Published online Nov 28, 2011. doi: 10.3748/wjg.v17.i44.4899
Revised: June 15, 2011
Accepted: June 22, 2011
Published online: November 28, 2011
AIM: To identify the characteristic clinical, laboratory and radiological findings and response to treatment in patients with fascioliasis.
METHODS: Patients who were diagnosed with Fasciola hepatica infection were included in this prospective study. Initial clinical, laboratory and radiological findings were recorded. All patients were followed until a complete response was achieved or for 6 mo after treatment discontinuation.
RESULTS: Fasciola hepatica infection was diagnosed in 30 patients (24 females; mean age: 42.6 years) between January 2008 and February 2011. Twenty-two (73%) patients had hepatic phase fascioliasis, 5 patients had biliary phase, and 3 patients had biliary phase associated with acute pancreatitis. Of the 8 patients with biliary phase fascioliasis, 2 patients displayed features that overlapped with both hepatic and biliary phase. Abdominal pain and right upper abdominal tenderness were the most prominent signs and symptoms in all patients. Eosinophilia was the most prominent laboratory abnormality in both patients with hepatic and biliary phase (100% and 50%, respectively). Multiple nodular lesions like micro-abscesses on abdominal computerized tomography were the main radiological findings in patients with hepatic phase. Small linear filling defects in the distal choledochus were the main endoscopic retrograde cholangiopancreatography (ERCP) findings in patients with biliary phase. Patients with hepatic phase were treated with triclabendazole alone, and patients with biliary phase were treated with triclabendazole and had live Fasciola hepatica extracted from the bile ducts during ERCP.
CONCLUSION: Fasciola hepatica infection should be considered in the differential diagnosis of patients with hepatic or biliary disease and/or acute pancreatitis associated with eosinophilia.
- Citation: Kaya M, Beştaş R, Çetin S. Clinical presentation and management of Fasciola hepatica infection: Single-center experience. World J Gastroenterol 2011; 17(44): 4899-4904
- URL: https://www.wjgnet.com/1007-9327/full/v17/i44/4899.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i44.4899
Fascioliasis is an infection caused by a trematode of the liver, Fasciola hepatica, that particularly affects sheep, goats and cattle. The flukes are leak-like, flat worms, measuring 2-4 cm[1]. The number of reports of humans infected with Fasciola hepatica has increased significantly since 1980, and several geographical areas have been described as endemic for the disease in humans, with prevalence and incidence ranging from low to very high[2,3]. In humans, the infection begins with the ingestion of watercress or contaminated water containing encysted larva. The larva excyst in the stomach, penetrate the duodenal wall, escape into the peritoneal cavity, and then pass through the liver capsule to enter the biliary tree[1]. Human fascioliasis has two phases. The hepatic phase of the disease begins one to three months after ingestion of metacercariae, with penetration and migration through the liver parenchyma toward the biliary ducts[1,4,5]. Common signs and symptoms of the hepatic phase are abdominal pain, fever, eosinophilia, and abnormal liver function tests[1,4,6-8]. The biliary phase of the disease usually presents with intermittent right upper quadrant pain with or without cholangitis or cholestasis[9-11].
In non-endemic areas, diagnosis of fascioliasis can be difficult and usually is delayed because the disease is not often encountered and the symptoms may be confused with other hepatic or biliary disorders. Diagnosis of Fasciola hepatica infection has traditionally relied on detecting the presence of eggs in fecal samples, but this method is unreliable and complex[1,4]. Among human cases in non-endemic areas, low egg outputs, e.g., 1-2 eggs per g of feces (epg) and 1-4 epg were being considered rare. These egg outputs are much lower than those found among humans in endemic areas[3]. Computerized tomographic (CT) findings in patients with hepatic phase and ultrasonographic findings in patients with biliary phase are used for the diagnosis of fascioliasis[5,6]. Confirmation of the diagnosis is necessary and should be based on serological findings and parasitic tests[12]. Triclabendazole and bithionol are effective agents for the treatment of fascioliasis[8].
The aim of this prospective study was to identify the characteristic clinical, laboratory and tomographic findings and response to treatment during follow-up in patients with fascioliasis.
