Published online Nov 28, 2011. doi: 10.3748/wjg.v17.i44.4858
Revised: August 21, 2011
Accepted: October 14, 2011
Published online: November 28, 2011
AIM: To investigate the role of epidermal growth factor receptor (EGFR) in colitis-associated dysplasia using the EGFR tyrosine kinase inhibitor erlotinib.
METHODS: Sprague-Dawley rats received trinitrobenzene sulfonic acid (TNBS; 30 mg in 50% ethanol, ic), followed 6 wk later by reactivation with TNBS (5 mg/kg, iv) for 3 d. To induce colitis-associated dysplasia, rats then received TNBS (iv) twice a week for 10 wk. One group received erlotinib (10 mg/kg, ip) for 1 wk before the start of the reactivation of the colitis and 2 wk after (21 d); the rest received the vehicle. After rats were euthanized, the colons were removed and analyzed for damage and expression of the EGFR downstream effectors Erk1/2 and c-Myc.
RESULTS: Ninety percent of the vehicle-treated animals had dysplasia in any region of the colon. Erlotinib-treated animals had a significant decrease in the incidence of dysplasia compared to vehicle-treated animals in all regions of the colon (50.00% ± 11.47% vs 90.00% ± 10.00% in proximal, P < 0.05; 15.00% ± 8.19% vs 50.00% ± 16.67% in mid, P < 0.05; and 20.00% ± 9.17% vs 70.00% ± 15.28% in distal, P < 0.01). Erlotinib-treated animals also had reduced cell proliferation, reduced active Erk1/2, and reduced c-Myc in colon epithelium compared with the vehicle-treated animals. In vitro, erlotinib treatment was shown to markedly decrease c-Myc and pErk1/2 levels in rat epithelial cells. Proliferation of rat epithelial cells was stimulated by epidermal growth factor and inhibited by erlotinib (P < 0.05).
CONCLUSION: Erlotinib can decrease the development of colitis-associated dysplasia, suggesting a potential therapeutic use for erlotinib in patients with long-standing colitis.
- Citation: Pagán B, Isidro AA, Cruz ML, Ren Y, Coppola D, Wu J, Appleyard CB. Erlotinib inhibits progression to dysplasia in a colitis-associated colon cancer model. World J Gastroenterol 2011; 17(44): 4858-4866
- URL: https://www.wjgnet.com/1007-9327/full/v17/i44/4858.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i44.4858
Patients with inflammatory bowel diseases (IBD), including ulcerative colitis and Crohn’s disease, are at increased risk of developing colorectal cancer (CRC)[1,2]. The known association of IBD with CRC presents an identifiable population for preventative intervention. This preventive effort, however, relies on understanding the molecular events critical for progression from IBD to dysplasia and CRC and on the identification of suitable molecular targets to block the disease development.
Epidermal growth factor receptor (EGFR) is a trans-membrane receptor tyrosine kinase. Aberrant EGFR activity has been linked to different types of carcinoma, including CRC[3]. EGFR activates several signaling pathways that include Ras/Raf/Mek/Erk1/2 and phosphoinositide 3-kinase/PDK1/Akt to control epithelial cell proliferation and survival[4]. Aberrant EGFR activity, resulting in proliferative effects and anti-apoptosis, can be targeted with small molecule tyrosine kinase inhibitors or with specific antibodies. A number of EGFR tyrosine kinase inhibitors, such as erlotinib, gefitinib and lapatinib, have been developed to treat cancer patients or are in clinical development to treat various types of human cancer[3]. Moreover, two monoclonal antibodies to EGFR, cetuximab and panitumumab, have been approved by the US Food and Drug Administration to treat CRC patients[3,5].
During inflammatory processes like IBD, EGFR and its ligands play a repair role in colonic mucosa[6]. EGFR expression is increased in inflamed tissues of the bowel in animal models and in patients with IBD and colon cancer[7-9]. For example, in a study by Malecka-Panas, it was found that EGFR is increased in colonic mucosa by 35.2% in patients with adenomatous polyps, by 40.6% in patients with ulcerative colitis, and by 123% in patients with colon cancer[10]. One of the complications of long-standing IBD is the development of cancer[11]. The risk of cancer in patients with colitis increases with longer duration of the disease[1]. While it is not understood completely how IBD leads to neoplastic transformation and progression to CRC, higher levels of EGFR and its ligands could cause hyper-activation of growth promoting signaling pathways and may contribute to development of dysplasia. In the rat model of colon carcinogenesis induced by the carcinogen azoxymethane, EGFR is involved in the development of dysplastic lesions and colon cancer[12].
