor (VEGF) antibody reduces severity of experimental ulcerative colitis in rats: direct evidence for the pathogenic role of VEGF. J Pharmacol Exp Ther. 2009;328:749-757. |
| 150. | Alexander J. Extracellular matrix, junctional integrity and matrix metalloproteinase interactions. J Anat. 2002;200:525. |
| 151. | Allport JR, Muller WA, Luscinskas FW. Monocytes induce reversible focal changes in vascular endothelial cadherin complex during transendothelial migration under flow. J Cell Biol. 2000;148:203-216. |
| 152. | Weber C, Fraemohs L, Dejana E. The role of junctional adhesion molecules in vascular inflammation. Nat Rev Immunol. 2007;7:467-477. |
| 153. | Meijer MJ, Mieremet-Ooms MA, van der Zon AM, van Duijn W, van Hogezand RA, Sier CF, Hommes DW, Lamers CB, Verspaget HW. Increased mucosal matrix metalloproteinase-1, -2, -3 and -9 activity in patients with inflammatory bowel disease and the relation with Crohn's disease phenotype. Dig Liver Dis. 2007;39:733-739. |
| 154. | Mosnier JF, Jarry A, Bou-Hanna C, Denis MG, Merlin D, Laboisse CL. ADAM15 upregulation and interaction with multiple binding partners in inflammatory bowel disease. Lab Invest. 2006;86:1064-1073. |
| 155. | Alexander JS, Zhu Y, Elrod JW, Alexander B, Coe L, Kalogeris TJ, Fuseler J. Reciprocal regulation of endothelial substrate adhesion and barrier function. Microcirculation. 2001;8:389-401. |
| 156. | Carden D, Xiao F, Moak C, Willis BH, Robinson-Jackson S, Alexander S. Neutrophil elastase promotes lung microvascular injury and proteolysis of endothelial cadherins. Am J Physiol. 1998;275:H385-H392. |
| 157. | Orlova VV, Economopoulou M, Lupu F, Santoso S, Chavakis T. Junctional adhesion molecule-C regulates vascular endothelial permeability by modulating VE-cadherin-mediated cell-cell contacts. J Exp Med. 2006;203:2703-2714. |
| 158. | Spalinger J, Patriquin H, Miron MC, Marx G, Herzog D, Dubois J, Dubinsky M, Seidman EG. Doppler US in patients with crohn disease: vessel density in the diseased bowel reflects disease activity. Radiology. 2000;217:787-791. |
| 159. | Danese S, Sans M, de la Motte C, Graziani C, West G, Phillips MH, Pola R, Rutella S, Willis J, Gasbarrini A. Angiogenesis as a novel component of inflammatory bowel disease pathogenesis. Gastroenterology. 2006;130:2060-2073. |
| 160. | Danese S, Papa A, Saibeni S, Repici A, Malesci A, Vecchi M. Inflammation and coagulation in inflammatory bowel disease: The clot thickens. Am J Gastroenterol. 2007;102:174-186. |
| 161. | Wakefield AJ, Sankey EA, Dhillon AP, Sawyerr AM, More L, Sim R, Pittilo RM, Rowles PM, Hudson M, Lewis AA. Granulomatous vasculitis in Crohn's disease. Gastroenterology. 1991;100:1279-1287. |
| 162. | Ginnan R, Guikema BJ, Halligan KE, Singer HA, Jourd'heuil D. Regulation of smooth muscle by inducible nitric oxide synthase and NADPH oxidase in vascular proliferative diseases. Free Radic Biol Med. 2008;44:1232-1245. |
| 163. | Ganta VC, Cromer W, Mills GL, Traylor J, Jennings M, Daley S, Clark B, Mathis JM, Bernas M, Boktor M. Angiopoietin-2 in experimental colitis. Inflamm Bowel Dis. 2010;16:1029-1039. |
| 164. | Morris GP, Beck PL, Herridge MS, Depew WT, Szewczuk MR, Wallace JL. Hapten-induced model of chronic inflammation and ulceration in the rat colon. Gastroenterology. 1989;96:795-803. |