Patients who were admitted to our clinic and were diagnosed with Fasciola hepatica infection between January 2008 and February 2011 were prospectively enrolled in this study. All patients received an initial complete clinical exam, laboratory tests (including complete blood counts and routine biochemical analyses), and abdominal CT. All of the CT scans were obtained using a 4-channel multi-slice CT scanner (Sensation 4; Siemens Medical Solutions, Erlargen, Germany). A specific indirect hemagglutination assay (IHA) using purified adult Fasciola hepatica F1 antigen (Laboratoires Fumouze Diagnostic, Levallois Perret, France; cut-off 1/320) was used for serological diagnosis of fascioliasis. The diagnosis of Fasciola hepatica infection with hepatic phase was based on: (1) the presence of characteristic findings on the abdominal CT examination, as previously described[5-8]; (2) exclusion of all other known diseases that cause hepatic lesions on tomographic examination; and (3) a positive specific IHA for Fasciola hepatica; and/or (d) the presence of Fasciola hepatica eggs in the fecal examination. The diagnosis of Fasciola hepatica infection with biliary phase was based on the extraction of live Fasciola hepatica during endoscopic retrograde cholangiopancreatography (ERCP). In all patients, clinical and laboratory response to treatment was assessed monthly. In patients with hepatic phase fascioliasis, radiological improvement was assessed at a 3-mo interval. All patients were followed until complete clinical and laboratory response or until 6 mo after treatment discontinuation.
This work has been carried out in accordance with the Declaration of Helsinki (2000) of the World Medical Association. This prospective study was approved by Institutional Review Board, and all patients provided informed written consent for participation.
Fasciola hepatica infection was detected in 30 patients (24 females, mean age: 42.6 years, range: 19-79 years). In 22 (73%) patients, the diagnosis of fascioliasis was based on radiological findings on abdominal CT examination and positive IHA test (≥ 1/620). We did not perform ERCP in these patients because they did not have clinical or laboratory findings compatible with extrahepatic biliary obstruction; therefore, these patients were accepted as hepatic phase fascioliasis. In the remaining 8 (27%) patients, the diagnosis of fascioliasis was confirmed by extraction of live, mobile Fasciola hepatica from extrahepatic biliary ducts during ERCP; therefore, these patients were accepted as biliary phase fascioliasis. Microscopic examination of fecal specimens for Fasciola hepatica eggs revealed a positive result for only 2 (7%) of the 30 patients, one with biliary and one with hepatic phase.
The mean antibody titer in the IHA was 1/2720 ± 1/549 (range: 1/640-1/5120) in the 22 patients with hepatic phase fascioliasis. Three patients were sisters and were admitted to the hospital on the same day. All patients were admitted at least five (mean: 7 ± 2) d before diagnosis. The mean duration of symptoms was 25 ± 36.6 (range: 3-144) wk. Abdominal pain was reported by all patients (100%), fever in 13 (59%), nausea in 3 (14%), chills in 4 (18%), weight loss in 4 (18%), pruritus and urticaria in 1 (5%), and recurrent oral aft and asthenia in 1 (5%) patient. On physical examination, there was mild right upper quadrant tenderness in 15 (68%) patients and hepatomegaly in 6 (27%) patients. Although 13 patients had a history of intermittent fever, only 3 (14%) patients had fever > 38 °C (2 of them were sisters), during clinical follow-up.
Table 1 shows the laboratory results for patients before treatment. Anemia was present in 6 (27%) patients, leukocytosis in 11 (50%), eosinophilia in 22 (100%) and elevations in the erythrocyte sedimentation rate (ESR) in 18 (82%) patients, alanine aminotransferase (ALT) in 6 (27%), aspartate aminotransferase (AST) in 2 (9%), alkaline phosphatase (ALP) in 13 (59%) , γ glutamyl transferase (GGT) in 13 (59%), and total bilirubin in 1 (5%) patient. Platelet counts were normal in all patients.