Erlotinib (Tarceva®) is the first EGFR tyrosine kinase inhibitor approved by the US Food and Drug Administration. It is currently used in clinics to treat lung and pancreatic cancer. In this study, we tested the effects of erlotinib on the occurrence of colitis-associated dysplasia in a rat model developed recently by us[13]. We hypothesized that, by inhibiting EGFR, the progression from chronic inflammation to dysplasia will be halted, as a result of the blockade of EGFR activity. Our data show that erlotinib significantly inhibits the colitis-induced dysplasia in this animal model.
Male Sprague-Dawley rats weighing 200-220 g at the start of the initial treatment were maintained in restricted-access rooms with controlled temperature (23 °C) and 12-h light-dark cycle. Standard laboratory chow (8640 Teklad Rodent Diet, Harlan Laboratories; Tampa, FL) and drinking water were provided ad libitum. One week before beginning the protocol animals were acclimatized to avoid additional stress. Animal protocols were approved by the Institutional Animal Care and Use Committee at Ponce School of Medicine.
Chronic colitis was induced by intracolonic administration of trinitrobenzene sulfonic acid (TNBS; 0.5 mL of 60 mg/mL; Sigma Aldrich; St. Louis, MO) in 50% ethanol followed by reactivation with systemically administered TNBS 6 wk later [14]. The induction was performed by using a rubber catheter, and TNBS was introduced rectally into the colon, approximately 8 cm proximal to the anus. The reactivation was performed 6 wk after the induction. Briefly, the rats were lightly anesthetized with ether, and TNBS (5 mg/kg in 0.9% saline) was administered intravenously via a tail vein every 24 h for three consecutive days[14]. Dysplasia was developed by continuing to administer the TNBS (5 mg/kg) twice a week intravenously for 10 wk[13]. The rats were weighed weekly until they were sacrificed at 10 wk with an overdose of pentobarbital (about 1.5 mL of 65 mg/kg for rats of > 500 g). The experiments reported herein were performed in accordance with the principles described in the “Guide for the Care and Use of Laboratory Animals,” publication No. DHHS (NIH) 86-23.
One group of animals was treated with the EGFR inhibitor erlotinib (a kind gift of OSI Pharmaceuticals, Farmingdale, NY). Erlotinib was administered at a dose of 10 mg/kg per day (i.p. dissolved in 0.5% methyl cellulose) from 1 wk before the start of the reactivation of the colitis until 2 wk after (21 d, Figure 1)[15]. Methyl cellulose alone was administered to a control group for the same amount of time.
After animals were euthanized, a macroscopic analysis of the colon was performed based on the criteria of Appleyard and Wallace[14]. Four variables were examined: the presence of diarrhea (0 or 1 for absence or presence), adhesions between the colon and other organs (0, 1 or 2 for none, minor or major, respectively), the thickness of each colon segment (in millimeters), and the degree of ulceration (0 for no damage; with increasing scores up to 10, depending on the extent of ulceration). These variables were added to give a total macroscopic damage score.
The colon length was measured in centimeters and cut in equal thirds representing the proximal, mid and distal parts of the colon. These segments were cut longitudinally; one half was weighed and stored at -80 °C for molecular analysis, and the other half was fixed in 10% buffered formalin for histological procedures. The Swiss-roll technique was used to evaluate each colon segment microscopically, allowing us to see the entire length of the intestine at once. Briefly, the tissue was rolled into a small piece with the help of forceps and then fixed and placed in a cassette for the remainder of the histological procedures[13].
The tissues were scored microscopically for damage by a blinded observer, as previously described[13]. Criteria included loss of mucosal architecture (0-3: absent, mild, to severe), cellular infiltration (0, none; 1, in muscularis mucosae; 2, in lamina propria/villi; 3, in serosa), muscle thickening (0, muscle < 1/2 of mucosal thickness; 1, muscle = 1/2 to 3/4 of mucosal thickness; 2, muscle = mucosal thickness; 3 = all muscle), goblet cell depletion (0, absent; 1, present), and crypt abscess formation (0, absent; 1, present). The score of each variable was added to give a total microscopic damage score (maximum of 11).
