Published online Dec 14, 2011. doi: 10.3748/wjg.v17.i46.5117
Revised: May 26, 2011
Accepted: June 2, 2011
Published online: December 14, 2011
AIM: To evaluate the influence of taking low-dose aspirin for 4 wk on small intestinal complications and to examine the preventive effect of rebamipide.
METHODS: This study was conducted as a single-center, randomized, double-blind, cross-over, placebo-controlled study. Eleven healthy male subjects were enrolled. Each subject underwent video capsule endoscopy after 1 and 4 wk of taking aspirin and omeprazole, along with either rebamipide or placebo therapy. The primary endpoint was to evaluate small bowel damage in healthy subjects before and after taking low-dose aspirin for 4 wk.
RESULTS: The number of subjects with mucosal breaks (defined as multiple erosions and/or ulcers) were 1 at 1 wk and 1 at 4 wk on the jejunum, and 6 at 1 wk (P = 0.0061) and 7 at 4 wk on the ileum (P = 0.0019). Rebamipide significantly prevented mucosal breaks on the ileum compared with the placebo group (P = 0.0173 at 1 wk and P = 0.0266 at 4 wk).
CONCLUSION: Longer-term, low-dose aspirin administration induced damage in the small bowel. Rebamipide prevented this damage, and may be a candidate drug for treating aspirin-induced small bowel complications.
- Citation: Mizukami K, Murakami K, Abe T, Inoue K, Uchida M, Okimoto T, Kodama M, Fujioka T. Aspirin-induced small bowel injuries and the preventive effect of rebamipide. World J Gastroenterol 2011; 17(46): 5117-5122
- URL: https://www.wjgnet.com/1007-9327/full/v17/i46/5117.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i46.5117
Video capsule endoscopy (VCE)[1-2] is a practical technique that can be used to identify the causes and sites of obscure gastrointestinal bleeding. VCE allows for prospective investigation of small intestinal injuries, which frequently occur following the administration of nonsteroidal anti-inflammatory drugs (NSAIDs), such as aspirin. For example, Graham et al[3] used VCE and reported that small intestinal injury occurred in 71% of chronic NSAID users.
Low-dose aspirin is currently recommended for the secondary prevention of cardiovascular and cerebral diseases[4-6]. An observational registry reported that 70% to 80% of patients with a high risk of atherothrombosis were receiving low-dose aspirin to prevent future vascular events[7]. Nonetheless, low-dose aspirin is not without risks. For instance, Lanas et al[8]reported that taking an anti-platelet agent induced lower, as well as upper, gastrointestinal (GI) events (16.9% and 15.5%, respectively). To date, there has been considerable interest in preventing upper gastrointestinal complications of NSAIDs; however, it has become clear that a strategy to prevent small bowel complications may also be needed[9].
It is not yet clear what duration of low-dose aspirin ingestion causes small bowel damage. Moreover, the frequency and severity of small bowel damage from taking low-dose aspirin is not yet known. Three recent reports investigating aspirin-induced small bowel damage were all short-term (1 to 2 wk) investigations[10-12]. These studies reported mild injuries (20% to 60%), such as erosion, in addition to more serious injuries (0 to 10%), such as ulcers[10-12]. The use of low-dose aspirin for cardiovascular prophylaxis is generally long-term, and it is clear that longer term observational studies are needed to examine damage to the small intestines.
The aims of this study were to investigate the frequency and type of small bowel damage associated with a 4 wk administration of low-dose aspirin in healthy subjects and to investigate the preventive effect of the cytoprotective agent, rebamipide, on aspirin-induced small bowel damage.
The study protocol was approved by the Ethics Committees of Oita University, and written informed consent was obtained from all subjects. Eligible subjects were aged 20 to 65 years, who had taken no drugs during the one-month period prior to the start of this study and who had normal physical examinations. The exclusion criteria were as follows: (1) subjects who did not have a full length, small bowel VCE prior to the start of the study; (2) subjects with stenosis, tumors, ulcers, erosions, or bleeding in the small bowel; (3) subjects who had active GI disease or a history of ulcers, surgery, or bleeding; and (4) subjects who had used any medication, including NSAIDs or aspirin, within 4 wk of the start of the study.