| Variables | Hepatic phase (mean ± SD) | Biliary phase (mean ± SD) |
| Age (yr) | 40 (19-79)1 | 41 (29-49)1 |
| Gender (M/F) | (5/17) | (1/7) |
| Hb (g/dL) | 12.5 ± 1.4 | 12.7 ± 1.57 |
| WBC (n/mm3) | 11862 ± 2829 | 8765 ± 1307 |
| Eosinophil (% of total WBC count) | 34.2 ± 16.2 | 14 ± 13.5 |
| Plt (n/mm3) | 290 ± 62 | 273 ± 70 |
| ESR (mm/h) | 48 ± 26 | 17 ± 12 |
| ALT (U/dL) | 44 ± 49 | 220 ± 217 |
| AST (U/dL) | 26 ± 9 | 260 ± 357 |
| ALP (U/dL) | 157 ± 65 | 166 ± 85 |
| GGT (U/dL) | 64 ± 40 | 226 ± 128 |
| Total bilirubin (U/dL) | 0.57 ± 0.32 | 1.65 ± 2.02 |
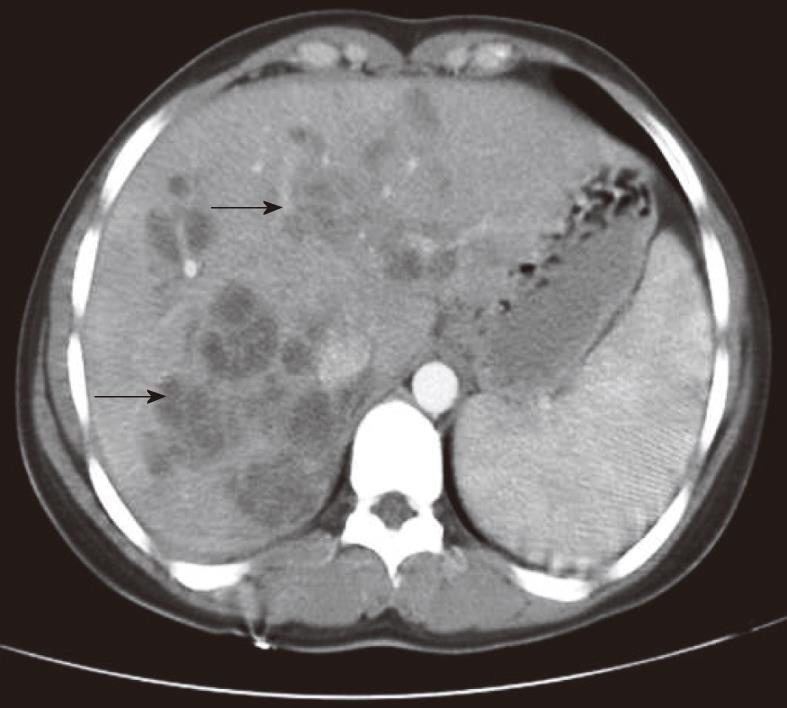
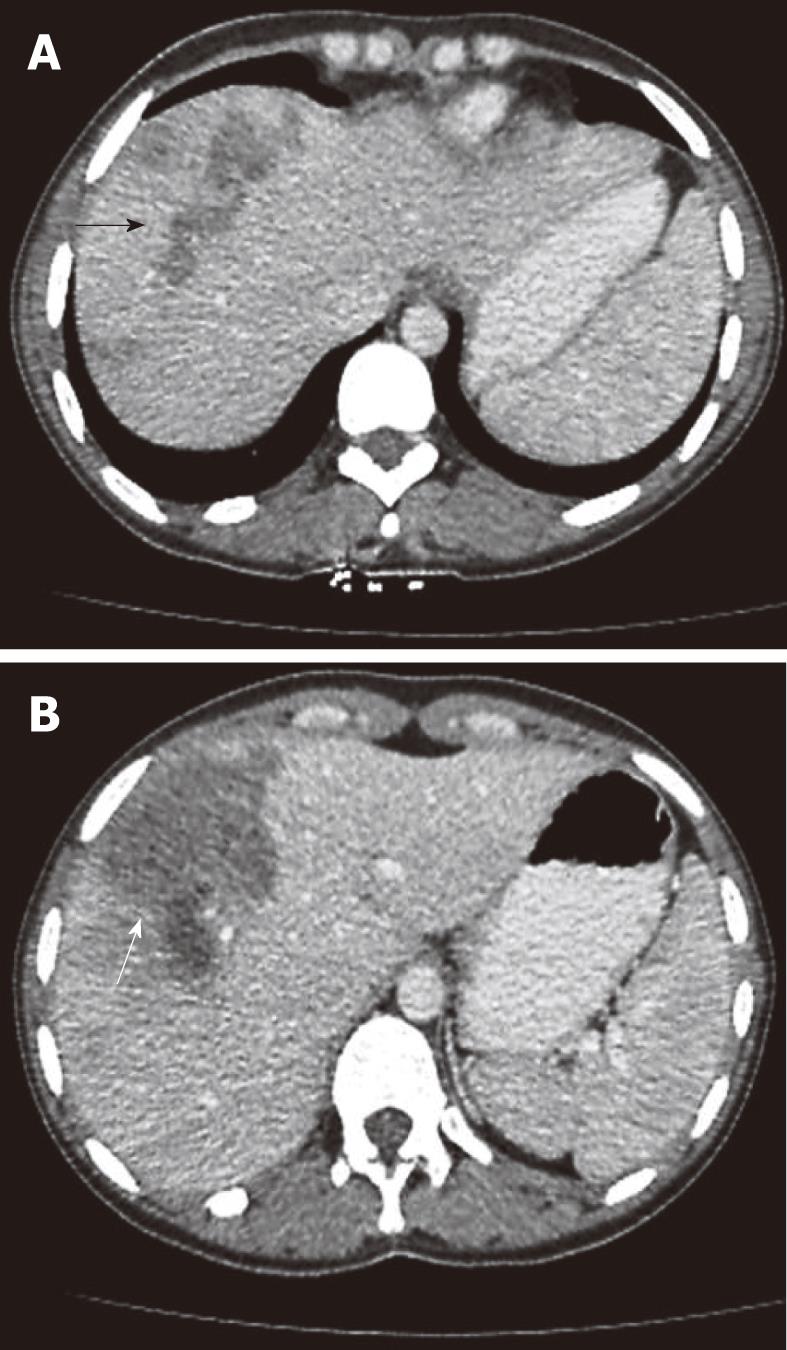
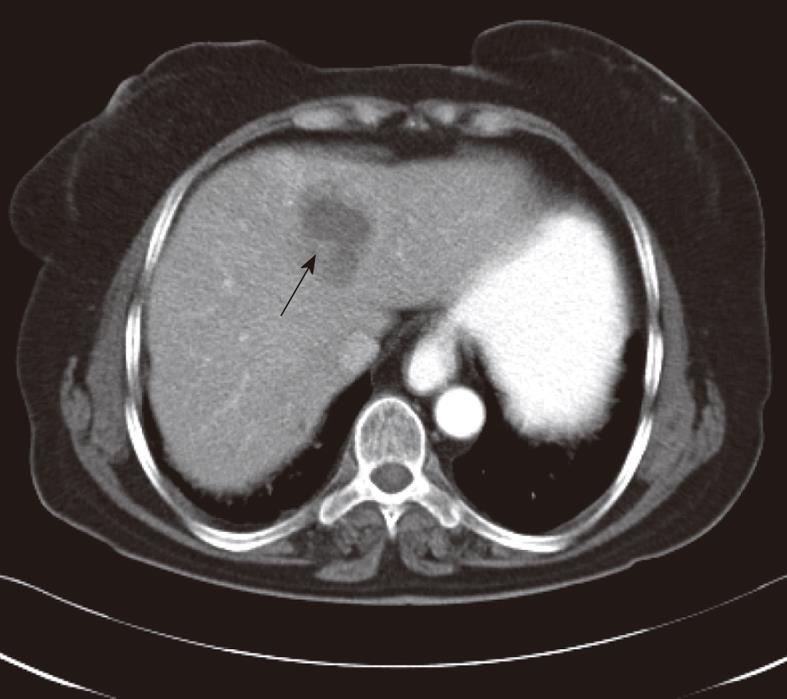
On abdominal CT examination, the main abnormalities were multiple nodular lesions like micro-abscesses (Figure 1) in 21 (95%) patients, tubular branching lesions (Figure 2A) in 11 (50%), subcapsular low density areas surrounded by a rim of parenchyma (Figure 2B) in 7 (32%), solitary nodular lesions with hazy margins (Figure 3) in 5 (23%), lymph node enlargement in the portal area in 4 (18%), and localized perihepatic fluid accumulation in 2 (9%) patients.
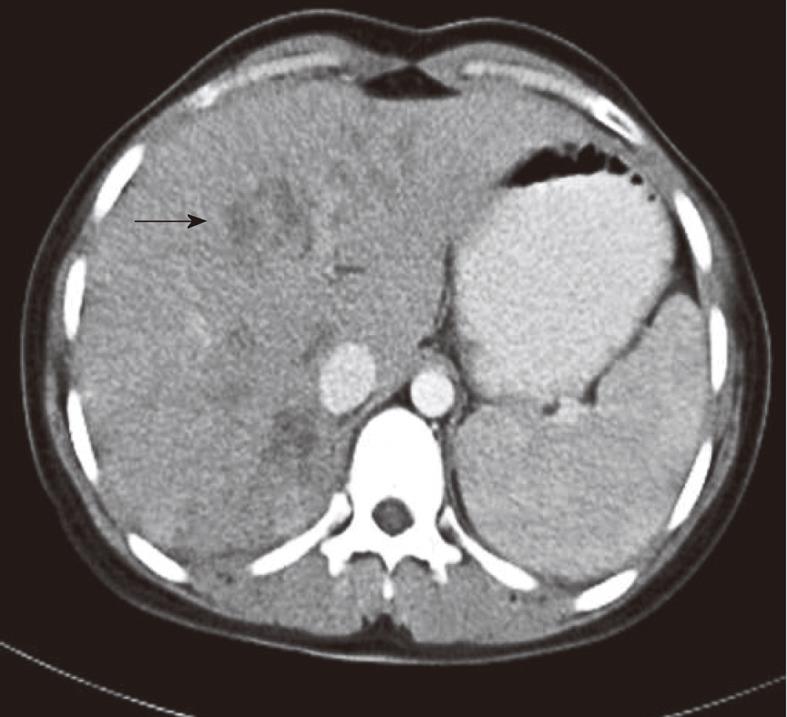
After diagnosis of fascioliasis, triclabendazole was administered at a dose of 10-12 mg/kg for 1 d to all patients. We did not observe any side-effects related to triclabendazole administration. None of the patients with hepatic phase was administered antibiotics. Three months after treatment, we observed complete clinical and laboratory recovery in 18 (82%) patients and complete improvement on abdominal CT examination in 12 (55%) patients. Six months after treatment, there was complete clinical and laboratory recovery in all patients, but abdominal CT examination showed complete improvement in only 16 (73%) patients and residual hypo-dense lesions (Figure 4) in 6 (27%) patients.