Colonic sections (2-4 μm) stained with hematoxylin and eosin were analyzed by our pathologists in a blinded manner for dysplasia. Histologic analysis for dysplasia was scored based on previously published criteria[16,17]. Briefly, tissue sections were classified as either negative for dysplasia or positive for dysplasia or carcinoma. The tissues classified as negative for dysplasia adhered to one of the following: normal (small basally located nuclei and normal architecture), non-specific inflammation (cryptitis and glandular invasion by neutrophils), or active colitis (cryptitis, glandular invasion by neutrophils, crypt abscesses, microabscesses). A classification of positive dysplasia was characterized by low-grade dysplasia, which included hyperchromasia, loss of mucin, increased nuclear/cytoplasmic ratio, nuclear elongation and stratification, irregular nuclear outline, and increased number of normal mitoses. The criteria for high-grade dysplasia included the characteristics of low-grade dysplasia plus mucosal architectural distortion including fusion of glands (cribiform pattern) and presence of vesicular polygonal nuclei. For a diagnosis of carcinoma, the characteristics of high-grade dysplasia were included in addition to presence of atypical mitosis and/or of single tumor cells within the lamina propria[16].
Formalin-fixed 4 μm tissue sections were deparaffinized with xylene, 2 changes, 15 min each, and then hydrated through descending grades of ethanol to deionized water. Antigen retrieval was performed on a hot plate using a beaker with distilled water with the appropriate buffer (0.01 mol/L citrate-ethylene-diamine-tetra-acetic acid (EDTA) buffer, pH 6.0 - high to boiling or EDTA - high to boiling). Slides were cooled at room temperature for 20 min, rinsed with deionized water, and placed in phosphate-buffered saline (PBS) for 5 min. Endogenous peroxidase was blocked with 3% aqueous hydrogen peroxide. After slides were washed with PBS for 5 min, they were blocked with normal serum for 20 min, followed by incubation with the primary antibody. Antibodies were used as follows: phosphorylated epidermal growth factor receptor-pY1068 (Cell Signaling; Danvers, MA), 1:400, overnight; antigen retrieval-EDTA buffer; and 5-bromo-2’deoxyuridine (BrdU), mouse monoclonal antibody (Santa Cruz Biotechnology; Santa Cruz, CA), 1:100, overnight, antigen retrieval-citrate-EDTA buffer. The secondary antibody (Bio-Genex Kit; San Ramon, CA) was added to the sections for 20 min and washed again with PBS for 4 minutes. Using the Bio-Genex Kit, we incubated sections with streptavidin-LSab-Peroxidase for 20 min and washed them with PBS for 4 min. The development of the sections was performed using 3,3’-diaminobenzidine tetrahydrochloride (Bio Genex, San Ramon, CA). All samples were lightly counterstained with Mayer’s hematoxylin for 15 s, dehydrated through graded alcohol, cleared with xylene, and mounted with resinous mounting medium.
Immunohistochemistry of c-Myc was performed using a Ventana Discovery XT automated slide staining instrument. The antigen retrieval method was Ventana Cell Conditioning-1. Immunohistochemical conditions for c-Myc (ab32072, Abcam) were as follows: 1:25 dilution (60 min), Ventana UltraMAP anti-rabbit (20 min).
The stains were semiquantitatively examined by two independent pathologists using the Allred 8-unit system with a combination of a proportion score from 0 to 5 and an intensity score on a scale from 0 to 3 (none, weak, moderate, strong). A total score of 2-3 was considered low, a score of 4-5 was considered intermediate, and a score of 6-8 was considered high[18].
Proteins were extracted using lysis buffer (50 mmol/L Tris-HCl, pH 7.5, 150 mmol/L NaCl, 1 mmol/L EDTA, 1 mmol/L EGTA, 25 mmol/L NaF, 5 mmol/L Na4P2O7, 1% Triton X-100, 1 mmol/L Na3VO4, 20 mmol/L p-nitrophenyl phosphate, 2 mg/mL leupeptin, 2 mg/mL aprotinin, and 1 mmol/L phenylmethyl-sulfonyl fluoride). Equal amounts (30 μg) of protein were separated on 12% sodium dodecyl sulfate gel and transferred to a polyvinylidene fluoride membrane (Bio Rad; Hercules, CA). Membranes were blocked with 5% non-fat dry milk in tris-buffered saline-tween (TBST) and incubated with one of the following primary antibodies: Erk1/2, pErk1/2, Akt, pAkt, Src, or Src-pY416 (Cell Signaling Technology; Danvers, MA). Membranes were washed with tris-buffered saline-tween and incubated with horseradish peroxidase-labeled secondary antibodies (Jackson ImmunoResearch Laboratories; West Grove, PA). The bands were detected using ECL-Plus reagent kit (GE Amersham; Piscataway, NJ). Western blotting bands were quantified by densitometry using ImageQuant 5.2 Software (Typhoon 9410; GE Amersham; Piscataway, NJ). pErk1/2, pAkt, and Src-pY416 bands were normalized for the corresponding total kinase.
