Published online Dec 7, 2011. doi: 10.3748/wjg.v17.i45.5014
Revised: May 6, 2011
Accepted: May 13, 2011
Published online: December 7, 2011
AIM: To discuss the safety, feasibility and regularity of destruction to porcine spleen in vivo with congestion and tumescence by microwave ablation (MWA).
METHODS: Ligation of the splenic vein was used to induce congestion and tumescence in vivo in five porcine spleens, and microwave ablation was performed 2-4 h later. A total of 56 ablation points were ablated and the ablation powers were 30-100 W. The ablation time (1, 2, 3, 4, 5, 6, 7, 8, 9 and 10 min) was performed at a power of 60 W. After ablation, the ablation size was measured in pigs A, C, D and E and spleen resection. In pig B, the ablation size was measured and 2 ablation points were sent for pathology analysis and all tissues were sutured following ablation. Pig B was killed 1 wk later and the ablation points were sent for pathology analysis. Bleeding, tissue carbonization surrounding electrodes, and pathological changes were observed, and the effect on destruction volume relative to different ablation powers, times and positions was analyzed.
RESULTS: The incidence of bleeding (only small am-ounts, < 20 mL) in the course of ablation was 5.4% (3/56) and was attributed to tissue carbonization surrounding electrodes, which also exhibited an incidence of 5.4% (3/56). The destruction volume was influenced by different ablation powers, times and points. It showed that the ablation lesion size increased with increased ablation time, from 1 to 10 min, when the ablation power was 60 W. Also, the ablation lesion size increased with the increase of ablation power, ranging from 30 to 100 W when the ablation time was set to 3 min. A direct correlation was seen between the destruction volume and ablation time by the power of 60 W (r = 0.97542, P < 0.0001, and also between the destruction volume and ablation powers at an ablation time of 3 min (r = 0.98258, P < 0.0001). The destruction volume of zone II (the extra-2/3 part of the spleen, relative to the first or second class vascular branches), which was near the hilum of the spleen, was noteably larger than the destruction volume of zoneI(the intra-1/3 part of the spleen) which was distal from the hilum of the spleen (P = 0.0015). Pathological changes of ablation occurring immediately and 1 wk after MWA showed large areas of coagulation. Immediately following ablation, intact spleen tissues were observed in the areas of coagulation necrosis, mainly around arterioles, and there were no obvious signs of hydropsia and inflammation, while 1 wk following the ablation, the coagulation necrosis was well distributed and complete, as many nuclear fragmentations were detected, and there were obvious signs of hydropsia and inflammation.
CONCLUSION: In vivo treatment of congestion and tumescence in the spleen using microwave ablation of water-cooled antenna is a safe and feasible method that is minimally invasive.
-
Citation: Gao F, Gu YK, Shen JX, Li CL, Jiang XY, Huang JH. Experimental study of destruction to porcine spleen
in vivo by microwave ablation. World J Gastroenterol 2011; 17(45): 5014-5020 - URL: https://www.wjgnet.com/1007-9327/full/v17/i45/5014.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i45.5014
Minimally invasive methods for treatment of hypersple-nism, such as partial splenic embolization (PSE) and radiofrequency ablation (RFA), have been developed in recent years. PSE has been widely used, but is not optimal due to a high recurrence rate and the finding that the embolism volume varies based on the surgeon’s experience and the estimation of contrast medium’s flow rate, making it difficult to accurately evaluate embolism volume in real time[1]. In recent years, RFA has been used for the treatment of hypersplenism, since it has greater control over the destruction volume of the spleen; however, RFA runs a high risk of bleeding. In this study, we propose microwave ablation (MWA) as an effective method for controlling the destruction volume of the spleen. MWA has the added advantages of higher rate of temperature increase, high thermal efficiency, stable and controllable thermal field, good hemostasis effect, and good blood vessel coagulation[2,3]. This study describes the destruction to porcine spleen in vivo using microwave ablation with water-cooled antenna.
This work has been carried out in accordance with the Declaration of Helsinki (2000) of the World Medical Association. This study was approved ethically by Cancer Center, Sun Yat-sen University.
Five healthy Tibet pigs (assigned A, B, C, D and E), one female and four males aged 6-8 mo (weighing 32-39 kg) were selected for this study.