| 165. | Peterson TC, Cleary CE, Shaw AM, Malatjalian DA, Veldhuyzen van Zanten SJ. Therapeutic role for bismuth compounds in TNBS-induced colitis in the rat. Dig Dis Sci. 2000;45:466-473. |
| 166. | Chidlow JH Jr, Langston W, Greer JJ, Ostanin D, Abdelbaqi M, Houghton J, Senthilkumar A, Shukla D, Mazar AP, Grisham MB. Differential angiogenic regulation of experimental colitis. Am J Pathol. 2006;169:2014-2030. |
| 167. | Scaldaferri F, Vetrano S, Sans M, Arena V, Straface G, Stigliano E, Repici A, Sturm A, Malesci A, Panes J. VEGF-A links angiogenesis and inflammation in inflammatory bowel disease pathogenesis. Gastroenterology. 2009;136:585-95.e5. |
| 168. | Kenet G, Wardi J, Avni Y, Aeed H, Shirin H, Zaidel L, Hershkoviz R, Bruck R. Amelioration of experimental colitis by thalidomide. Isr Med Assoc J. 2001;3:644-648. |
| 169. | Maconi G, Sampietro GM, Russo A, Bollani S, Cristaldi M, Parente F, Dottorini F, Bianchi Porro G. The vascularity of internal fistulae in Crohn's disease: an in vivo power Doppler ultrasonography assessment. Gut. 2002;50:496-500. |
| 170. | Wallace JL, Le T, Carter L, Appleyard CB, Beck PL. Hapten-induced chronic colitis in the rat: alternatives to trinitrobenzene sulfonic acid. J Pharmacol Toxicol Methods. 1995;33:237-239. |
| 171. | Funayama Y, Sasaki I, Naito H, Fukushima K, Matsuno S, Masuda T. Remodeling of vascular wall in Crohn's disease. Dig Dis Sci. 1999;44:2319-2323. |
| 172. | Angerson WJ, Allison MC, Baxter JN, Russell RI. Neoterminal ileal blood flow after ileocolonic resection for Crohn's disease. Gut. 1993;34:1531-1534. |
| 173. | Olsson AK, Dimberg A, Kreuger J, Claesson-Welsh L. VEGF receptor signalling - in control of vascular function. Nat Rev Mol Cell Biol. 2006;7:359-371. |
| 174. | Usui T, Ishida S, Yamashiro K, Kaji Y, Poulaki V, Moore J, Moore T, Amano S, Horikawa Y, Dartt D. VEGF164(165) as the pathological isoform: differential leukocyte and endothelial responses through VEGFR1 and VEGFR2. Invest Ophthalmol Vis Sci. 2004;45:368-374. |
| 175. | Bousvaros A, Leichtner A, Zurakowski D, Kwon J, Law T, Keough K, Fishman S. Elevated serum vascular endothelial growth factor in children and young adults with Crohn's disease. Dig Dis Sci. 1999;44:424-430. |
| 176. | Ozawa CR, Banfi A, Glazer NL, Thurston G, Springer ML, Kraft PE, McDonald DM, Blau HM. Microenvironmental VEGF concentration, not total dose, determines a threshold between normal and aberrant angiogenesis. J Clin Invest. 2004;113:516-527. |
| 177. | Danese S, Sans M, Spencer DM, Beck I, Doñate F, Plunkett ML, de la Motte C, Redline R, Shaw DE, Levine AD. Angiogenesis blockade as a new therapeutic approach to experimental colitis. Gut. 2007;56:855-862. |
| 178. | Appleton I, Brown NJ, Willis D, Colville-Nash PR, Alam C, Brown JR, Willoughby DA. The role of vascular endothelial growth factor in a murine chronic granulomatous tissue air pouch model of angiogenesis. J Pathol. 1996;180:90-94. |
| 179. | Edelman JL, Castro MR, Wen Y. Correlation of VEGF expression by leukocytes with the growth and regression of blood vessels in the rat cornea. Invest Ophthalmol Vis Sci. 1999;40:1112-1123. |
| 180. | Petersen W, Tsokos M, Pufe T. Expression of VEGF121 and VEGF165 in hypertrophic chondrocytes of the human growth plate and epiphyseal cartilage. J Anat. 2002;201:153-157. |
