Published online Dec 7, 2011. doi: 10.3748/wjg.v17.i45.4999
Revised: June 9, 2011
Accepted: June 16, 2011
Published online: December 7, 2011
AIM: To investigate the endoscopic features of pharyngeal superficial carcinoma and evaluate the utility of narrow-band imaging (NBI) for this disease.
METHODS: In the present prospective study, 335 patients underwent conventional white light (CWL) endoscopy and non-magnified/magnified NBI endoscopy, followed by an endoscopic biopsy, for 445 superficial lesions in the oropharynx and hypopharynx. The macroscopic appearance of superficial lesions was categorized as either elevated (< 5 mm in height), flat, or depressed (not ulcerous). Superficial carcinoma (SC) was defined as a superficial lesion showing high-grade dysplasia or squamous cell carcinoma on histology. The color, delineation, and macroscopic appearances of the lesions were evaluated by CWL endoscopy. The ratio of the brownish area/intervascular brownish epithelium (IBE), as well as microvascular proliferation, dilation, and irregularities, was determined by non-magnified/magnified NBI endoscopy. An experienced pathologist who was unaware of the endoscopic findings made the histological diagnoses. By comparing endoscopic findings with histology, we determined the endoscopic features of SC and evaluated the diagnostic utility of NBI.
RESULTS: The 445 lesions were divided histologically into two groups: a non-SC group, including non-neoplasia and low-grade dysplasia cases, and an SC group. Of the 445 lesions examined, 333 were classified as non-SC and 112 were classified as SC. There were no significant differences in age, gender, or the location of the lesions between the patients in the two groups. The mean diameter of the SC lesions was significantly greater than that of non-SC lesions (11.0 ± 7.6 mm vs 4.6 ± 3.6 mm, respectively, P < 0.001). Comparisons of CWL endoscopy findings for SC and non-SC lesions by univariate analysis revealed that the incidence of redness (72% vs 41%, respectively, P < 0.001) and a flat or depressed type of lesion (58% vs 44%, respectively, P = 0.013) was significantly higher in the SC group. Using non-magnified NBI endoscopy, the incidence of a brownish area was significantly higher for SC lesions (79% vs 57%, respectively, P < 0.001). On magnified NBI endoscopy, the incidence of IBE (68% vs 33%, P < 0.001) and microvascular proliferation (82% vs 51%, P < 0.001), dilation (90% vs 76%, P = 0.002), and irregularity (82% vs 31%, P < 0.001) was also significantly higher for the SC compared with the non-SC lesions. Multivariate analysis revealed that the incidence of redness (P = 0.022) on CWL endoscopy and IBE (P < 0.001) and microvascular irregularities (P < 0.001) on magnified NBI endoscopy was significantly higher in SC than non-SC lesions. Redness alone exhibited significantly higher sensitivity and significantly lower specificity for the diagnosis of SC compared with redness plus IBE and microvascular irregularities (72% vs 52%, P = 0.002; and 59% vs 92%, P < 0.001, respectively). The accuracy of redness plus IBE and irregularities for the diagnosis of SC was significantly greater than using redness alone (82% vs 62%, respectively, P < 0.001).
CONCLUSION: Redness, IBE, and microvascular irregularities appear to be closely related to SC lesions. Magnified NBI endoscopy may increase the diagnostic accuracy of CWL endoscopy for SC.
- Citation: Yoshimura N, Goda K, Tajiri H, Yoshida Y, Kato T, Seino Y, Ikegami M, Urashima M. Diagnostic utility of narrow-band imaging endoscopy for pharyngeal superficial carcinoma. World J Gastroenterol 2011; 17(45): 4999-5006
- URL: https://www.wjgnet.com/1007-9327/full/v17/i45/4999.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i45.4999
Most epithelial malignancies of the head and neck are squamous cell carcinomas (SCCs)[1]. The most important known risk factors for these carcinomas are tobacco use and alcohol consumption, direct exposure to which may result in the synchronous or metachronous development of SCC and dysplasia (a precancerous lesion) in the upper aerodigestive tract, especially in the oropharynx and hypopharynx, and the esophagus[1,2]. This was reported more than 50 years ago as part of the concept of “field cancerization”[2].
In the esophagus, the clinical utility of Lugol chromoendoscopy for the early detection of superficial neoplasms has been established, and this technique is now widely used[3]. Consequently, the rate of detection of early stage esophageal SCC (ESCC) is increasing. Endoscopic treatment of tumors at the earliest stages, particularly mucosal carcinomas, has resulted in considerable improvements in prognosis[4]. However, although early treatment increases the long-term survival of patients with ESCC, in accordance with the concept of field cancerization, the incidence of secondary head and neck SCCs in patients with ESCC has also increased[5]. Among the secondary head and neck SCCs associated with ESCC, oropharyngeal and hypopharyngeal SCCs (OHSCCs) are the most common and adversely affect the improved prognosis of ESCC[5].