The mean duration of symptoms was 63.5 ± 80.6 (range: 1-208) wk in the 8 patients with biliary phase fascioliasis. One female patient underwent a cholecystectomy 6 mo ago after developing acute cholecystitis of unknown etiology. Abdominal pain was reported by all patients, fever in 2 (25%), nausea in 3 (38%), and weight loss in 1 (13%) patient. On physical examination, there was right upper quadrant tenderness in 5 (63%) and scleral icterus in 1 (13%) patient.
The laboratory findings before treatment (Table 1) showed that mild anemia was present in 2 (25%) patients, eosinophilia in 4 (50%), and elevations in the ESR in 4 (50%), ALT in 7 (87%), AST in 6 (75%), ALP in 4 (50%), GGT in 7 (87%), and total bilirubin in 3 (38%) patients. Total white blood cell (WBC) and platelet counts were normal in all patients. One patient had a normal initial eosinophil count but elevated eosinophil count after the ERCP procedure (2.7% of total WBC before ERCP vs 23% after ERCP).
Abdominal CT examination showed no abnormalities in 6 (75%) and subcapsular low density areas surrounded by a rim of parenchyma in 2 (25%) patients. Although these 2 patients had clinical findings consistent with biliary phase fasciolisis, they had radiological findings consistent with hepatic phase fascioliasis. One of the patients was a 46-year-old woman who had cholangitis with a normal eosinophil count and live Fasciola hepatica extracted from the extrahepatic bile ducts. The other patient was a 29-year-old man who had acute pancreatitis, cholangitis, and eosinophilia (25% of total WBC); this patient also was one of the 3 patients who had biliary phase fascioliasis associated with acute pancreatitis.
Three (38%) patients (two females) with biliary phase fascioliasis also had acute pancreatitis. Two of these patients had elevated eosinophil counts, and all 3 patients had elevated liver enzymes and amylase (> 1275 U/dL). They also had acute edematous pancreatitis and were treated by extraction of live Fasciola hepatica by balloon during ERCP and conservative management.
ERCP was performed in all patients because there were clinical and laboratory findings of extrahepatic biliary obstruction. Before ERCP, we considered the diagnosis of fascioliasis in 4 (50%) patients with eosinophilia. Cholangiography showed slight extrahepatic and intrahepatic biliary dilatation in 1 (13%) patient with acute pancreatitis. In the remaining 7 (87%) patients, the intrahepatic and extrahepatic biliary systems were within normal diameter. ERCP demonstrated a radiolucent, roughly crescent-shaped shadow in the common bile duct in all patients (Figure 5A). After standard sphincterotomy, live Fasciola hepatica (3-5 Fasciola hepatica per patient) were removed using a balloon catheter from the extrahepatic bile ducts (Figure 5B).
We routinely administered 1 g ceftriaxone at least one hour before ERCP to prevent post-ERCP cholangitis. We did not administer antibiotics to any patient after ERCP. Triclabendazole was administered at a dose of 10-12 mg/kg for 1 d to all patients. Complete clinical and laboratory recovery was observed in all patients 3 mo after treatment. There was complete resolution on abdominal CT examination in the 2 patients who had initial lesions.
Fascioliasis is an emerging disease in humans. The epidemiological and transmission characteristics of fascioliasis suggest that the disease has a patchy distribution, with foci related to the local distribution of the intermediate snail host population in freshwater bodies as well as climatic conditions[2,3]. Epidemiological studies on the incidence of fascioliasis in our region have not been reported previously, but the results from this prospective single-center study suggest that Fasciola hepatica infection is not very rare in our region. Our hospital is a tertiary care center in the southeast of Turkey that serves approximately 3 million people. All of the patients in this study resided in rural areas and had a history of consuming watercress grown in areas where sheep were raised. Twenty-four (80%) patients were female, and all of them were home-working, suggesting that the females had more contact with watercress than the men.