Rat intestinal epithelial-1 (RIE-1) cell line (American type culture collection CRL-1592) was cultured in RPMI 1640 containing 5% fetal bovine serum. For analysis of c-Myc, cells were treated with erlotinib (LC Laboratories) as indicated in the figure legends and epidermal growth factor (EGF, 10 ng/mL; Rocky Hill, NJ) for 24 h. For analysis of pErk1/2, erlotinib- or mock-treated RIE-1 cells were stimulated with EGF (10 ng/mL) for 5 min. Cell lysates (20 μg/each) were analyzed by immunoblotting.
Cell proliferation was assayed by plating cells in quadruplet in 96-well plates (1000 cells/well). Twenty-four hours after plating, EGF (10 ng/mL) or erlotinib (10 μmol/L) was added. Four days later, viable cells were measured using CellTiterGlo reagent (Promega) as reported previously[19].
Values are presented as means ± SEM where “n” represents one tissue from one animal used for a single replicate of an experiment. Statistical analyses were performed using GraphPad Instat V3.0 and Graph Pad Prism V4.0 (Graph Pad Software, San Diego, CA). Groups were analyzed using one-way analysis of variance with Turkey’s post-test, and P < 0.05 was considered to represent a significant difference.
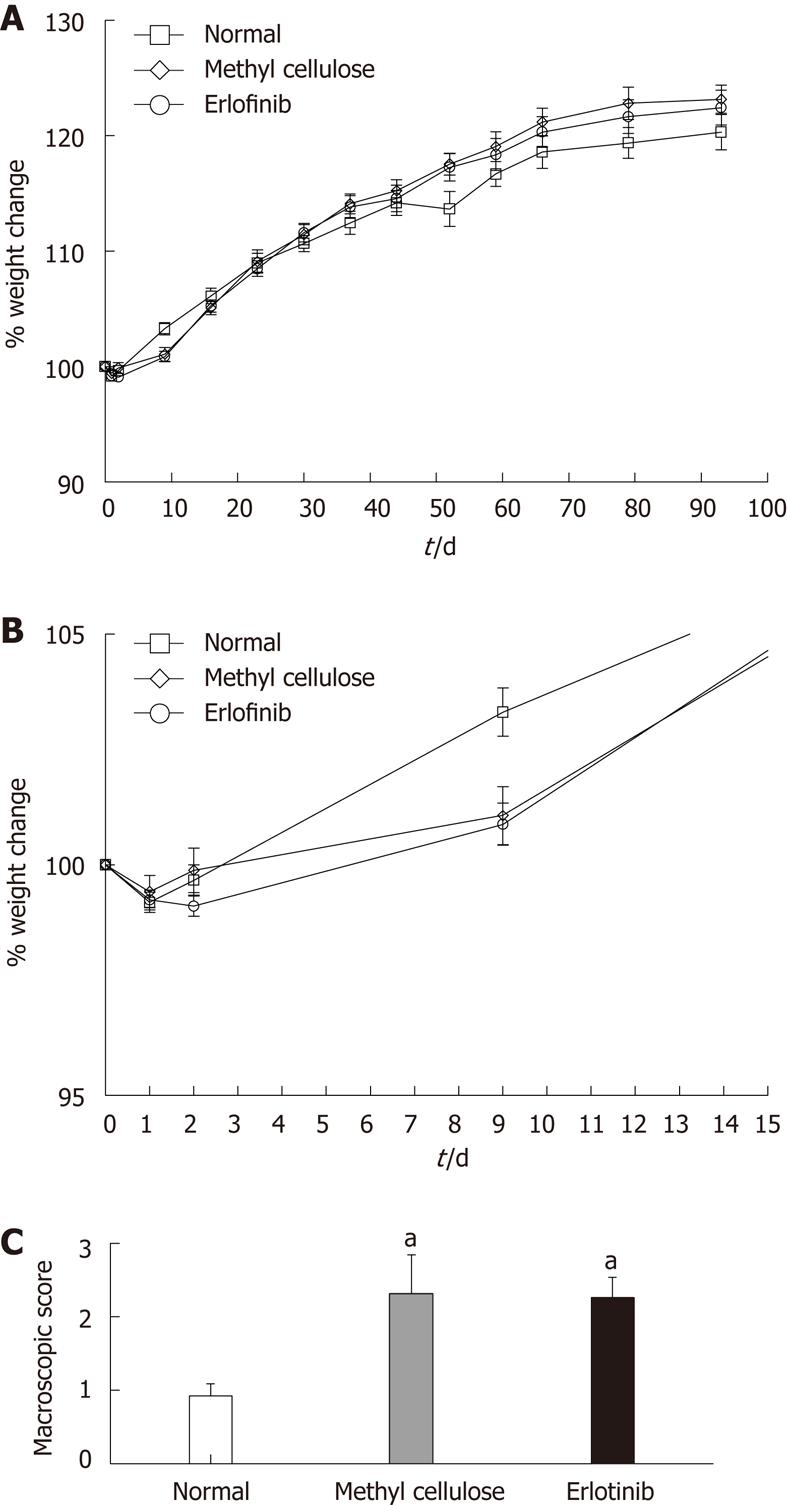
During the study (10 wk), all of the rats increased their weight in comparison with their original starting weight, apart from the first 3 d of treatment, where all groups (normal, vehicle-treated, and erlotinib-treated) lost weight (Figure 2A and B). We have observed this phenomenon in prior studies and attribute it to a combination of the intravenous administration of TNBS and the stress initially associated with the procedure[13,16]. No differences in weight change were observed between the normal, vehicle-treated, and erlotinib-treated animals during the study, suggesting no major toxicity of the drug or vehicle.