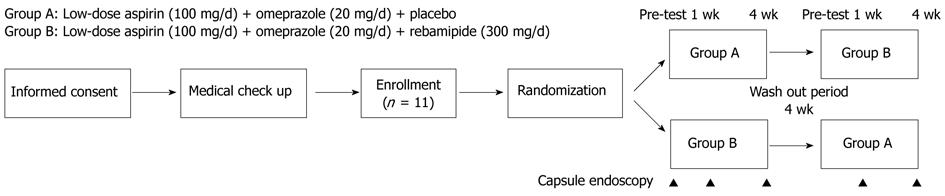
This study was conducted using a cross-over design as shown in Figure 1. Medication groups A and B were defined as follows: group A: placebo plus aspirin (Bayer Pharmaceutical Co., Ltd., Tokyo, Japan) plus omeprazole (Sawai Pharmaceutical Co., Ltd., Osaka, Japan) for 4 wk; and group B: rebamipide (Otsuka Pharmaceutical Co., Ltd., Tokyo, Japan) plus aspirin plus omeprazole for 4 wk (rebamipide 300 mg or placebo tid, aspirin 100 mg od, omeprazole 20 mg od). Omeprazole was used primarily for ethical reasons to avoid the effect of aspirin on the stomach.
Medications were administered for 4 wk. Then following a 4-wk washout period, the treatments were reversed for the two groups, and the medications were administered for a second 4-wk period. The washout period was designed according to a previous study by Niwa[13]. VCEs of the small bowel were performed five times: prior to the intervention, at 1 and 4 wk during the first period, and at 1 and 4 wk during the second treatment period.
Allocation and randomization were conducted by an independent pharmacologist of Yamanami Pharmacy who had no connection to our institution or the results of this study. The placebo was prepared by Yamanami Pharmacy.
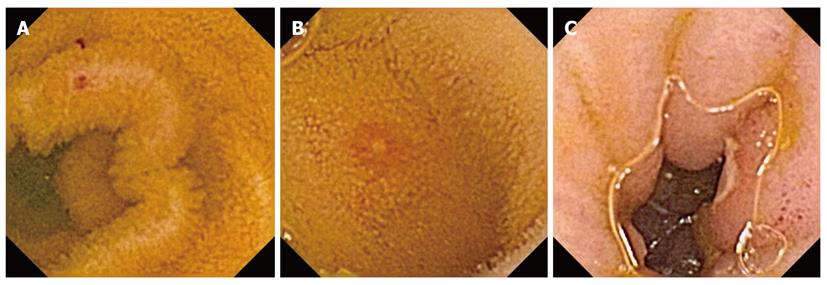
In this study, erosion and ulcer were defined according to Graham’s report[3]. Red spots were defined as red areas without clear mucosal break. Erosions were defined as circumscribed areas of mucosal disruption denuded of villi with or without exudates or red color that involved a diameter equivalent to the valvulae conniventes. Ulcers were defined as erosions with a central area with exudates typically having a surrounding border of elevated mucosa, producing a target lesion or coral polyp appearance[3]. Typical cases of red spots and erosion are shown in Figures 2A and B, and an example of an ulcer is shown in Figure 2C.
Mucosal break was defined as two or more erosions and/or an ulcer. Red spots were scored, but were not considered as a significant injury, since they can be observed normally. The location of the injury was also scored in terms of the locations as proximal (jejunal) or distal (ileal) based on the transit time. The transit time from the pylorus to cecum was divided in half, and the first portion was arbitrarily defined as the jejunal section. Cases with erosion, multiple erosions, and ulcers were calculated. Multiple erosions were defined as more than 2 erosions in a subject. Subjects were only analyzed if the entire small bowel was observable by VCE.
The primary endpoint was to evaluate healthy subjects before and after 4 wk of low-dose aspirin-induced small bowel damage. The secondary endpoint was to evaluate the preventive effect of rebamipide.
We used the Olympus video capsule system (EndoCapsule, Olympus Ltd.; Tokyo, Japan) for this study. The capsule endoscopy procedure and the methodology for reviewing the images were conducted as previously described. All video images were analyzed twice by each skilled reviewer (KM, TA, and KI). These three investigators were instructed to mark any significant lesions under blinded conditions, and to evaluate lesions according to criteria for determination of the endpoints. If the results differed between the reviewers, then they consulted one another to achieve a consensus. All images were saved for a final comprehensive analysis upon completion of all of the post-treatment capsule endoscopies.
The subjects’ symptoms were observed daily throughout the study periods, and the information was evaluated using a patient diary.