Despite its usefulness in screening for superficial neo-plasms in the esophagus, Lugol chromoendoscopy cannot be used to screen for OHSCCs because of the accompanying irritation due to iodine and the risk of aspiration. Early stage OHSCCs show an obscure appearance and most affected patients have few symptoms, making the early detection of OHSCCs difficult. Most such lesions are detected at advanced stages, thereby resulting in an extremely poor prognosis[6]. Furthermore, even if the advanced lesions can be removed, dysphagia or aphonia following surgery for advanced OHSCCs causes a marked deterioration in the patients’ quality of life[5,6]. Accordingly, a new approach that facilitates the detection and diagnosis of early stage OHSCCs is needed.
The narrow-band imaging (NBI) system is a revolutionary optical image-enhanced technology that uses narrow bandwidth NBI filters. Magnifying endoscopy combined with NBI clearly visualizes the surface microvascular structures in different organs because NBI illumination consists of two limited wavelength ranges that are well absorbed by hemoglobin[7]. Recent studies have reported the usefulness of NBI for the early detection and diagnosis of OHSCCs using gastrointestinal endoscopy or rhinolaryngoscopy[8-10]. However, to the best of our knowledge, there have been no studies to date that have investigated the relationships between detailed findings of conventional white light (CWL) endoscopy, magnified NBI endoscopy, and histology in superficial pharyngeal lesions. There is also scant information currently available in the literature regarding the differential diagnosis of non-neoplasia, dysplasia, and carcinoma using endoscopy.
The process underlying the carcinogenesis of OHSCCs has been proposed to be a dysplasia-carcinoma sequence similar to that seen in ESCC, proceeding from mild or moderate dysplasia (low-grade dysplasia) to severe dysplasia [high-grade dysplasia (HGD)] and SCC[11]. Previous epidemiological follow-up studies of esophageal squamous neoplasms have suggested that the relative risk of HGD developing into invasive SCC is comparable to that of SCC in situ[11,12]. In contrast, a low-grade dysplasia indicates a significantly lower risk of malignant transformation[11]. Among squamous neoplasias, HGD appears to be a good candidate for intervention, as with SCC[13].
The aim of our present study was to investigate the relationships between the endoscopic and histologic findings of superficial lesions in the oropharynx and hypopharynx, and to evaluate the diagnostic utility of NBI for superficial squamous neoplasms showing HGD or SCC.
The macroscopic appearance of superficial lesions was evaluated and lesions were classified as either elevated (< 5 mm in height), flat, or depressed (not ulcerous). Between March 2005 and April 2009, CWL and non-magnified/magnified NBI endoscopies were performed in 1010 consecutive patients for the evaluation of the oropharyngeal and hypopharyngeal region. The endoscopic findings for 455 superficial lesions in the oropharynx and hypopharynx in 342 patients were evaluated prospectively in our present study. Biopsy samples were obtained for all 455 superficial lesions, although 10 lesions from seven patients were excluded from analysis because insufficient material was obtained by biopsy to enable a histological diagnosis to be made. The findings for 445 superficial oropharyngeal and hypopharyngeal lesions in 335 patients were therefore analyzed in the present study.
Superficial lesions that demonstrated HGD or SCC histologically were defined as superficial carcinomas (SC). Endoscopic findings of SC lesions were compared with those of non-SC lesions in the oropharynx and hypopharynx to reveal the endoscopic features of SC lesions. Moreover, we investigated the diagnostic utility of NBI for SC lesions. The present analyses were performed with local ethics committee approval and all patients provided informed consent prior to their inclusion in the study.
All patients were orally administered 20 000 U pronase (Pronase MS; Kaken Pharmaceutical Products Inc., Tokyo, Japan) prior to pharyngeal anesthesia to eliminate saliva and mucus. Pharyngeal anesthesia was achieved using four to five pump-spray applications of lidocaine (Xylocaine; AstraZeneca, Osaka, Japan). All endoscopic inspections were performed in conscious, sedated patients after the administration of pethidine hydrochloride (35-70 mg; Opystan; Mitsubishi Tanabe Pharma, Osaka, Japan) and flunitrazepam (0.2-0.8 mg; Rohypnol; Chugai Pharmaceutical, Tokyo, Japan).