After animals were euthanized, the colons were removed to score for ulceration, adhesions, diarrhea, and thickness and to measure colon length. As expected, animals treated with TNBS and receiving the vehicle methyl cellulose had significantly higher macroscopic damage scores than the normal animals (Figure 2C). Erlotinib treatment had no effect on the macroscopic score when compared with the vehicle-treated group, with damage scores still significantly higher than normal (P < 0.05; Figure 2C). The average length of the colon in TNBS/vehicle-treated animals was shorter that normal (10.55 ± 0.42 cm vs 12.17 ± 0.38 cm). This shortening was not attenuated in erlotinib-treated animals (10.85 ± 0.45 cm).
Microscopic analysis of the colon revealed that total microscopic damage score was higher in all regions of the colon in animals receiving TNBS. The damage found was significantly higher in all regions of the colon from these animals than that shown in normal animals (P < 0.01, Table 1). Erlotinib had no effect on damage found.
| Loss of mucosal architecture | Cell infiltration | Muscle thickness | Goblet cell depletion | Crypt abscess formation | Total microscopic score | |
| Proximal | ||||||
| Normal | 0.67 ± 0.19 | 1.58 ± 0.23 | 0.83 ± 0.11 | 0.83 ± 0.11 | 0.33 ± 0.14 | 4.25 ± 0.55 |
| Vehicle | 1.40 ± 0.16b | 2.50 ± 0.12b | 1.60 ± 0.22a | 1.00 ± 0.00 | 0.80 ± 0.13 | 7.50 ± 0.27b |
| Erlotinib | 1.40 ± 0.11b | 2.35 ± 0.11b | 1.55 ± 0.17a | 1.00 ± 0.00 | 0.60 ± 0.11 | 6.90 ± 0.27b |
| Mid | ||||||
| Normal | 0.75 ± 0.18 | 1.50 ± 0.67 | 0.92 ± 0.29 | 0.83 ± 0.39 | 0.45 ± 0.51 | 4.42 ± 0.51 |
| Vehicle | 1.50 ± 0.22a | 2.70 ± 0.15b | 2.00 ± 0.26b | 1.00 ± 0.00 | 0.70 ± 0.15 | 7.90 ± 0.53b |
| Erlotinib | 1.50 ± 0.14b | 2.35 ± 0.49b | 1.85 ± 0.59b | 1.00 ± 0.00 | 0.45 ± 0.51 | 7.15 ± 0.31b |