The primary endpoint was to evaluate the proportion of healthy subjects with small intestinal injury after 4 wk of low-dose aspirin therapy. The number of healthy subjects with small bowel mucosal breaks, ulcers, erosions, multiple erosions, and red spots were calculated and treated as parametric parameters. The small bowel area was divided into the jejunum and the ileum. The number of small bowel ulcers, erosions, and red spots were described as the mean ± SD. These values were analyzed at each measured point of the VCE in each group using Mann-Whitney’s U test and compared with the evaluation prior to the aspirin administration.
The secondary endpoint was to compare the number of small intestinal injuries between the placebo and the rebamipide groups. The injuries were described as means ± SD and treated as nonparametric parameters. These injuries were analyzed at each measured point of the VCE in each group using Mann-Whitney’s U test. In addition, the small intestinal injuries were compared between the placebo and rebamipide groups.
This study was a pilot study. Therefore, there were no reference data to calculate the sample size. A P-value < 0.05 was considered significant. All statistical analyses were performed using SAS version 8.2 (SAS Institute, Cary, NC, United States).
Eleven healthy subjects were enrolled in this study. The mean age of the subjects was 30 ± 6 years. The median age was 27 years, and the range was 24 to 43 years.
The number of subjects with multiple erosions, ulcers, and mucosal breaks of the small bowel are shown in Table 1. There were 8 subjects with red spots on the jejunum before the administration of aspirin, 10 subjects at 1 wk, and 10 at 4 wk. There were 8 subjects with red spots on the ileum before administration of aspirin, 11 subjects at 1 wk, and 11 at 4 wk. There were 2 subjects with small bowel erosion on the jejunum at 1 wk and 4 at 4 wk (P = 0.0379). There were 7 subjects with small bowel erosion on the ileum at 1 wk (P = 0.0019) and 9 at 4 wk (P < 0.0001).
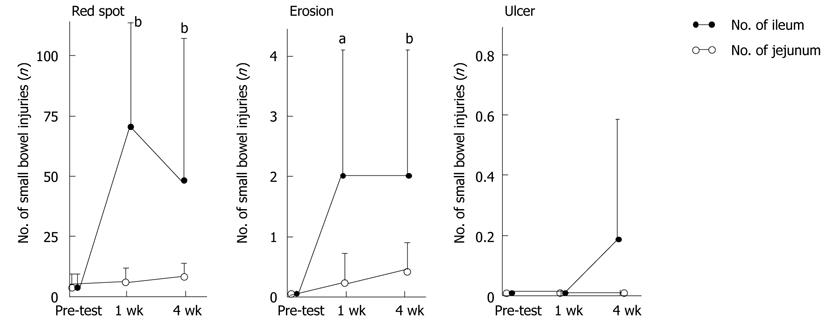
The numbers of small bowel injuries before and after aspirin treatment are shown in Figure 3. There were 6.3 ± 6.9 small intestinal red spots at 1 wk and 9.6 ± 6.6 at 4 wk on the jejunum, and 68.0 ± 133.6 at 1 wk (P = 0.0010) and 48.4 ± 67.7 at 4 wk (P = 0.0010) on the ileum. There were 0.2 ± 0.6 small bowel erosions at 1 wk and 0.4 ± 0.6 at 4 wk on the jejunum, and 2.1 ± 2.6 at 1 wk (P = 0.0156) and 2.0 ± 2.5 at 4 wk (P = 0.0039) on the ileum. There were no small intestinal ulcers at 1 wk and at 4 wk on the jejunum, and there were no small intestinal ulcers at 1 wk and 0.2 ± 0.4 ulcers at 4 wk on the ileum.
The preventive effect of rebamipide is shown in Table 1. There were no subjects with small bowel mucosal breaks on the jejunum at 1 wk and 1 subject at 4 wk, and there was 1 small bowel mucosal break on the ileum at 1 wk and 2 at 4 wk. Rebamipide significantly prevented small bowel mucosal breaks on the ileum compared with the placebo group (P = 0.0173 at 1 wk and P = 0.0266 at 4 wk). There were 15.7 ± 8.5 red spots on the ileum at 4 wk in the rebamipide group, which was significantly fewer than in the placebo group (P = 0.0354). There were 0.6 ± 1.2 erosions on the ileum at 4 wk, which was significantly fewer that in the placebo group (P = 0.0362).
We examined symptoms daily for all subjects throughout the study period. Three subjects with ulcers were observed in the placebo group. Two of these subjects were symptomatic, but not anemic. The symptoms of these two subjects diminished 1 month after the study ended. One subject with an ulcer was observed in the rebamipide group, but the subject was neither symptomatic nor anemic.