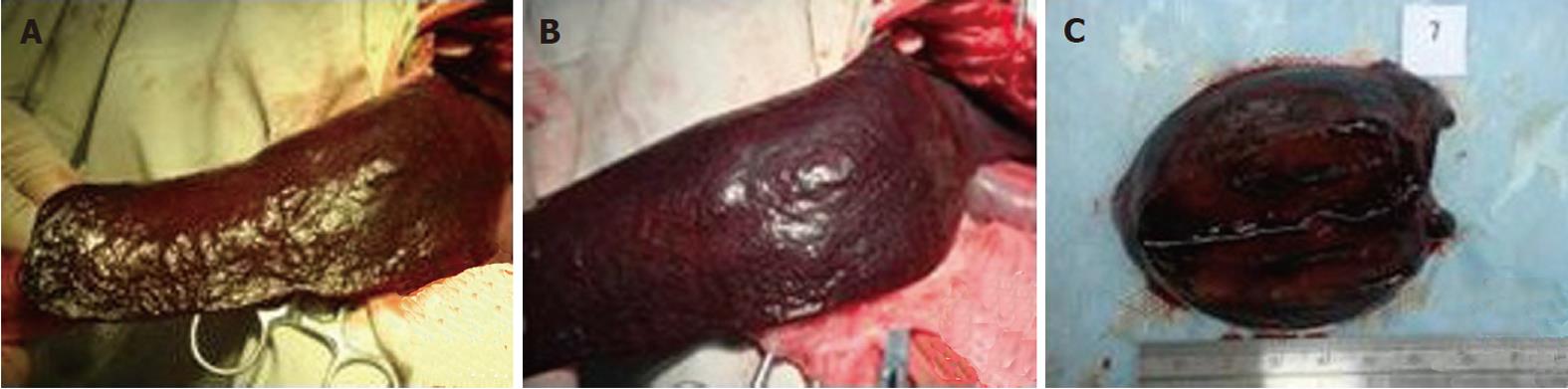
All the pigs were fasted for 12 h before operation and received atropine i.m. (0.5 mg; 30 min before operation), Su Mian Xin i.m. (1 mL/kg; 20 min before operation), and ketamine i.m. (8 mg/kg; 20 min before operation). After successful induction of anesthesia, the animal was fixed on the operation table. After abdominal skin preparation and sterilization, the skin, hypoderma, fat, muscle and peritoneum were cut open for complete exposure of the spleen. Then the hilum of the spleen was separated and the splenic artery and splenic vein were exposed. Next, the splenic vein was ligated using blood vessel forceps. Microwave ablation was performed when congestion and tumescence were seen, which was 2-4 h after ligation of the splenic vein. The ablation size was measured in pigs A, C, D and E after ablation and spleen resection. In pig B, the ablation size was measured and 2 ablation points were sent for pathology analysis and all tissues were sutured following ablation. Pig B was killed 1 wk later and the ablation points were sent for pathology analysis (Figure 1).
Specifications of water-cooled antenna: diameter 1.7 mm, model III with high-power (made by Qi Ya Medical Treatment Facility Limited Company, Nanjing, China; batch No. 090226003EX); Emission Facility: made by Qi Ya Medical Treatment Facility Limited Company, Nanjing, China; Frequency: 2450 MHz; Output power: 0-120 W; Precision of temperature control: ± 0.1 °C; Discharge waveform: continuous wave; operating conditions: main voltage AC 220% ± 10%, environmental temperature 5 °C-40 °C.
There were a total of 56 ablation points (8 in pig A, 12 in B, 14 in C, 13 in D and 9 in E). The ablation powers were 30, 40, 50, 60, 70, 80, 90 and 100 W. The ablation time (1, 2, 3, 4, 5, 6, 7, 8, 9 and 10 min) was performed at a power of 60 W. The whole spleen was divided into two sections: zeroI, which was adjacent to the hilum of the spleen (the intra-1/3 part of the spleen); and zero II, which was distal from the hilum of the spleen (the extra-2/3 part of the spleen), relative to the first or second class vascular branches.
The incidence of bleeding of the pin hole, tissue carbonization surrounding electrodes, ablation volume (multiplied by long diameter and short diameter), and the pathological changes of ablation points and the surrounding tissues were observed using hemotoxylin and eosin.
Statistical analysis was performed using SAS 8.0 (not all the carbonization points were included).
The correlation analysis between the ablation volume (zeroIand zero II) and the ablation time (1, 2, 3, 4, 5, 6, 7, 8, 9 and 10 min) was performed at a power of 60 W.
The correlation analysis between the ablation volume and the ablation power (30, 40, 50, 60, 70, 80, 90 and 100 W) was performed using an ablation time of 3 min.