Endoscopic examinations were performed using a high-resolution and zoom gastrointestinal endoscope (GIF-Q240Z or GIF-H260Z; Olympus Medical Systems, Tokyo, Japan) with a maximum magnification of × 90 on a 19 inch (48.3 cm) monitor. The light source unit (Olympus Medical Systems) has two rotary filters in front of a xenon bulb, one for CWL and the other for NBI. Thus, it is easy to switch from the CWL to NBI mode by simply pushing the control knob on the endoscope. A black rubber attachment (Olympus Medical Systems) was mounted on the tip of the scope to enable the endoscopist to adjust the focus easily and to maintain the focal distance between the tip of the scope and the lesion surface at 2 mm during magnified observation.
Three endoscopic techniques, namely CWL and non-magnified and magnified NBI, were performed for each patient by one of three endoscopists (NY, YY or KG). First, the oropharynx and hypopharynx were inspected thoroughly using CWL endoscopy, after which the endoscope was withdrawn to the oral cavity and a switch made from the CWL to NBI mode. The oropharynx and hypopharynx were then examined using non-magnified NBI endoscopy. For lesions that had not been detected by CWL endoscopy and were first detected using non-magnified NBI endoscopy, the NBI mode was switched back to the CWL mode and the lesions were evaluated using CWL endoscopy. All lesions were then inspected using magnified NBI endoscopy. Finally, tissue samples were obtained endoscopically from the lesions using biopsy forceps (Radial Jaw 3; Boston Scientific, Natick, MA). All endoscopic images were preserved in digital form.
After completion of each endoscopic inspection, each endoscopic finding of CWL, non-magnified NBI, or magnified NBI was recorded immediately on a case report form.
CWL endoscopy: Endoscopic findings for each super-ficial lesion were evaluated with regards to color, delineation, and macroscopic appearance. Superficial lesions were determined to be either “redness” or “white or isochromatic” in terms of color, “well-delineated” or “poorly delineated”, and either “elevated” or “flat or depressed” macroscopically. Each of these parameters was classified based on the predominant finding.
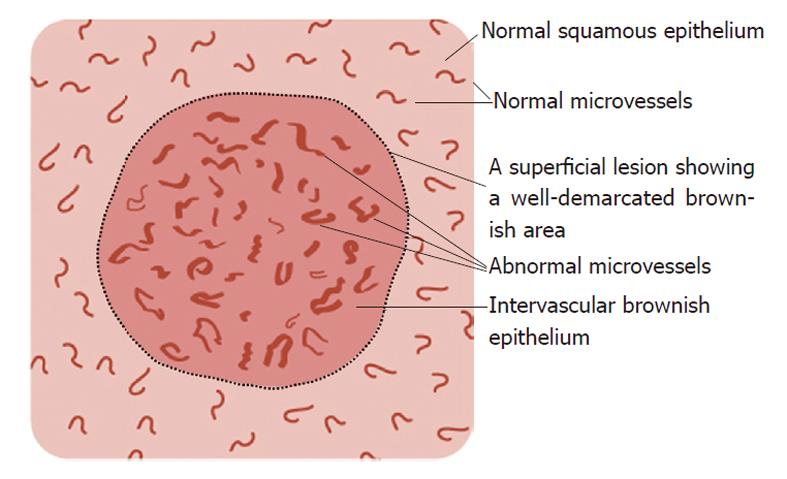
Non-magnified NBI endoscopy: Superficial lesions exhibiting a well-demarcated brownish area by non-magnified NBI endoscopy were classified as having a “brownish area”[8].
Magnified NBI endoscopy: In a preliminary study[14], we found that the “brownish area” observed by non-magnified NBI endoscopy can comprise any one or a combination of the following observations on magnified NBI endoscopy: (1) an intervascular brownish epithelium (IBE); (2) microvascular dilation; and (3) microvascular proliferation. IBE is the presence of brown-colored epithelium between superficial microvessels in a lesion that differs from the whitish surrounding squamous epithelium. Microvascular dilation is defined as the presence of a group of superficial microvessels, the calibers of which are more than twice the caliber of the surrounding reference microvessels. Microvascular proliferation is defined as the presence of a group of superficial microvessels with a greater density than that of the surrounding reference microvessels. In addition, microvascular irregularities were assessed using magnified NBI endoscopy, where “irregularities” were defined as the presence of a group of superficial microvessels showing marked variations in caliber and/or highly variable forms compared with the surrounding reference microvessels.