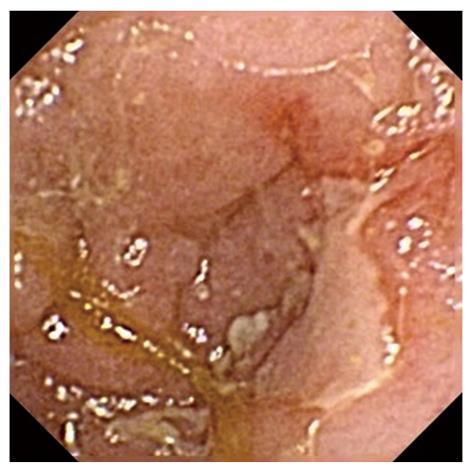
In our study, we observed that the administration of low-dose aspirin induced small bowel red spots and erosions in healthy subjects in the early phase (1 wk). Red spots were observed in all subjects with a mean of 68.0 ± 133.6 at 1 wk and 48.4 ± 67.7 at 4 wk. There were 7 subjects with erosions on the ileum at 1 wk and 9 at 4 wk in the placebo group. These small bowel injuries were induced in the early phase and maintained while taking low-dose aspirin. On the other hand, small intestinal ulcers were observed in 3 subjects in the late phase (4 wk), although no ulcers were observed at 1 wk. These results indicate that serious small bowel injury, such as ulcers, may be induced by longer-term administration of low-dose aspirin. In addition, a very large ulcer was observed in one case at 4 wk (Figure 4), although no ulcer was observed in this subject at 1 wk. This may demonstrate a risk of longer-term low-dose aspirin ingestion. Patients taking low-dose aspirin should be given periodic management. In this study, small bowel bleeding was not observed; however, some patients may present small bowel bleeding due to the use of low-dose aspirin during treatment periods longer than 4 wk. The clinical implications of small bowel mucosal injuries are not yet clear. However, bleeding from mucosal injury can be fatal because aspirin is an anti-platelet agent. In this study, multiple erosions were also evaluated. They resulted in a more serious condition than a single erosion.
Our results indicate that most small bowel injuries occurred in the ileum rather than the jejunum area. There were 7 cases with a mucosal break in the ileum and 1 in the jejunum at 4 wk in the placebo group. Bjarnason et al[14]demonstrated that small bowel damage depended on various factors, such as microvascular aspects, neutrophil recruitment, mucosal prostaglandins, decreased blood flow, increased permeability, and bacteria. The number and variation of bacteria in the ileum is reported to be greater than in the jejunum[15]. The results of this study may be a reflection of the different bacterial environment in the ileum compared with the jejunum.
We conducted a comparative study with placebo and rebamipide to observe whether rebamipide could prevent aspirin-induced small bowel injuries. Niwa et al[13] reported that rebamipide had a preventive effect on diclofenac-induced small-bowel injury compared to the placebo. The preventive ratio of diclofenac-induced small bowel mucosal breaks was 60% in the placebo group and 20% in the rebamipide group, and this difference was statistically significant. Moreover, Fujimori et al[16] demonstrated that the prostaglandin analogue misoprostol prevented diclofenac-induced small-intestinal complications. These two reports indicate the importance of prostaglandins.
Rebamipide {2-(4-chlorobenzoylamino)-3-[2-(1H)-quinolinon-4-yl]-propionic acid} is a cytoprotective antiulcer drug that stimulates the production of endogenous prostaglandins[17]. Here, we observed that rebamipide significantly prevented small bowel mucosal breaks in the ileum area compared to the placebo group at both 1 wk and 4 wk. There were 3 subjects with ulcers in the ileum area at 4 wk in the placebo group, compared with 1 subject with an ulcer at 4 wk in the rebamipide group. The administration of rebamipide showed potential in preventing the incidence of ulcers; however, rebamipide does not have an anti-bacterial effect. The action of rebamipide may be explained by Bjarnason’s hypothesis[14]. Previous reports have indicated that rebamipide induces the production of intracellular prostaglandins[14], improves blood flow[18], suppresses increases in permeability[19], scavenges free radicals[20], and has an anti-inflammatory action[21]. Presently, there is no drug to prevent aspirin-induced small bowel complications or treat patients with these complications, so it is essential to research drugs with these properties in the future.