For each correlation analysis, the data was represented by scatterplot and the value of r was calculated.
The t test of independent samples was performed to compare the ablation range of zeroI(9 points of 3 min by 60 W) and that of zero II (8 points of 3 min by 60 W).
The incidence of bleeding was 5.4% (3/56), only small amounts (< 20 mL) were caused by tissue carbonization surrounding the electrodes. The carbonization incidence rate was also 5.4% (3/56).
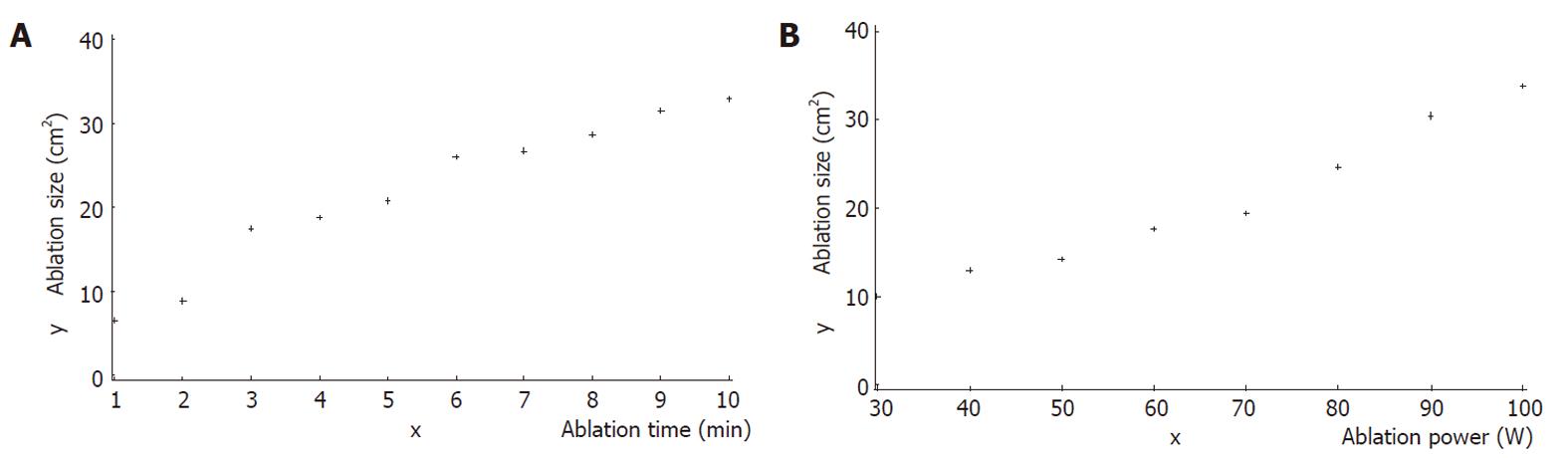
As shown in Figure 2A, the ablation range had a direct correlation with ablation time by the power of 60 W (r = 0.97542, x = 5.5 ± 3.028, y = 23.4 ± 9.023, P = 0.0001). The ablation lesion size increased with increased ablation time, from 1 to 10 min, when the ablation power was 60 W.
As shown in Figure 2B, the ablation range was directly correlated with the power (30-100 W), at an ablation time of 3 min (r = 0.98258, x = 65.0 ± 24.495, y = 18.47 ± 8.47, P = 0.0001). The ablation lesion size increased with the increase of ablation power, ranging from 30 to 100 W when the ablation time was set to 3 min.
The ablation lesion size of zone II (the extra-2/3 part of the spleen, relative to the first or second class vascular branches), which was near the hilum of the spleen was significantly larger than that of zoneI(the intra-1/3 part of the spleen) which was distal from the hilum of the spleen (10.763 ± 2.001 vs 16.569 ± 4.112, P = 0.0015, t = -3.81, P = 0.0015).