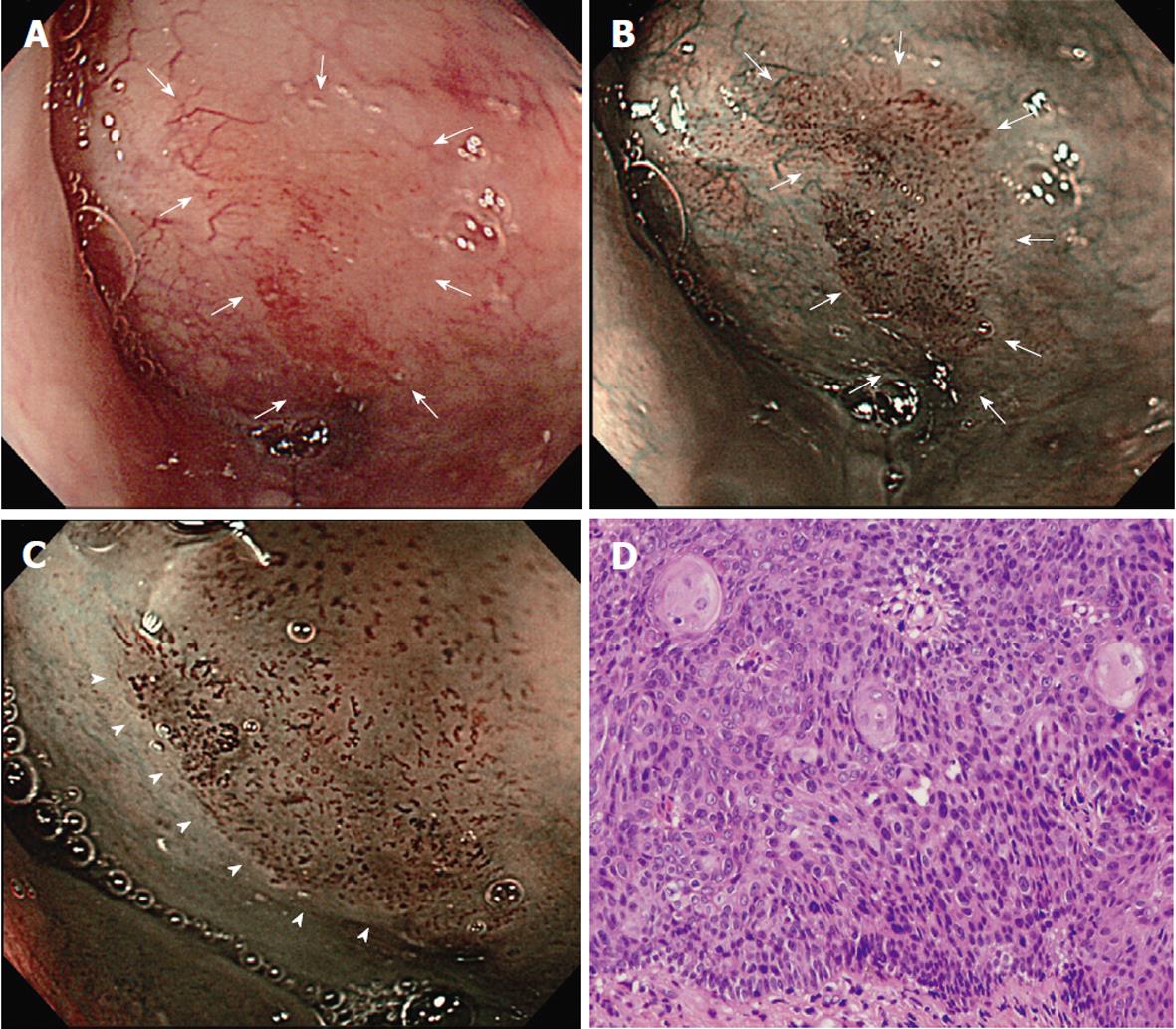
Figure 1 schematically summarizes the features of superficial lesions and the surrounding mucosa seen using magnified NBI endoscopy. A representative image of an SC lesion is shown in Figure 2.
Endoscopic biopsy specimens of the 445 superficial lesions examined in this study were subjected to histologic assessment. Tissue samples were placed on custom-made black filter paper (Toyo Roshi Kaisha, Tokyo, Japan) and fixed in 40 g/L formaldehyde for 24 h. Black filter paper makes it easier to flatten out the whitish specimens, which makes it easier to prepare vertical microsections and make a more accurate diagnosis of the histological grade of the intraepithelial neoplasm. All specimens were embedded in paraffin, sectioned and stained with hematoxylin and eosin.
Histologic diagnoses were made by a pathologist (MI) specializing in gastrointestinal pathology who was unaware of the endoscopic findings and were based on the revised Vienna classification[13].
Continuous variables are expressed as the mean ± SD and were analyzed using the Student’s t test. The χ2 test and logistic regression were used to analyze categorical data. P < 0.05 was considered significant. All analyses were performed using STATA 10.0 software (STATA, College Station, TX).