A limitation of this study is that the study size was small. We did not use a large number of patients, since this was a preliminary study. In addition, since the small bowel is a long organ, it will be necessary to further investigate the appropriate dosage for this candidate drug.
In conclusion, the use of long-term low-dose aspirin induced small bowel damage. Rebamipide prevented this damage, and this candidate drug may be suitable for preventing aspirin-induced small bowel complications.
The authors thank Dr. Shunji Fujimori for assistance with capsule endoscopy.
Low-dose aspirin is currently recommended for the secondary prevention of cardiovascular and cerebral diseases. Recently, aspirin-induced small bowel complications have become the focus of investigations around the world.
Few studies have observed healthy subjects with low-dose aspirin-induced, small bowel injuries using capsule endoscopy. These results demonstrated the prevalence of slight and low frequency small bowel injuries. However, these studies examined the effects of low-dose aspirin ingestion in the very short-term (1 or 2 wk). Here, we investigated the influence of small bowel damage following ingestion of low-dose aspirin for a longer period of 4 wk.
In this study, the ingestion of low-dose aspirin for 4 wk induced small bowel ulcers. In one case, a huge ulcer developed at 4 wk, although no ulcer was observed at 1 wk. Although it is common for patients to take low-dose aspirin for more than 4 wk, this duration of ingestion may be the limit in healthy subjects. Moreover, the results of this study demonstrate the differences in small bowel injuries after ingesting low-dose aspirin for 1 wk or 4 wk. Taking rebamipide was found to prevent aspirin-induced small bowel injuries.
The results of this study indicate that long-term use of low-dose aspirin can cause small intestinal injuries. Rebamipride could prevent this damage.
Rebamipide is a cytoprotective antiulcer drug that stimulates the production of endogenous prostaglandins. Its actions include scavenging free radicals, elevating blood flow, and suppressing permeability. In this study, capsule endoscopy was performed using the Olympus video capsule system (EndoCapsule, Olympus Ltd.; Tokyo, Japan).
This study focused on the influence of low-dose aspirin ingestion for 4 wk on small bowel damage in healthy subjects. The study was designed as a randomized, placebo-controlled, double-blind, cross-over study using video capsule endoscopy. The long-term (4 wk) use of low-dose aspirin induced small bowel damage. Rebamipide prevented this damage, and it may be a candidate drug for preventing aspirin-induced small bowel complications.
Peer reviewer: Rami Eliakim, Professor, Department of Gastroenterology, Rambam Medical Center, Haifa 31096, Israel
S- Editor Sun H L- Editor Logan S E- Editor Zhang DN
| 1. | Iddan G, Meron G, Glukhovsky A, Swain P. Wireless capsule endoscopy. Nature. 2000;405:417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1994] [Cited by in RCA: 1384] [Article Influence: 55.4] [Reference Citation Analysis (1)] |
| 2. | Gong F, Swain P, Mills T. Wireless endoscopy. Gastrointest Endosc. 2000;51:725-729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 97] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 3. | Graham DY, Opekun AR, Willingham FF, Qureshi WA. Visible small-intestinal mucosal injury in chronic NSAID users. Clin Gastroenterol Hepatol. 2005;3:55-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 404] [Cited by in RCA: 391] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 4. | Mehta P. Aspirin in the prophylaxis of coronary artery disease. Curr Opin Cardiol. 2002;17:552-558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 5. | Grosser T, Fries S, FitzGerald GA. Biological basis for the cardiovascular consequences of COX-2 inhibition: therapeutic challenges and opportunities. J Clin Invest. 2006;116:4-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 753] [Cited by in RCA: 730] [Article Influence: 38.4] [Reference Citation Analysis (0)] |
| 6. | Rothwell PM, Warlow CP. Timing of TIAs preceding stroke: time window for prevention is very short. Neurology. 2005;64:817-820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 287] [Cited by in RCA: 275] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 7. | Pearson TA, Blair SN, Daniels SR, Eckel RH, Fair JM, Fortmann SP, Franklin BA, Goldstein LB, Greenland P, Grundy SM. AHA Guidelines for Primary Prevention of Cardiovascular Disease and Stroke: 2002 Update: Consensus Panel Guide to Comprehensive Risk Reduction for Adult Patients Without Coronary or Other Atherosclerotic Vascular Diseases. American Heart Association Science Advisory and Coordinating Committee. Circulation. 2002;106:388-391. [PubMed] |