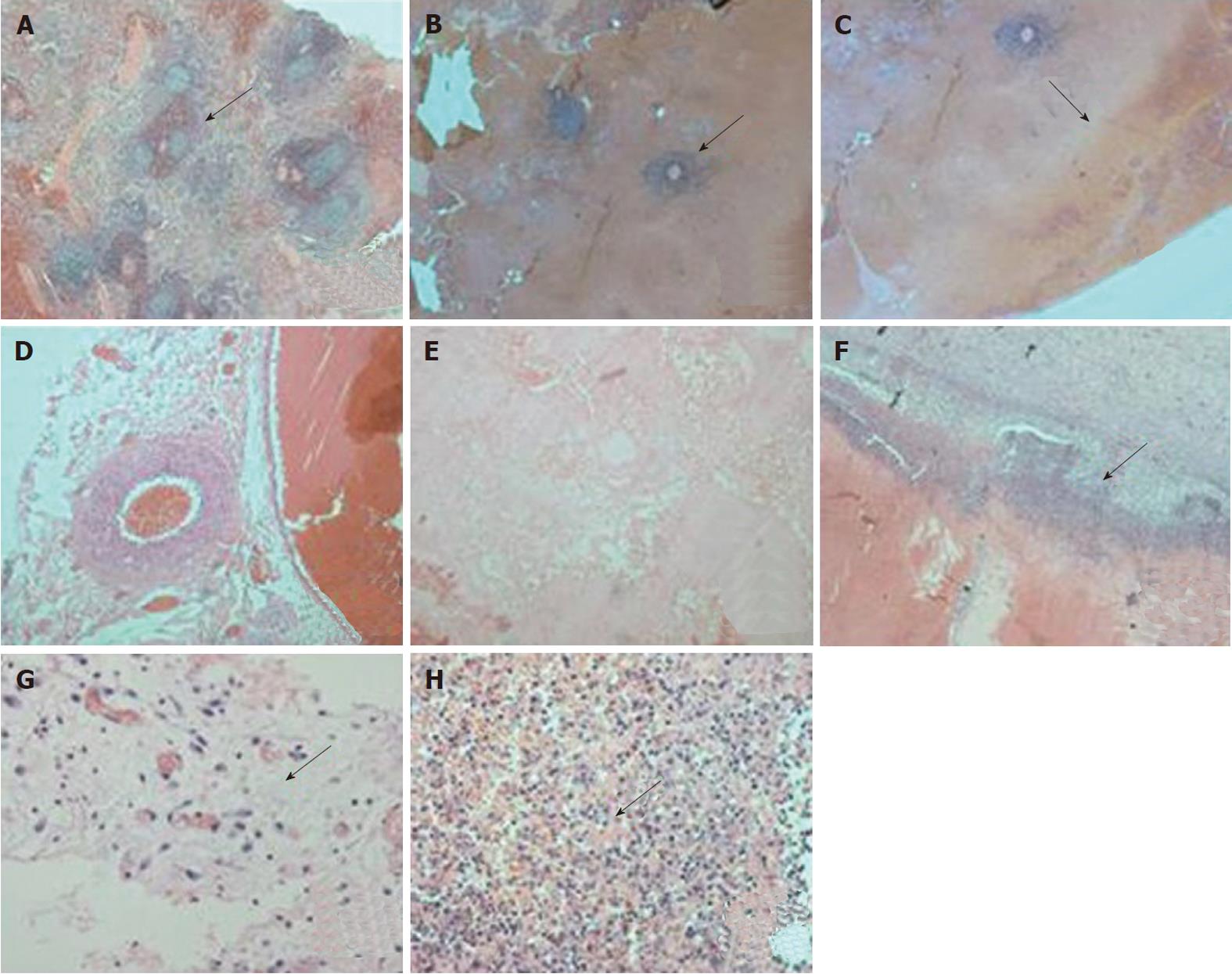
As shown in Figure 3, pathological changes occurred immediately and up to 1 wk after the ablation, and were marked by a large area of coagulation necrosis. Immediately following ablation, intact spleen tissues were observed in the areas of coagulation necrosis, mainly around arterioles, and there were no obvious signs of hydropsia and inflammation. One week following the ablation, the coagulation necrosis was well distributed and complete, as many nuclear fragmentations were detected, and there were obvious signs of hydropsia and inflammation.
With the development of minimally invasive therapy, new methods such as PSE and RFA are being optimized for the treatment of hypersplenism. Many research studies and clinical practices have proved that PSE has good therapeutic effects in the treatment of hypersplenism[4-7]. However, it has a high recurrence rate and the embolism volume varies based on the surgeon’s experience and the estimation of the contrast medium’s flow rate. For this reason, PSE is usually influenced by subjective factors, making it difficult to accurately evaluate embolism volume in real time[1]. Recently, some studies reported that RFA can effectively control the destruction volume of the spleen and overcome the limitation posed by PSE[8,9]. With RFA, coagulative necrosis, not liquefaction necrosis, occurred in the spleen after thermo-ablation, and the probability of splenic abscess was obviously decreased. Furthermore, the recurrence rate of hypersplenism was obviously decreased because the heat-sink effect can damage the vascular endothelium and lead to thrombogenesis, which can lead to spleen consolidation, interstitial fibrosis and decreased splenic recirculation.