Table 1 lists the clinicopathologic features of our current cohort of 335 patients comprising 445 superficial lesions in the oropharynx and hypopharynx. These 445 lesions were divided histologically into two groups, namely non-SC (normal epithelium, inflammation, hyperplasia, papilloma, “other”, and low-grade dysplasia) and SC (HGD and SCC). The category of “other” included hyperkeratosis (n = 2), regenerative atypia (n = 1), atrophy (n = 1), xanthoma (n = 1), and melanosis (n = 1). Most patients in both groups were male and there were no significant differences found in age, male:female ratio, or in the location of the lesions between the two groups. However, the lesion diameter was significantly greater in the SC group (P < 0.001).
| Total | Non-SC | SC | P value | |
| (n = 445) | (n = 333) | (n = 112) | ||
| Age (yr) | 63 ± 9 | 63 ± 10 | 64 ± 8 | |
| Male : female ratio | 16:1 | 14:1 | 27:1 | |
| Lesion location (n) | ||||
| Oropharynx/ hypopharynx | 269/176 | 206/127 | 63/49 | |
| Lesion diameter (mm) | 6.2 ± 5.6 | 4.6 ± 3.6 | 11.0 ± 7.6 | < 0.0011 |
| Histology (n) | Normal: 40 | HGD: 35 | ||
| Inflammation: 115 | SCC: 77 | |||
| Hyperplasia: 50 | ||||
| Papilloma: 33 | ||||
| Other2: 6 | ||||
| LGD: 89 |
Table 2 lists the results of univariate analysis of the relationships between the endoscopic findings in the two study groups. Univariate analysis of the CWL endoscopy findings identified significant differences in the color and macroscopic appearance of lesions between the SC and non-SC groups. Redness was more commonly seen for lesions in the SC than in the non-SC group (72% vs 41%, respectively, P < 0.001). In addition, lesions in the SC group were significantly more likely to appear flat or depressed (58% vs 44%, respectively, P = 0.013). In both groups, approximately three-quarters of the lesions appeared to be well delineated, with no significant difference detected.
| Endoscopy | Endoscopic findings | Non-SC (n = 333) | SC (n = 112) | OR | 95% CI | P value |
| CWL (Non-magnified) | Color (redness; %) | 41 | 72 | 3.69 | 2.31, 5.89 | < 0.0011 |
| Delineation (well delineated; %) | 66 | 69 | 1.13 | 0.71, 1.79 | ||
| Macroscopic type (flat or depressed; %) | 44 | 58 | 1.73 | 1.12, 2.67 | 0.0131 | |
| NBI | ||||||
| (Non-magnified) | Brownish area (%) | 57 | 79 | 2.79 | 1.69, 4.61 | < 0.0011 |
| (Magnified) | IBE (%) | 33 | 68 | 4.22 | 2.67, 6.67 | < 0.0011 |
| Microvascular | ||||||
| Proliferation (%) | 51 | 82 | 4.41 | 2.60, 7.49 | < 0.0011 | |
| Dilation (%) | 76 | 90 | 2.9 | 1.48, 5.68 | 0.0021 | |
| Irregularities (%) | 31 | 82 | 10.27 | 6.01, 17.56 | < 0.0011 |
On non-magnified NBI endoscopy, the incidence of a brownish area was significantly higher in the SC group than in the non-SC group (79% vs 57%, respectively, P < 0.001). On magnified NBI endoscopy, the incidence of IBE was also significantly higher in the SC group (68% vs 33%, respectively, P < 0.001). Furthermore, the incidences of microvascular proliferation (82% vs 51%, P < 0.001), dilation (90% vs 76%, P < 0.001), and irregularities (82% vs 31%, P < 0.001) were again significantly greater in the SC group.
The results of multivariate logistic regression analysis are listed in Table 3. Significant differences between the non-SC and SC groups were identified for redness (P = 0.022) on CWL endoscopy, and for IBE (P < 0.001) and microvascular irregularities (P < 0.001) on magnified NBI. The diagnostic sensitivity, specificity, and accuracy using redness alone and redness plus IBE and microvascular irregularities are indicated in Table 4. The redness of lesions on CWL endoscopy had 72% sensitivity, 59% specificity, and 62% accuracy for a diagnosis of superficial carcinoma in the oropharynx and hypopharynx. If IBE and irregularities on magnified NBI endoscopy were taken into account in addition to the redness of lesions, the diagnostic yield was 52% for sensitivity, 92% for specificity, and 82% for accuracy. The sensitivity for redness alone was significantly higher than that for redness plus IBE and microvascular irregularities (P = 0.002). Specificity and accuracy for redness plus IBE and microvascular irregularities were significantly higher than values for redness alone (P < 0.001 and P < 0.001, respectively).