Fascioliasis has a hepatic phase and a biliary phase, each displaying different clinical signs and symptoms. The acute stage of fascioliasis (hepatic phase) begins with the slow migration of Fasciola hepatica through the liver parenchyma; the mature flukes digest and consume hepatocytes, dig tunnels and caves, and reside in the liver for months[1,7,13]. The hepatic phase is characterized by fever with chills, upper abdominal pain, hepatomegaly, mild hepatitis, weight loss and prominent eosinophilia[6-8,14]. Reports suggest that the clinical presentation of hepatic phase fascioliasis is similar to that of liver abscesses of other etiology[1,8]. In our patients with hepatic phase fascioliasis, the common clinical signs and symptoms were right upper abdominal pain, intermittent fever, right upper quadrant tenderness and hepatomegaly. Based on our clinical experience with pyogenic liver abscesses[15], patients with Fasciola hepatica infection had a longer duration of symptoms (25 ± 36.6 wk vs 5.7 ± 1.6 wk), a healthier condition, and less upper abdominal tenderness than patients with pyogenic liver abscesses. Although 13 (59%) of 22 patients with hepatic phase fascioliasis had a history of intermittent fever, we recorded fever in only 3 patients. These findings suggest that fever in hepatic phase fascioliasis is not a prominent finding.
In the biliary phase of the disease, patients often present with biliary colic, epigastric pain, jaundice and abdominal tenderness due to the obstruction of the bile ducts by adult worms and the resultant inflammatory response. In this stage, the main laboratory findings are cholestasis including predominantly elevated serum ALP, GGT and total bilirubin[9-11]. Adult flukes in the extrahepatic bile ducts are visualized as a filling defect on cholangiogram[1,6,9,10]. Because of the chronic inflammation, the thickened walls of the extrahepatic ducts and gallbladder are visible on abdominal CT examination[1,5,6]. Although our patients with biliary phase fascioliasis had clinical signs and symptoms of biliary obstruction, we did not find the typical cholestatic biochemical abnormalities in these patients. We also found no specific abnormalities on abdominal CT examination in our patients with biliary phase except for slight dilatation of intrahepatic and extrahepatic bile ducts in 1 patient and subcapsular low density areas surrounded by a rim of parenchyma in 2 patients. The absence of bile duct wall thickness on CT examination and the absence of biochemical findings indicative of cholestasis in our patients may reflect intermittent rather than chronic biliary obstruction or our patients may have had early stage biliary duct involvement. The 2 patients with parenchymal lesions probably had overlapping hepatic and biliary phase fascioliasis. The most specific cholangiographic finding in our patients with biliary phase fascioliasis was a radiolucent, roughly crescent-shaped shadow in the extrahepatic bile ducts without dilatation.
Major causes of acute pancreatitis include alcohol ingestion and gallstones[16-18]. A small number of patients who have Fasciola hepatica infection complicated with acute pancreatitis have been reported. The pathogenesis of acute pancreatitis secondary to fascioliasis is unknown. Intermittent biliary obstruction and cholangitis caused by adult Fasciola hepatica may be the principle mechanism involved in the development of acute pancreatitis[19-21]. In our case series, 3 (38%) of 8 patients (2 with eosinophilia) with biliary phase fascioliasis also had acute edematous pancreatitis. One of the 3 patients, had both hepatic and biliary phase fascioliasis. Although our number of cases is small, we suggest that Fasciola hepatica infection should be considered during differential diagnosis in patients with acute pancreatitis associated with cholangitis and eosinophilia.
Diagnosis of fascioliasis may be delayed because of the wide spectrum of the differential diagnosis and the low incidence of Fasciola hepatica infection[3]. The abnormal laboratory and radiological findings in Fasciola hepatic infection may represent viral hepatitis, liver abscess, malignancy, cholecystitis, sclerosant cholangitis, AIDS-related cholangitis, ruptured hydatic cyst, and infection with parasites such as ascariasis and clonorchiasis[1,5,8]. The specificity of the indirect hemagglutination test (IHA) using purified adult Fasciola hepatica antigen F1 is 96.9% for serological diagnosis of Fasciola hepatica infection[12]. Diagnosis is confirmed only by demonstrating live parasites or eggs in the bile or feces[1,5,8]. The disease cannot be ruled out by a negative stool examination[3,5,8]. A high index of suspicion and specific radiological findings are very helpful in the diagnosis. We suspected of the possibility of fascioliasis in all patients with hepatic phase because of the presence of eosinophilia, characteristic abdominal CT findings, and typical clinical sign and symptoms. We found eggs in stool samples in 1 of 22 patients with hepatic phase fascioliasis and 1 of 8 patients with biliary phase fascioliasis. Diagnosis in patients with hepatic phase was confirmed by the clinical, laboratory and radiological responses to triclabendazole treatment and the high titer in the IHA. Diagnosis in patients with biliary phase fascioliasis was confirmed by extraction of live Fasciola hepatica form bile ducts. We suggest that stool examination for eggs is not a reliable method and that both serological testing and extraction of live parasites form bile ducts are very reliable methods for the diagnosis of fascioliasis.