MWA is one method of thermo-ablation and can also control the destruction volume of the spleen. MWA has the added advantages of a rapid rise in temperature, high thermal efficiency, stable and controllable thermal field, good hemostatic effect, and effective blood vessel coagulation[2,3]. Shibata et al[10] first compared MWA with RFA in pig liver and found that the temperature of the area surrounding the MWA electrode was significantly higher than the temperature of the area surrounding the RFA electrode; the MWA electrode achieved superior results to the RFA electrode with respect to the diameter of the coagulated area and the temperature of the area in which the electrode was inserted, at the specified times. Yu et al[11] also compared the effectiveness of MWA and RFA using a single internally cooled probe in a hepatic porcine model and the results showed that MWA had higher potential for complete destruction of liver tumors than RFA. Crocetti et al[12] designed a study to compare the feasibility, safety, and effectiveness of MWA versus RFA of lung tissue at histopathology in a rabbit model and found the feasibility and safety of MWA and RFA are similar in a lung rabbit model, but MWA produced a greater damage to peripheral small vessels inducing thrombosis. So, MWA has the advantage in the treatment of splenomegalia and hypersplenism and shows good prospects in the treatment of spleen diseases[13]. Some reports have also shown that MWA improves the peripheral blood conditions[13,14]. Further experimental and clinical research should investigate the relationship between the destructive proportion of the spleen and the improvement of the peripheral blood conditions. Regarding the type of cooled antennas, two types, air-cooled and water-cooled, have been used for microwave ablation to create as large ablation patterns as possible[15]. Firstly, cooling water significantly reduces the temperature near the antenna, as confirmed by a previous study[16]. This approach avoids carbonization near the antenna, since the coagulation region is limited by the carbonized tissues. Therefore, a water-cooled antenna makes it possible to create the maximum ablation pattern. Secondly, a water-cooled antenna could effectively reduce the adhesion between antenna and tissue, and reportedly reduces patient pain during treatment and reduces the risk of infection after the operation[16].
The main complication of MWA treatment is bleeding of the pin hole, because the spleen tissue is soft and fragile and it has many close-set blood sinuses. In the current study, we report that the incidence of bleeding was 5.4% (3/56), only small amounts (< 20 mL), and that the bleeding was controlled by further ablation of the pin hole, suggesting that MWA is a safe method. Compared to RFA, MWA has higher thermal efficiency and better blood vessel coagulation[17,18]. So, the risk of pin hole errhysis is lower than that occurring with RFA, especially since the pin hole coagulation after MWA further decreases the probability of bleeding. In this study, the risk of pin hole bleeding is likely increased due to splenic congestion and tumescence, and the splenic sinus expanding 2-4 h after splenic vein ligation. In the cases of splenomegalia and hypersplenism caused by long-term portal hypertension, in which there is endangium hyperplasia and arteriole and veinule obstruction, MWA may run a lower risk of bleeding. Animal models of splenomegalia and hypersplenism caused by portal hypertension should be developed to test this hypothesis.
Regarding the relation between the carbonization surrounding electrodes and bleeding, the current study presumes that the crushing and avulsion of carbonization tissues to the spleen tissues without ablation, such as in the course of needle withdrawal, can lead to bleeding, especially since the spleen has an abundant blood supply. So, cutting down the carbonization incidence rate is an effective method for reducing the risk of bleeding, which can be regulated based on ablation power.
In this study, the carbonization incidence rate was 5.4% (3/56). Because the spleen has an abundant blood supply, the tissues of coagulative necrosis are easy to adhere to the pinhead after dehydration, and then carbonization occurs, which decreases the heat conduction and affects the ablation range. Further statistical analysis was not performed because there were few carbonization cases in this study, presumably due to the higher speed of water circulation and ice water circulation, which could decrease the carbonization incidence rate.