| Endoscopy | Endoscopic findings | Non-SC (n = 333) | SC (n = 112) | OR | 95% CI | P value |
| CWL (Non-magnified) | Color (redness; %) | 41 | 72 | 2.16 | 1.12, 4.16 | 0.0221 |
| Macroscopic type (flat or depressed; %) | 44 | 58 | 0.84 | 0.47, 1.51 | ||
| NBI | ||||||
| (Non-magnified) | Brownish area (%) | 57 | 79 | 0.63 | 0.26, 1.56 | |
| (Magnified) | IBE (%) | 33 | 68 | 3.39 | 1.88, 6.11 | < 0.0011 |
| Microvascular | ||||||
| Proliferation (%) | 51 | 82 | 1.64 | 0.81, 3.35 | ||
| Dilation (%) | 76 | 90 | 0.91 | 0.39, 2.13 | ||
| Irregularities (%) | 31 | 82 | 7.51 | 4.02, 14.06 | < 0.0011 |
| Endoscopic findings | P value1 | ||
| Redness | Redness plus IBE and microvascular irregularities | ||
| % Sensitivity (95% CI) | 72 (63-80) | 52 (42-61) | 0.002 |
| % Specificity (96% CI) | 59 (53-64) | 92 (88-94) | < 0.001 |
| % Accuracy (99% CI) | 62 (58-65) | 82 (78-85) | < 0.001 |
There were no complications, such as massive hemorrhaging or aspiration, associated with the endoscopic examinations undertaken in the present study cohort.
Advances in endoscopic techniques enabling accurate diagnoses to be made in the oropharynx and hypopharynx are crucially needed. At present, endoscopic biopsy in the pharyngeal region may be quite challenging due to the gag reflex and the complicated structure of the region. Indeed, in our present study we failed to obtain sufficient biopsy material for the histologic assessment of 10 lesions. Thus, progress in endoscopic diagnostics in the pharyngeal region will be of greater clinical significance than for other regions of the gastrointestinal tract.
Previous studies have revealed that a brownish area with a proliferation of dilated microvessels on NBI endoscopy facilitates the endoscopic detection of OHSCCs at an early stage[8-10]. However, the differences that are evident on NBI and CWL endoscopy and that can differentiate between non-SC and SC lesions in the oropharynx and hypopharynx remain unclear. The results of the present study suggest that there are several endoscopic features that are closely related to SC lesions that could be used in the differential diagnosis of SC and non-SC lesions. Our univariate analysis revealed that SC lesions are associated with a brownish area and microvascular proliferation and dilation. These results are similar to those of a previous study that reported that all malignant lesions appear as well-demarcated brownish areas with proliferation of dilated microvessels[8].
However, the multivariate analysis performed in the present study revealed that redness, IBE, and microvascular irregularities seem to be independent factors related to SC lesions, rather than a brownish area or microvascular dilation and proliferation. In our non-SC group, most lesions (61%) were found to be inflammatory lesions (n = 115) or low-grade dysplasias (n = 89), which are often found to have a brownish area (80% and 67%, respectively) or to exhibit microvascular proliferation (63% and 62%, respectively) and dilation (89% and 78%, respectively). The relatively high rates of these endoscopic features in inflammatory lesions and low-grade dysplasias may have affected the outcome of the multivariate analysis in the present study.
The sensitivity of redness by CWL endoscopy alone for SC lesions was significantly higher than that combined with IBE and microvascular irregularities by magnified NBI endoscopy. Conversely, the specificity and accuracy of redness plus IBE and microvascular irregularities for SC lesions were significantly higher than redness alone. These results suggest that magnified NBI endoscopy may complement CWL endoscopy in the diagnosis of SC lesions.
Ishihara et al[15] have reported that in the esophagus, the criterion of a brownish epithelium (with the same definition used for IBE in the present study) was an independent factor significantly associated with high-grade intraepithelial neoplasia consisting of HGD and intramucosal SCC. IBE seems to be a key finding associated with superficial high-grade neoplasms. Histologically, the appearance of IBE could be attributed to increased intraepithelial cell density or changes in the intraepithelial cells themselves as a result of malignant transformation. Further investigations in the future are needed to identify the mechanisms underlying IBE.
There are some notable limitations to the present study. First, the three endoscopic procedures performed in each patient were performed by the same endoscopist. Thus, the possibility exists that the subsequent assessment of endoscopic images may have been influenced by information bias and a carryover effect from prior endoscopies. Moreover, it remains unclear whether non-magnified or magnified NBI endoscopy provides an additional benefit over CWL endoscopy alone. Second, our histological diagnoses were based on biopsy samples obtained during the endoscopic procedure. The histologic findings for these samples may therefore not be representative of the histology of the entire lesion.