Treatment of human fascioliasis has been difficult for a long time. Today, triclabendazole is the drug of choice for its effectiveness against both adult and immature worms[7,13,21]. Its anti-parasitic effect is derived from the inhibition by an active sulfoxide metabolite of the synthesis of the tegumental ultra-structure of Fasciola hepatica[22]. Triclabendazole at a dose of 10 mg/kg body weight (single or split postprandial dose) reportedly is effective in about 80%-90% of patients and is well tolerated. The most common drug-related side-effects are nausea, vomiting and abdominal pain[23]. All of our patients with hepatic phase were treated with triclabendazole alone, and those patients with biliary phase were treated with endoscopic sphincterotomy, extraction of live parasite from bile ducts, and administration of triclabendazole. We observed that triclabendazole improved both clinical and laboratory findings in a few weeks; radiological improvement, however, required a longer period.
In conclusion, in addition to classically defined hepatic phase and biliary phase fascioliasis, some cases may have overlap of these two phases with or without acute pancreatitis. In cases of right abdominal pain, elevated eosinophil count, and multiple micro-abscesses and/or tunnel-like hypo-dense lesions on abdominal CT examination, hepatic phase fascioliasis should be considered, and a serological test for Fasciola hepatica should be used for diagnosis. In cases of biliary colic and/or acute pancreatitis associated with eosinophilia, we suggest that biliary phase fascioliasis should be considered, and ERCP should be used for both diagnosis and treatment.
In non-endemic areas, diagnosis of fascioliasis is difficult and usually is delayed because the disease is relatively rare and the symptoms may be confused with other hepatic or biliary disorders. Confirmation of the diagnosis is necessary and patients should be followed for response to treatment.
Fascioliasis has a hepatic phase and a biliary phase, each displaying different clinical signs and symptoms. In addition to classically defined hepatic phase and biliary phase fascioliasis, some cases may have overlap of these two phases with or without acute pancreatitis.
Fascioliasis may have different clinical presentations. In cases of abdominal pain and elevated eosinophil count, fascioliasis should be considered in differential diagnosis. Serological tests and abdominal computerized tomographic (CT) examination are the methods of choice for diagnosis. But, fecal examination for Fasciola hepatica eggs is not a reliable diagnostic method. It may take a long time for complete clinical and radiological improvement after triclabendazole administration.
Fasciola hepatica infection should be considered in the differential diagnosis of patients with hepatic or biliary disease and/or acute pancreatitis associated with eosinophilia.
Fascioliasis is an infection caused by a trematode of the liver. Fasciola hepatica, particularly affects sheep, goats and cattle. The flukes are leak-like, flat worms, measuring 2-4 cm. In humans, the infection begins with the ingestion of watercress or contaminated water containing encysted larva.
The authors investigated the characteristic clinical, laboratory, and tomographic findings and response to treatment during follow-up in patients with fascioliasis. They revealed that fascioliasis has different clinical presentations and in cases of right abdominal pain, elevated eosinophil count, and multiple micro-abscesses and/or tunnel-like hypo-dense lesions on abdominal CT examination, the diagnosis of hepatic phase fascioliasis should be considered, and a serological test for Fasciola hepatica should be used for diagnosis.