Some reports find that thermo-ablation targeted next to blood vessels affects the ablation range, and that the extent of this effect is closely related to the caliber of the blood vessel[19-23], but there are no related reports describing the effect of the point of MWA in spleen ablation. In the current study, it is presumed that the blood vessels adjoining the hilum of the spleen are always branches of the first class, and that the caliber is larger and the blood flow rate is faster, and therefore they can convey the heat acquired by the spleen easily and have a predictable affect on the ablation range. The blood vessels distal from the hilum of spleen are always branches of the second and third class, with a smaller caliber and slower blood flow rates, and therefore they cannot convey the heat acquired by the spleen easily and consequently have little effect on ablation lesion size. In this study, the ablation lesion size of zoneIwas obviously smaller than that of zone II by the power of 60 W in the same time period.
This study found that the ablation lesion size increased with increased ablation time, from 1 to 10 min, when the ablation power was 60 W. Also, the ablation lesion size increased with the increase of ablation power, ranging from 30 to 100 W when the ablation time was set to 3 min. So, by adjusting ablation time and power, MWA can accurately control the destruction volume of the spleen in cases of splenomegalia and hypersplenism, and effectively overcome the limitations in existing treatments, such as a reduced therapeutic effect caused by a smaller destruction volume and more complications associated with excess destruction volume.
Some studies that use PSE for the treatment of hypersplenism caused by portal hypertention[1,24] report that 50%-70% is the best embolism proportion. The best destruction proportion of MWA needs further research. Different studies report different results on the best ablation power and time. Hope et al[25,26] found that 45 W was the best ablation power and 10 min was the best ablation time for in vivo experimental treatment of porcine kidney using microwave ablation. Hines-Peralta et al[27] found the ablation range increased with the increase in ablation time and power when used for in vivo and ex vivo experimental treatment of porcine liver using microwave ablation (50-150 W; 2-20 min). The current study is similar to that of Hines-Peralta, and the best ablation power and time should be further studied using more ablation points.
Similar to other reports[28,29], in the current study pathology marked by coagulation necrosis of a large area was noted both immediately and 1 wk after MWA. Immediately following MWA, some remaining spleen tissue was observed around arterioles, within areas of coagulation necrosis, and there were no obvious signs of hydropsia and inflammation. However, 1 wk after MWA, coagulation necrosis was well-distributed and complete, with clear detection of many nuclear fragmentations and hydropsia and inflammation. The remaining spleen tissues detected around arterioles immediately following MWA was likely due to the heat conveying. This tissue mainly consisted of lymphocytes which had integrated cytolemma, cell organelles and cell nuclei. Though the current study did not assess pathological changes occurring beyond 1 wk post-ablation, it is possible that there is extended hydropsia and inflammation.
The differences between the pathology changes immediately and 1 wk later in this study were the changes around arterioles in the ablation area and the hydropsia and inflammatory reaction beside which was the normal tissue reaction after thermal ablation. The disaggregation and necrosis noted in this study was probably related to the finding that the remaining cells around the arterioles were surrounded by coagulation necrosis and ischemic necrosis due to a loss of blood supply. The difference between the pathological changes occurring immediately versus 1 wk after MWA is also reflected in the change of the ablation borderline. Specifically, the ablation borderline was clearer 1 wk post-ablation, and included many nuclear fragmentations with surrounding inflammatory cells that were trachychromatic. Here we show an optimized method for treatment of hypersplenism. Results from this study have the potential to enhance therapeutic treatment of hypersplenism.
The incidence of hypersplenism is currently rising faster in China and current treatments have lots of limitations. Microwave ablation (MWA) of water-cooled antenna is expected to be a safe and feasible method to treat this disease that is also minimally invasive.
MWA is a good method of thermo-ablation which has more advantages of a rapid rise in temperature, high thermal efficiency, stable and controllable thermal field, good hemostatic effect, and effective blood vessel coagulation than radiofrequency ablation (RFA) in tumor treatment. However, whether MWA can be used in spleen ablation and how to control the destruction volume of the spleen has not been unequivocally addressed. In this study, the authors demonstrate that MWA is a safe and feasible method that is minimally invasive in spleen ablation and the appropriate volume of ablation of spleen can be obtained by controlling the ablation time and power.
Recent reports have highlighted the value of RFA in the treatment of hypersplenism. However, MWA has more advantages, such as a rapid rise in temperature, high thermal efficiency, stable and controllable thermal field, good hemostatic effect, and effective blood vessel coagulation than RFA. This is the first study to report MWA as a safe and feasible method in spleen ablation by animal experiment. Furthermore, this studies would suggest the regularity of the spleen damage in vivo using MWA and confirm the efficacy of microwave ablation from the pathological point of view.
By understanding the regularity of spleen damage in vivo using MWA, this study may represent a future strategy for therapeutic intervention in the treatment of patients with hypersplenism.