| 181. | Lu J, Kasama T, Kobayashi K, Yoda Y, Shiozawa F, Hanyuda M, Negishi M, Ide H, Adachi M. Vascular endothelial growth factor expression and regulation of murine collagen-induced arthritis. J Immunol. 2000;164:5922-5927. |
| 182. | Kevil CG, De Benedetti A, Payne DK, Coe LL, Laroux FS, Alexander JS. Translational regulation of vascular permeability factor by eukaryotic initiation factor 4E: implications for tumor angiogenesis. Int J Cancer. 1996;65:785-790. |
| 183. | Griga T, Gutzeit A, Sommerkamp C, May B. Increased production of vascular endothelial growth factor by peripheral blood mononuclear cells in patients with inflammatory bowel disease. Eur J Gastroenterol Hepatol. 1999;11:175-179. |
| 184. | Birmingham JM, Busik JV, Hansen-Smith FM, Fenton JI. Novel mechanism for obesity-induced colon cancer progression. Carcinogenesis. 2009;30:690-697. |
| 185. | Kusumanto YH, Dam WA, Hospers GA, Meijer C, Mulder NH. Platelets and granulocytes, in particular the neutrophils, form important compartments for circulating vascular endothelial growth factor. Angiogenesis. 2003;6:283-287. |
| 186. | Kucharzik T, Walsh SV, Chen J, Parkos CA, Nusrat A. Neutrophil transmigration in inflammatory bowel disease is associated with differential expression of epithelial intercellular junction proteins. Am J Pathol. 2001;159:2001-2009. |
| 187. | Perminow G, Reikvam DH, Lyckander LG, Brandtzaeg P, Vatn MH, Carlsen HS. Increased number and activation of colonic macrophages in pediatric patients with untreated Crohn's disease. Inflamm Bowel Dis. 2009;15:1368-1378. |
| 188. | Upile T, Jerjes W, Kafas P, Harini S, Singh SU, Guyer M, Bentley M, Sudhoff H, Hopper C. Salivary VEGF: a non-invasive angiogenic and lymphangiogenic proxy in head and neck cancer prognostication. Int Arch Med. 2009;2:12. |
| 189. | Krzystek-Korpacka M, Neubauer K, Matusiewicz M. Platelet-derived growth factor-BB reflects clinical, inflammatory and angiogenic disease activity and oxidative stress in inflammatory bowel disease. Clin Biochem. 2009;42:1602-1609. |
| 190. | Del Zotto B, Mumolo G, Pronio AM, Montesani C, Tersigni R, Boirivant M. TGF-beta1 production in inflammatory bowel disease: differing production patterns in Crohn's disease and ulcerative colitis. Clin Exp Immunol. 2003;134:120-126. |
| 191. | Beck PL, Podolsky DK. Growth factors in inflammatory bowel disease. Inflamm Bowel Dis. 1999;5:44-60. |
| 192. | Ma Y, Guan Q, Bai A, Weiss CR, Hillman CL, Ma A, Zhou G, Qing G, Peng Z. Targeting TGF-beta1 by employing a vaccine ameliorates fibrosis in a mouse model of chronic colitis. Inflamm Bowel Dis. 2010;16:1040-1050. |
| 193. | Rieder F, Fiocchi C. Intestinal fibrosis in IBD--a dynamic, multifactorial process. Nat Rev Gastroenterol Hepatol. 2009;6:228-235. |
| 194. | Zeisberg EM, Tarnavski O, Zeisberg M, Dorfman AL, McMullen JR, Gustafsson E, Chandraker A, Yuan X, Pu WT, Roberts AB. Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nat Med. 2007;13:952-961. |
| 195. | Sundberg C, Ivarsson M, Gerdin B, Rubin K. Pericytes as collagen-producing cells in excessive dermal scarring. Lab Invest. 1996;74:452-466. |
| 196. | Bousvaros A, Zurakowski D, Fishman SJ, Keough K, Law T, Sun C, Leichtner AM. Serum basic fibroblast growth factor in pediatric Crohn's disease. Implications for wound healing. Dig Dis Sci. 1997;42:378-386. |