The image resolution of a gastrointestinal endoscope is greater than that of a rhinolaryngoscope. Because of the growing interest in the pharyngeal region by gastroenterologists, the number of cases of OHSCCs detected and treated by gastrointestinal endoscopy is also increasing[16,17]. However, the field of view obtained using gastrointestinal endoscopy is limited compared with the comprehensive examinations performed by otorhinolaryngologists, using rhinolaryngoscopy as well as direct visual inspection. It thus remains difficult for gastrointestinal endoscopists to detect or treat tumors of the tongue, the floor of the oral cavity, and the nasopharynx. Hence, to improve the prognosis and quality of life of patients with head and neck SCCs and/or esophageal SCCs, gastrointestinal endoscopists and otorhinolaryngologists need to collaborate in the future to further develop endoscopic diagnosis and treatment methods for these conditions.
The endoscopic features of redness, IBE, and microvascular irregularities seem to be significantly associated with SC lesions in the oropharynx and hypopharynx. The diagnostic accuracy of redness plus IBE and microvascular irregularities for SC lesions was found to be greater than the accuracy of redness alone. Therefore, magnified NBI endoscopy may increase the diagnostic accuracy of CWL endoscopy for SC lesions.
Prolonged tobacco use and alcohol consumption may result in the synchronous or metachronous development of squamous cell carcinoma (SCC), both in the pharynx and the esophagus. The prognosis of patients with esophageal SCC has improved due to the early detection of these lesions as a result of the widespread adoption of an upper gastrointestinal endoscopy with Lugol staining. However, an early detection of oropharyngeal and hypopharyngeal SCCs (OHSCCs) remains difficult because Lugol staining is not possible during a screening endoscopy for OHSCCs. Moreover, OHSCCs at an early stage show an obscure appearance and manifest few symptoms. Hence, most OHSCCs are detected at advanced stages, resulting in an extremely poor prognosis, including that of patients with esophageal SCCs. Identifying the endoscopic features of superficial OHSCCs is thus essential for both early detection and differential diagnosis.
The narrow-band imaging (NBI) system is a revolutionary optical image-enhanced technology that uses narrow bandwidth NBI filters. Magnifying endoscopy combined with NBI clearly visualizes the surface microvascular structures in different organs as NBI illumination consists of two limited wavelength ranges that are well absorbed by hemoglobin. NBI endoscopy can visualize a hemoglobin rich lesion such as a superficial squamous neoplasia as a brownish area with no use of Lugol staining.
Recent studies have reported the usefulness of NBI for the early detection and diagnosis of superficial OHSCCs using gastrointestinal endoscopy or rhinolaryngoscopy. However, there has been no report to date that has compared the endoscopic features of non-neoplastic lesions and pharyngeal squamous neoplasias using magnified NBI as well as conventional white light (CWL) endoscopy. This is the first report to investigate the relationships between detailed findings of CWL endoscopy, magnified NBI endoscopy, and histology in an analysis of superficial pharyngeal lesions. Moreover, we have for the first time evaluated the diagnostic utility of magnified NBI endoscopy for superficial OHSCCs compared with CWL endoscopy.
This study may help to reveal endoscopic features of superficial carcinomas in the pharynx and the accuracy of endoscopy with CWL, NBI, or magnified NBI for diagnosing superficial carcinoma. Identifying these endoscopic features will lead to an enhanced detection rate of these lesions and will be useful in making a differential diagnosis.
NBI is a revolutionary optical image-enhanced technology that allows people to see an obscure tumor as a brownish area and observe the detailed structure of superficial microvessels when this technology is combined with magnified endoscopy. Superficial carcinoma is histologically defined as a high-grade dysplasia (HGD) as well as an SCC as HGD seems to have the same clinical implications as SCC in situ.
The topic itself is interesting for otorhinolaryngologists as well as for gastroenterologists. The NBI technique can still be considered as a novel method and therefore is a valuable source of new and interesting studies such as this one. The authors outlined the limitations of this study, because many researchers do not do so. The abstract and key words are appropriate. The abstract gives clear insight into the materials and diagnostic procedures used. The materials and methods section is also appropriately detailed and will enable replication of the methodologies used in this study. The discussion is well organized, systematic and clearly written. The cited references are relevant and appropriate. The figures are nicely incorporated in the text and appropriately presented. The language used is good and clear. It is a well structured article.