The authors examined the safety, feasibility and regularity of destruction to porcine spleen in vivo with congestion and tumescence by microwave ablation. It reveals that MWA is safe in spleen ablation and the destruction volume of the spleen can be easily controlled. The results are interesting and may represent a treatment option for hypersplenism.
Peer reviewer: Yukihiro Shimizu, MD, PhD, Kyoto Katsura Hospital, 17 Yamada-Hirao, Nishikyo, 615-8256 Kyoto, Japan
S- Editor Lv S L- Editor O’Neill M E- Editor Li JY
| 1. | Huang JH, Gao F, Gu YK, Li WQ, Lu LW. Combined treatment of hepatocellular carcinoma with partial splenic embolization and transcatheter hepatic arterial chemoembolization. World J Gastroenterol. 2007;13:6593-6597. [PubMed] |
| 2. | Brace CL. Radiofrequency and microwave ablation of the liver, lung, kidney, and bone: what are the differences? Curr Probl Diagn Radiol. 2009;38:135-143. [PubMed] |
| 3. | Brace CL. Microwave ablation technology: what every user should know. Curr Probl Diagn Radiol. 2009;38:61-67. [PubMed] |
| 4. | Miraglia R, Pietrosi G, Maruzzelli L, Petridis I, Caruso S, Marrone G, Mamone G, Vizzini G, Luca A, Gridelli B. Efficacy of transcatheter embolization/chemoembolization (TAE/TACE) for the treatment of single hepatocellular carcinoma. World J Gastroenterol. 2007;13:2952-2955. [PubMed] |
| 5. | Koconis KG, Singh H, Soares G. Partial splenic embolization in the treatment of patients with portal hypertension: a review of the english language literature. J Vasc Interv Radiol. 2007;18:463-481. [PubMed] |
| 6. | Lee CM, Leung TK, Wang HJ, Lee WH, Shen LK, Liu JD, Chang CC, Chen YY. Evaluation of the effect of partial splenic embolization on platelet values for liver cirrhosis patients with thrombocytopenia. World J Gastroenterol. 2007;13:619-622. [PubMed] |
| 7. | Madoff DC, Denys A, Wallace MJ, Murthy R, Gupta S, Pillsbury EP, Ahrar K, Bessoud B, Hicks ME. Splenic arterial interventions: anatomy, indications, technical considerations, and potential complications. Radiographics. 2005;25 Suppl 1:S191-S211. [PubMed] |
| 8. | Liu QD, Ma KS, He ZP, Ding J, Huang XQ, Dong JH. Experimental study on the feasibility and safety of radiofrequency ablation for secondary splenomagely and hypersplenism. World J Gastroenterol. 2003;9:813-817. [PubMed] |
| 9. | Matsuoka T, Yamamoto A, Okuma T, Oyama Y, Nakamura K, Inoue Y. CT-guided percutaneous radiofrequency ablation of spleen: a preliminary study. AJR Am J Roentgenol. 2007;188:1044-1046. [PubMed] |
| 10. | Shibata T, Morita T, Okuyama M, Kitada M, Tsukahara Y, Ikeda K, Suzuki R, Shimano T, Ishida T. [Comparison of microwave coagulation using a new type electrode with radiofrequency ablation in the liver of living animals]. Gan To Kagaku Ryoho. 2001;28:1595-1598. [PubMed] |
| 11. | Yu J, Liang P, Yu X, Liu F, Chen L, Wang Y. A comparison of microwave ablation and bipolar radiofrequency ablation both with an internally cooled probe: results in ex vivo and in vivo porcine livers. Eur J Radiol. 2011;79:124-130. [PubMed] |
| 12. | Crocetti L, Bozzi E, Faviana P, Cioni D, Della Pina C, Sbrana A, Fontanini G, Lencioni R. Thermal ablation of lung tissue: in vivo experimental comparison of microwave and radiofrequency. Cardiovasc Intervent Radiol. 2010;33:818-827. [PubMed] |
| 13. | Gao Y, Wang Y, Duan Y, Li C, Sun Y, Zhang D, Lu T, Liang P. 915MHz microwave ablation with high output power in in vivo porcine spleens. Eur J Radiol. 2010;75:87-90. [PubMed] |