| 197. | Mannaioni PF, Di Bello MG, Masini E. Platelets and inflammation: role of platelet-derived growth factor, adhesion molecules and histamine. Inflamm Res. 1997;46:4-18. |
| 198. | Di Lorenzo A, Fernández-Hernando C, Cirino G, Sessa WC. Akt1 is critical for acute inflammation and histamine-mediated vascular leakage. Proc Natl Acad Sci USA. 2009;106:14552-14557. |
| 199. | Dulic-Sills A, Blunden MJ, Mawdsley J, Bastin AJ, McAuley D, Griffiths M, Rampton DS, Yaqoob MM, Macey MG, Agrawal SG. New flow cytometric technique for the evaluation of circulating endothelial progenitor cell levels in various disease groups. J Immunol Methods. 2006;316:107-115. |
| 200. | Jakobsson L, Bentley K, Gerhardt H. VEGFRs and Notch: a dynamic collaboration in vascular patterning. Biochem Soc Trans. 2009;37:1233-1236. |
| 201. | Gerhardt H, Golding M, Fruttiger M, Ruhrberg C, Lundkvist A, Abramsson A, Jeltsch M, Mitchell C, Alitalo K, Shima D. VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J Cell Biol. 2003;161:1163-1177. |
| 203. | Fiedler U, Augustin HG. Angiopoietins: a link between angiogenesis and inflammation. Trends Immunol. 2006;27:552-558. |
| 204. | Fiedler U, Scharpfenecker M, Koidl S, Hegen A, Grunow V, Schmidt JM, Kriz W, Thurston G, Augustin HG. The Tie-2 ligand angiopoietin-2 is stored in and rapidly released upon stimulation from endothelial cell Weibel-Palade bodies. Blood. 2004;103:4150-4156. |
| 205. | Fujiyama S, Matsubara H, Nozawa Y, Maruyama K, Mori Y, Tsutsumi Y, Masaki H, Uchiyama Y, Koyama Y, Nose A. Angiotensin AT(1) and AT(2) receptors differentially regulate angiopoietin-2 and vascular endothelial growth factor expression and angiogenesis by modulating heparin binding-epidermal growth factor (EGF)-mediated EGF receptor transactivation. Circ Res. 2001;88:22-29. |
| 206. | Chen YH, Wu HL, Chen CK, Huang YH, Yang BC, Wu LW. Angiostatin antagonizes the action of VEGF-A in human endothelial cells via two distinct pathways. Biochem Biophys Res Commun. 2003;310:804-810. |
| 207. | Sandor Z, Deng XM, Khomenko T, Tarnawski AS, Szabo S. Altered angiogenic balance in ulcerative colitis: a key to impaired healing? Biochem Biophys Res Commun. 2006;350:147-150. |
| 208. | Cornelius LA, Nehring LC, Harding E, Bolanowski M, Welgus HG, Kobayashi DK, Pierce RA, Shapiro SD. Matrix metalloproteinases generate angiostatin: effects on neovascularization. J Immunol. 1998;161:6845-6852. |
| 209. | Folkman J. Antiangiogenesis in cancer therapy--endostatin and its mechanisms of action. Exp Cell Res. 2006;312:594-607. |
| 210. | Deng X, Tolstanova G, Khomenko T, Chen L, Tarnawski A, Szabo S, Sandor Z. Mesalamine restores angiogenic balance in experimental ulcerative colitis by reducing expression of endostatin and angiostatin: novel molecular mechanism for therapeutic action of mesalamine. J Pharmacol Exp Ther. 2009;331:1071-1078. |
| 211. | Ambati BK, Nozaki M, Singh N, Takeda A, Jani PD, Suthar T, Albuquerque RJ, Richter E, Sakurai E, Newcomb MT. Corneal avascularity is due to soluble VEGF receptor-1. Nature. 2006;443:993-997. |