Peer reviewer: Marko Duvnjak, MD, Department of Gastroenterology and Hepatology, Sestre Milosrdnice University Hospital, Vinogradska cesta 29, 10 000 Zagreb, Croatia
S- Editor Yang XC L- Editor O’Neill M E- Editor Li JY
| 1. | Argiris A, Eng C. Epidemiology, staging, and screening of head and neck cancer. Cancer Treat Res. 2003;114:15-60. [PubMed] |
| 2. | SLAUGHTER DP, SOUTHWICK HW, SMEJKAL W. Field cancerization in oral stratified squamous epithelium; clinical implications of multicentric origin. Cancer. 1953;6:963-968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 3. | Dubuc J, Legoux JL, Winnock M, Seyrig JA, Barbier JP, Barrioz T, Laugier R, Boulay G, Grasset D, Sautereau D. Endoscopic screening for esophageal squamous-cell carcinoma in high-risk patients: a prospective study conducted in 62 French endoscopy centers. Endoscopy. 2006;38:690-695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 80] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 4. | Kodama M, Kakegawa T. Treatment of superficial cancer of the esophagus: a summary of responses to a questionnaire on superficial cancer of the esophagus in Japan. Surgery. 1998;123:432-439. [PubMed] |
| 5. | Matsubara T, Yamada K, Nakagawa A. Risk of second primary malignancy after esophagectomy for squamous cell carcinoma of the thoracic esophagus. J Clin Oncol. 2003;21:4336-4341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 6. | de Graeff A, de Leeuw JR, Ros WJ, Hordijk GJ, Blijham GH, Winnubst JA. Long-term quality of life of patients with head and neck cancer. Laryngoscope. 2000;110:98-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 190] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 7. | Gono K, Yamazaki K, Doguchi N Nonami T, Obi T, Yamaguchi M, Ohyama N, Machida H, Sano Y, Yoshida S, Hamamoto Y. Endoscopic observation of tissue by narrowband illumination. Opt Rev. 2003;10:211-215. |
| 8. | Muto M, Nakane M, Katada C, Sano Y, Ohtsu A, Esumi H, Ebihara S, Yoshida S. Squamous cell carcinoma in situ at oropharyngeal and hypopharyngeal mucosal sites. Cancer. 2004;101:1375-1381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 281] [Cited by in RCA: 283] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 9. | Watanabe A, Tsujie H, Taniguchi M, Hosokawa M, Fujita M, Sasaki S. Laryngoscopic detection of pharyngeal carcinoma in situ with narrowband imaging. Laryngoscope. 2006;116:650-654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 10. | Muto M, Minashi K, Yano T, Saito Y, Oda I, Nonaka S, Omori T, Sugiura H, Goda K, Kaise M. Early detection of superficial squamous cell carcinoma in the head and neck region and esophagus by narrow band imaging: a multicenter randomized controlled trial. J Clin Oncol. 2010;28:1566-1572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 427] [Cited by in RCA: 525] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 11. | Dawsey SM, Lewin KJ, Wang GQ, Liu FS, Nieberg RK, Yu Y, Li JY, Blot WJ, Li B, Taylor PR. Squamous esophageal histology and subsequent risk of squamous cell carcinoma of the esophagus. A prospective follow-up study from Linxian, China. Cancer. 1994;74:1686-1692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 140] [Reference Citation Analysis (0)] |
| 12. | Wang GQ, Abnet CC, Shen Q, Lewin KJ, Sun XD, Roth MJ, Qiao YL, Mark SD, Dong ZW, Taylor PR. Histological precursors of oesophageal squamous cell carcinoma: results from a 13 year prospective follow up study in a high risk population. Gut. 2005;54:187-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 272] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 13. | Dixon MF. Gastrointestinal epithelial neoplasia: Vienna revisited. Gut. 2002;51:130-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 445] [Cited by in RCA: 498] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 14. | Goda K, Tajiri H, Kato M, Yoshida Y, Kaise M, Ikegami M, Seino Y, Saito T, Kato T, Moriyama H. Clinical significance of magnified endoscopy with narrow band imaging for neoplastic lesions at oropharyngeal and hypopharyngeal sites [in Japanese with English abstract]. Endoscopia Digestiva. 2006;18:1427-1435. |
| 15. | Ishihara R, Inoue T, Uedo N, Yamamoto S, Kawada N, Tsujii Y, Kanzaki H, Hanafusa M, Hanaoka N, Takeuchi Y. Significance of each narrow-band imaging finding in diagnosing squamous mucosal high-grade neoplasia of the esophagus. J Gastroenterol Hepatol. 2010;25:1410-1415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 57] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 16. | Shimizu Y, Yamamoto J, Kato M, Yoshida T, Hirota J, Ono Y, Nakagawa M, Nakagawa S, Oridate N, Asaka M. Endoscopic submucosal dissection for treatment of early stage hypopharyngeal carcinoma. Gastrointest Endosc. 2006;64:255-259; discussion 255-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 71] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 17. | Katada C, Tanabe S, Koizumi W, Higuchi K, Sasaki T, Azuma M, Katada N, Masaki T, Nakayama M, Okamoto M. Narrow band imaging for detecting superficial squamous cell carcinoma of the head and neck in patients with esophageal squamous cell carcinoma. Endoscopy. 2010;42:185-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 73] [Article Influence: 4.9] [Reference Citation Analysis (0)] |