| 14. | Duan YQ, Gao YY, Ni XX, Wang Y, Feng L, Liang P. Changes in peripheral lymphocyte subsets in patients after partial microwave ablation of the spleen for secondary splenomegaly and hypersplenism: a preliminary study. Int J Hyperthermia. 2007;23:467-472. [PubMed] |
| 15. | Wang Y, Sun Y, Feng L, Gao Y, Ni X, Liang P. Internally cooled antenna for microwave ablation: results in ex vivo and in vivo porcine livers. Eur J Radiol. 2008;67:357-361. [PubMed] |
| 16. | He N, Wang W, Ji Z, Li C, Huang B. Microwave ablation: An experimental comparative study on internally cooled antenna versus non-internally cooled antenna in liver models. Acad Radiol. 2010;17:894-899. [PubMed] |
| 17. | Yeasmin S, Nakayama K, Ishibashi M, Katagiri A, Iida K, Nakayama N, Aoki S, Kanaoka Y, Miyazaki K. Microwave endometrial ablation as an alternative to hysterectomy for the emergent control of uterine bleeding in patients who are poor surgical candidates. Arch Gynecol Obstet. 2009;280:279-282. [PubMed] |
| 18. | Bourdrez P, Bongers MY, Mol BW. Treatment of dysfunctional uterine bleeding: patient preferences for endometrial ablation, a levonorgestrel-releasing intrauterine device, or hysterectomy. Fertil Steril. 2004;82:160-166, quiz 265. [PubMed] |
| 19. | Yu NC, Raman SS, Kim YJ, Lassman C, Chang X, Lu DS. Microwave liver ablation: influence of hepatic vein size on heat-sink effect in a porcine model. J Vasc Interv Radiol. 2008;19:1087-1092. [PubMed] |
| 20. | Lu DS, Raman SS, Vodopich DJ, Wang M, Sayre J, Lassman C. Effect of vessel size on creation of hepatic radiofrequency lesions in pigs: assessment of the "heat sink" effect. AJR Am J Roentgenol. 2002;178:47-51. [PubMed] |
| 21. | Sato K, Nakamura K, Hamuro M, Sakai Y, Nishida N, Yamada R, Ikura Y, Ueda M, Inoue Y. The influence of radiofrequency ablation on hepatic vessels in porcine liver. Hepatogastroenterology. 2005;52:571-574. [PubMed] |
| 22. | Bangard C, Gossmann A, Kasper HU, Hellmich M, Fischer JH, Hölscher A, Lackner K, Stippel DL. Experimental radiofrequency ablation near the portal and the hepatic veins in pigs: differences in efficacy of a monopolar ablation system. J Surg Res. 2006;135:113-119. [PubMed] |
| 23. | dos Santos I, Haemmerich D, Pinheiro Cda S, da Rocha AF. Effect of variable heat transfer coefficient on tissue temperature next to a large vessel during radiofrequency tumor ablation. Biomed Eng Online. 2008;7:21. [PubMed] |
| 24. | Zhu K, Meng X, Qian J, Huang M, Li Z, Guan S, Jiang Z, Shan H. Partial splenic embolization for hypersplenism in cirrhosis: a long-term outcome in 62 patients. Dig Liver Dis. 2009;41:411-416. [PubMed] |
| 25. | Hope WW, Schmelzer TM, Newcomb WL, Heath JJ, Lincourt AE, Norton HJ, Heniford BT, Iannitti DA. Guidelines for power and time variables for microwave ablation in an in vivo porcine kidney. J Surg Res. 2009;153:263-267. [PubMed] |
| 26. | Hope WW, Schmelzer TM, Newcomb WL, Heath JJ, Lincourt AE, Norton HJ, Heniford BT, Iannitti DA. Guidelines for power and time variables for microwave ablation in a porcine liver. J Gastrointest Surg. 2008;12:463-467. [PubMed] |
| 27. | Hines-Peralta AU, Pirani N, Clegg P, Cronin N, Ryan TP, Liu Z, Goldberg SN. Microwave ablation: results with a 2.45-GHz applicator in ex vivo bovine and in vivo porcine liver. Radiology. 2006;239:94-102. [PubMed] |
| 28. | Gravante G, Ong SL, Metcalfe MS, Strickland A, Dennison AR, Lloyd DM. Hepatic microwave ablation: a review of the histological changes following thermal damage. Liver Int. 2008;28:911-921. [PubMed] |
| 29. | Awad MM, Devgan L, Kamel IR, Torbensen M, Choti MA. Microwave ablation in a hepatic porcine model: correlation of CT and histopathologic findings. HPB (Oxford). 2007;9:357-362. [PubMed] |