| 212. | Ambati BK, Patterson E, Jani P, Jenkins C, Higgins E, Singh N, Suthar T, Vira N, Smith K, Caldwell R. Soluble vascular endothelial growth factor receptor-1 contributes to the corneal antiangiogenic barrier. Br J Ophthalmol. 2007;91:505-508. |
| 213. | Albuquerque RJ, Hayashi T, Cho WG, Kleinman ME, Dridi S, Takeda A, Baffi JZ, Yamada K, Kaneko H, Green MG. Alternatively spliced vascular endothelial growth factor receptor-2 is an essential endogenous inhibitor of lymphatic vessel growth. Nat Med. 2009;15:1023-1030. |
| 214. | Bates DO, Cui TG, Doughty JM, Winkler M, Sugiono M, Shields JD, Peat D, Gillatt D, Harper SJ. VEGF165b, an inhibitory splice variant of vascular endothelial growth factor, is down-regulated in renal cell carcinoma. Cancer Res. 2002;62:4123-4131. |
| 215. | Perrin RM, Konopatskaya O, Qiu Y, Harper S, Bates DO, Churchill AJ. Diabetic retinopathy is associated with a switch in splicing from anti- to pro-angiogenic isoforms of vascular endothelial growth factor. Diabetologia. 2005;48:2422-2427. |
| 216. | Varey AH, Rennel ES, Qiu Y, Bevan HS, Perrin RM, Raffy S, Dixon AR, Paraskeva C, Zaccheo O, Hassan AB. VEGF 165 b, an antiangiogenic VEGF-A isoform, binds and inhibits bevacizumab treatment in experimental colorectal carcinoma: balance of pro- and antiangiogenic VEGF-A isoforms has implications for therapy. Br J Cancer. 2008;98:1366-1379. |
| 217. | Nowak DG, Woolard J, Amin EM, Konopatskaya O, Saleem MA, Churchill AJ, Ladomery MR, Harper SJ, Bates DO. Expression of pro- and anti-angiogenic isoforms of VEGF is differentially regulated by splicing and growth factors. J Cell Sci. 2008;121:3487-3495. |
| 218. | Leong TT, Fearon U, Veale DJ. Angiogenesis in psoriasis and psoriatic arthritis: clues to disease pathogenesis. Curr Rheumatol Rep. 2005;7:325-329. |
| 219. | Veale DJ, Fearon U. Inhibition of angiogenic pathways in rheumatoid arthritis: potential for therapeutic targeting. Best Pract Res Clin Rheumatol. 2006;20:941-947. |
| 220. | Cordiali-Fei P, Trento E, D'Agosto G, Bordignon V, Mussi A, Ardigó M, Mastroianni A, Vento A, Solivetti F, Berardesca E. Effective therapy with anti-TNF-alpha in patients with psoriatic arthritis is associated with decreased levels of metalloproteinases and angiogenic cytokines in the sera and skin lesions. Ann N Y Acad Sci. 2007;1110:578-589. |
| 222. | Rafiee P, Heidemann J, Ogawa H, Johnson NA, Fisher PJ, Li MS, Otterson MF, Johnson CP, Binion DG. Cyclosporin A differentially inhibits multiple steps in VEGF induced angiogenesis in human microvascular endothelial cells through altered intracellular signaling. Cell Commun Signal. 2004;2:3. |
| 223. | Carvalho AT, Souza H, Carneiro AJ, Castelo-Branco M, Madi K, Schanaider A, Silv F, Pereira Junior FA, Pereira MG, Tortori C. Therapeutic and prophylactic thalidomide in TNBS-induced colitis: synergistic effects on TNF-alpha, IL-12 and VEGF production. World J Gastroenterol. 2007;13:2166-2173. |
| 224. | Ogawa H, Rafiee P, Fisher PJ, Johnson NA, Otterson MF, Binion DG. Butyrate modulates gene and protein expression in human intestinal endothelial cells. Biochem Biophys Res Commun. 2003;309:512-519. |
| 225. | Kruh J, Defer N, Tichonicky L. [Molecular and cellular action of butyrate]. C R Seances Soc Biol Fil. 1992;186:12-25. |
| 226. | Ogawa H, Rafiee P, Fisher PJ, Johnson NA, Otterson MF, Binion DG. Sodium butyrate inhibits angiogenesis of human intestinal microvascular endothelial cells through COX-2 inhibition. FEBS Lett. 2003;554:88-94. |