| 8. | Lanas A, García-Rodríguez LA, Polo-Tomás M, Ponce M, Alonso-Abreu I, Perez-Aisa MA, Perez-Gisbert J, Bujanda L, Castro M, Muñoz M. Time trends and impact of upper and lower gastrointestinal bleeding and perforation in clinical practice. Am J Gastroenterol. 2009;104:1633-1641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 454] [Cited by in RCA: 415] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 9. | Leung WK, Graham DY. Obscure gastrointestinal bleeding: where do we go from here? Gastroenterology. 2010;138:1655-1658. [PubMed] |
| 10. | Endo H, Hosono K, Inamori M, Kato S, Nozaki Y, Yoneda K, Akiyama T, Fujita K, Takahashi H, Yoneda M. Incidence of small bowel injury induced by low-dose aspirin: a crossover study using capsule endoscopy in healthy volunteers. Digestion. 2009;79:44-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 65] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 11. | Shiotani A, Haruma K, Nishi R, Fujita M, Kamada T, Honda K, Kusunoki H, Hata J, Graham DY. Randomized, double-blind, pilot study of geranylgeranylacetone versus placebo in patients taking low-dose enteric-coated aspirin. Low-dose aspirin-induced small bowel damage. Scand J Gastroenterol. 2010;45:292-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 41] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 12. | Smecuol E, Pinto Sanchez MI, Suarez A, Argonz JE, Sugai E, Vazquez H, Litwin N, Piazuelo E, Meddings JB, Bai JC. Low-dose aspirin affects the small bowel mucosa: results of a pilot study with a multidimensional assessment. Clin Gastroenterol Hepatol. 2009;7:524-529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 90] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 13. | Niwa Y, Nakamura M, Ohmiya N, Maeda O, Ando T, Itoh A, Hirooka Y, Goto H. Efficacy of rebamipide for diclofenac-induced small-intestinal mucosal injuries in healthy subjects: a prospective, randomized, double-blinded, placebo-controlled, cross-over study. J Gastroenterol. 2008;43:270-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 103] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 14. | Bjarnason I, Thjodleifsson B. Gastrointestinal toxicity of non-steroidal anti-inflammatory drugs: the effect of nimesulide compared with naproxen on the human gastrointestinal tract. Rheumatology (Oxford). 1999;38 Suppl 1:24-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 43] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 15. | Lin HC. Small intestinal bacterial overgrowth: a framework for understanding irritable bowel syndrome. JAMA. 2004;292:852-858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 265] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 16. | Fujimori S, Seo T, Gudis K, Ehara A, Kobayashi T, Mitsui K, Yonezawa M, Tanaka S, Tatsuguchi A, Sakamoto C. Prevention of nonsteroidal anti-inflammatory drug-induced small-intestinal injury by prostaglandin: a pilot randomized controlled trial evaluated by capsule endoscopy. Gastrointest Endosc. 2009;69:1339-1346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 98] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 17. | Arakawa T, Watanabe T, Fukuda T, Yamasaki K, Kobayashi K. Rebamipide, novel prostaglandin-inducer accelerates healing and reduces relapse of acetic acid-induced rat gastric ulcer. Comparison with cimetidine. Dig Dis Sci. 1995;40:2469-2472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 52] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 18. | Kim HK, Kim JI, Kim JK, Han JY, Park SH, Choi KY, Chung IS. Preventive effects of rebamipide on NSAID-induced gastric mucosal injury and reduction of gastric mucosal blood flow in healthy volunteers. Dig Dis Sci. 2007;52:1776-1782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 47] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 19. | Banan A, Fitzpatrick L, Zhang Y, Keshavarzian A. OPC-compounds prevent oxidant-induced carbonylation and depolymerization of the F-actin cytoskeleton and intestinal barrier hyperpermeability. Free Radic Biol Med. 2001;30:287-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 55] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 20. | Yoshikawa T, Naito Y, Tanigawa T, Kondo M. Free radical scavenging activity of the novel anti-ulcer agent rebamipide studied by electron spin resonance. Arzneimittelforschung. 1993;43:363-366. [PubMed] |
| 21. | Murakami K, Okajima K, Uchiba M, Harada N, Johno M, Okabe H, Takatsuki K. Rebamipide attenuates indomethacin-induced gastric mucosal lesion formation by inhibiting activation of leukocytes in rats. Dig Dis Sci. 1997;42:319-325. [PubMed] [DOI] [Full Text] |