Peer reviewer: Kiichi Tamada, MD, Department of Gastroenterology, Jichi Medical Shool, 3311-1 Yakushiji, Minamikawachi, Kawachigun, Tochigi 329-0498, Japan
S- Editor Wu X L- Editor Ma JY E- Editor Xiong L
| 1. | Lim JH, Mairiang E, Ahn GH. Biliary parasitic diseases including clonorchiasis, opisthorchiasis and fascioliasis. Abdom Imaging. 2007;33:157-165. [PubMed] |
| 2. | Parkinson M, O'Neill SM, Dalton JP. Endemic human fasciolosis in the Bolivian Altiplano. Epidemiol Infect. 2007;135:669-674. [PubMed] |
| 3. | Mas-Coma MS, Esteban JG, Bargues MD. Epidemiology of human fascioliasis: a review and proposed new classification. Bull World Health Organ. 1999;77:340-346. [PubMed] |
| 4. | Koç Z, Ulusan S, Tokmak N. Hepatobiliary fascioliasis: imaging characteristics with a new finding. Diagn Interv Radiol. 2009;15:247-251. [PubMed] |
| 5. | Kabaalioğlu A, Cubuk M, Senol U, Cevikol C, Karaali K, Apaydin A, Sindel T, Lüleci E. Fascioliasis: US, CT, and MRI findings with new observations. Abdom Imaging. 2000;25:400-404. [PubMed] |
| 6. | Van Beers B, Pringot J, Geubel A, Trigaux JP, Bigaignon G, Dooms G. Hepatobiliary fascioliasis: noninvasive imaging findings. Radiology. 1990;174:809-810. [PubMed] |
| 7. | Teichmann D, Grobusch MP, Göbels K, Müller HP, Koehler W, Suttorp N. Acute fascioliasis with multiple liver abscesses. Scand J Infect Dis. 2000;32:558-560. [PubMed] |
| 8. | Aksoy DY, Kerimoğlu U, Oto A, Ergüven S, Arslan S, Unal S, Batman F, Bayraktar Y. Fasciola hepatica infection: clinical and computerized tomographic findings of ten patients. Turk J Gastroenterol. 2006;17:40-45. [PubMed] |
| 9. | Bektaş M, Dökmeci A, Cinar K, Halici I, Oztas E, Karayalcin S, Idilman R, Sarioglu M, Ustun Y, Nazligul Y. Endoscopic management of biliary parasitic diseases. Dig Dis Sci. 2010;55:1472-1478. [PubMed] |
| 10. | Ozer B, Serin E, Gümürdülü Y, Gür G, Yilmaz U, Boyacioğlu S. Endoscopic extraction of living fasciola hepatica: case report and literature review. Turk J Gastroenterol. 2003;14:74-77. [PubMed] |
| 11. | Gulsen MT, Savas MC, Koruk M, Kadayifci A, Demirci F. Fascioliasis: a report of five cases presenting with common bile duct obstruction. Neth J Med. 2006;64:17-19. [PubMed] |
| 12. | Azab M el-S, el Zayat EA. Evaluation of purified antigens in haemagglutination test (IHA) for determination of cross reactivities in diagnosis of fascioliasis and schistosomiasis. J Egypt Soc Parasitol. 1996;26:677-685. [PubMed] |
| 13. | Das K, Sakuja P, Aggarwal A, Puri AS, Tatke M. Non-resolving liver abscess with Echinococcus cross-reactivity in a non-endemic region. Indian J Gastroenterol. 2007;26:92-93. [PubMed] |
| 14. | Kim KA, Lim HK, Kim SH, Lee WJ, Lim JH. Necrotic granuloma of the liver by human fascioliasis: imaging findings. Abdom Imaging. 1999;24:462-464. [PubMed] |
| 15. | Kaya M. Use of Venflon Branule needle for aspiration of liver abscesses. Indian J Gastroenterol. 2009;28:225-226. [PubMed] |
| 16. | Munsell MA, Buscaglia JM. Acute pancreatitis. J Hosp Med. 2010;5:241-250. [PubMed] |
| 17. | Clemens DL, Mahan KJ. Alcoholic pancreatitis: lessons from the liver. World J Gastroenterol. 2010;16:1314-1320. [PubMed] |
| 18. | Talukdar R, Vege SS. Recent developments in acute pancreatitis. Clin Gastroenterol Hepatol. 2009;7:S3-S9. [PubMed] |
| 19. | Badalov NL, Anklesaria A, Torok A, Wall IM, Braha J, Li J, Iswara K, Tenner S. Fasciola hepatica causing acute pancreatitis complicated by biliary sepsis. Gastrointest Endosc. 2009;70:386-37; discussion 387. [PubMed] |
| 20. | Echenique-Elizondo M, Amondarain J, Lirón de Robles C. Fascioliasis: an exceptional cause of acute pancreatitis. JOP. 2005;6:36-39. [PubMed] |
| 21. | Karabuli TA, Shaikhani MA, Karadaghi SH, Kasnazan KH. Education and imaging. Hepatobiliary and pancreatic: fascioliasis. J Gastroenterol Hepatol. 2009;24:1309. [PubMed] |
| 22. | Wilkinson M, Horackova M, Giles A. Reduction of ventricular M2 muscarinic receptors in cardiomyopathic hamster (CHF 147) at the necrotic stage of the myopathy. Pflugers Arch. 1994;426:516-523. [PubMed] |
| 23. | López-Vélez R, Domínguez-Castellano A, Garrón C. Successful treatment of human fascioliasis with triclabendazole. Eur J Clin Microbiol Infect Dis. 1999;18:525-526. [PubMed] |