| 227. | Rafiee P, Stein DJ, Nelson VM, Otterson MF, Shaker R, Binion DG. Thalidomide inhibits inflammatory and angiogenic activation of human intestinal microvascular endothelial cells (HIMEC). Am J Physiol Gastrointest Liver Physiol. 2010;298:G167-G176. |
| 228. | Rafiee P, Johnson CP, Li MS, Ogawa H, Heidemann J, Fisher PJ, Lamirand TH, Otterson MF, Wilson KT, Binion DG. Cyclosporine A enhances leukocyte binding by human intestinal microvascular endothelial cells through inhibition of p38 MAPK and iNOS. Paradoxical proinflammatory effect on the microvascular endothelium. J Biol Chem. 2002;277:35605-35615. |
| 229. | Choi PM, Zelig MP. Similarity of colorectal cancer in Crohn's disease and ulcerative colitis: implications for carcinogenesis and prevention. Gut. 1994;35:950-954. |
| 230. | Karlén P, Löfberg R, Broström O, Leijonmarck CE, Hellers G, Persson PG. Increased risk of cancer in ulcerative colitis: a population-based cohort study. Am J Gastroenterol. 1999;94:1047-1052. |
| 231. | Gyde SN, Prior P, Allan RN, Stevens A, Jewell DP, Truelove SC, Lofberg R, Brostrom O, Hellers G. Colorectal cancer in ulcerative colitis: a cohort study of primary referrals from three centres. Gut. 1988;29:206-217. |
| 232. | Wagner AD, Arnold D, Grothey AA, Haerting J, Unverzagt S. Anti-angiogenic therapies for metastatic colorectal cancer. Cochrane Database Syst Rev. 2009;CD005392. |
| 233. | Sonoshita M, Takaku K, Oshima M, Sugihara K, Taketo MM. Cyclooxygenase-2 expression in fibroblasts and endothelial cells of intestinal polyps. Cancer Res. 2002;62:6846-6849. |
| 234. | Oshima M, Dinchuk JE, Kargman SL, Oshima H, Hancock B, Kwong E, Trzaskos JM, Evans JF, Taketo MM. Suppression of intestinal polyposis in Apc delta716 knockout mice by inhibition of cyclooxygenase 2 (COX-2). Cell. 1996;87:803-809. |
| 235. | Oshima M, Taketo MM. COX selectivity and animal models for colon cancer. Curr Pharm Des. 2002;8:1021-1034. |
| 236. | Paiotti AP, Miszputen SJ, Oshima CT, de Oliveira Costa H, Ribeiro DA, Franco M. Effect of COX-2 inhibitor after TNBS-induced colitis in Wistar rats. J Mol Histol. 2009;40:317-324. |
| 237. | Binion DG, Otterson MF, Rafiee P. Curcumin inhibits VEGF-mediated angiogenesis in human intestinal microvascular endothelial cells through COX-2 and MAPK inhibition. Gut. 2008;57:1509-1517. |
| 238. | Klenke FM, Gebhard MM, Ewerbeck V, Abdollahi A, Huber PE, Sckell A. The selective Cox-2 inhibitor Celecoxib suppresses angiogenesis and growth of secondary bone tumors: an intravital microscopy study in mice. BMC Cancer. 2006;6:9. |
| 240. | Chan AT, Ogino S, Fuchs CS. Aspirin and the risk of colorectal cancer in relation to the expression of COX-2. N. Engl J Med. 2007;356:2131-2142. |
| 241. | Park YS. [COX-2 inhibitors in inflammatory bowel disease: friends or foes?]. Korean J Gastroenterol. 2007;50:350-355. |
| 242. | Wu AW, Gu J, Li ZF, Ji JF, Xu GW. COX-2 expression and tumor angiogenesis in colorectal cancer. World J Gastroenterol. 2004;10:2323-2326. |
| 243. | Ishikawa TO, Herschman HR. Tumor formation in a mouse model of colitis-associated colon cancer does not require COX-1 or COX-2 expression. Carcinogenesis. 2010;31:729-736. |