| Distal | ||||||
| Normal | 1.08 ± 0.26 | 1.92 ± 0.23 | 1.50 ± 0.19 | 0.92 ± 0.08 | 0.25 ± 0.13 | 5.67 ± 0.66 |
| Vehicle | 1.80 ± 0.20 | 2.70 ± 0.15a | 2.20 ± 0.20b | 1.00 ± 0.00 | 0.50 ± 0.11 | 8.20 ± 0.49b |
| Erlotinib | 2.05 ± 0.15b | 2.50 ± 0.14a | 2.45 ± 0.14b | 1.00 ± 0.00 | 0.75 ± 0.10a | 8.75 ± 0.32b |
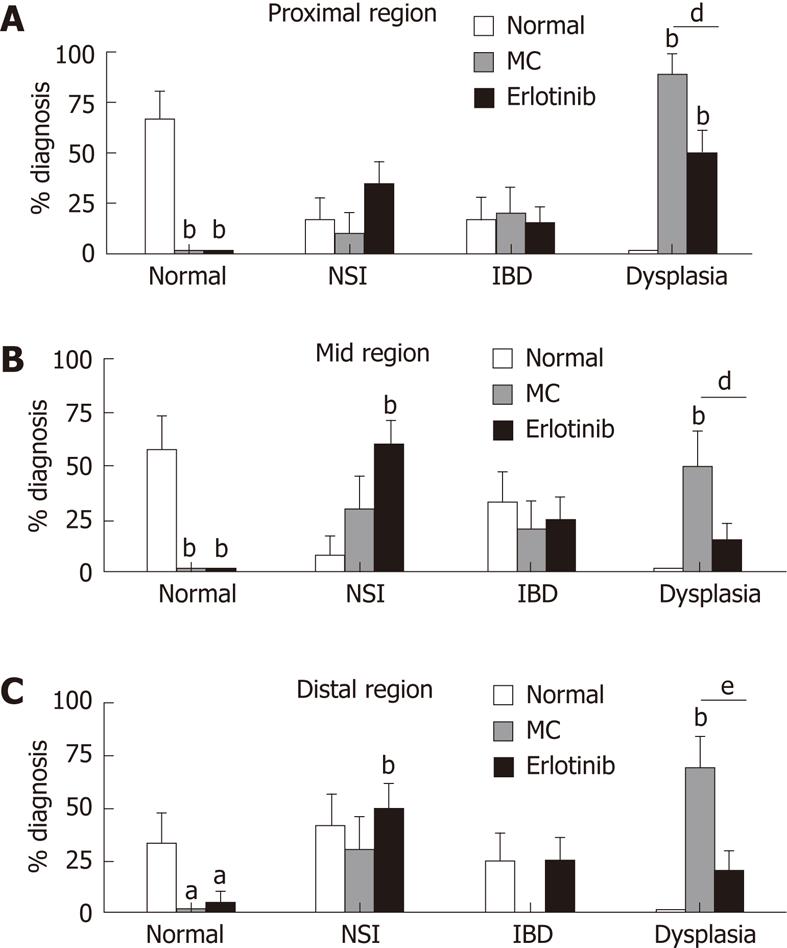
Pathological analysis identified areas of the colon as normal, showing inflammation (IBD and non-specific inflammation), and showing dysplasia. Ninety percent of the vehicle-treated animals had dysplasia in any region of the colon. This was decreased in the erlotinib-treated group such that only 55% of the animals had dysplasia in any area of the colon. When specific regions were analyzed, a decrease in dysplasia incidence was found in the proximal, mid, and distal regions with erlotinib treatment (50%, 15% and 20% in erlotinib vs 90%, 50% and 70% in vehicle). Moreover, in the erlotinib-treated group, a close to normal mucosal architecture was found, while in vehicle-treated animals a normal pathology was never observed (Figure 3). Erlotinib treatment increased non-specific inflammation in the mid-region when compared with both normal and vehicle-treated animals. No differences were observed in the identification of IBD in each region of the colon, suggesting that erlotinib may maintain animals in a milder stage of pathology, preventing progression to a more severe diagnosis (Figure 3). It was noted that some “normal” animals were found to have a finding of IBD in some areas; this may be explained by the fact that the normal animals were age-matched and, with increased age, inflammation in response to normal microflora begins to appear[20]. None of the normal animals developed dysplasia.
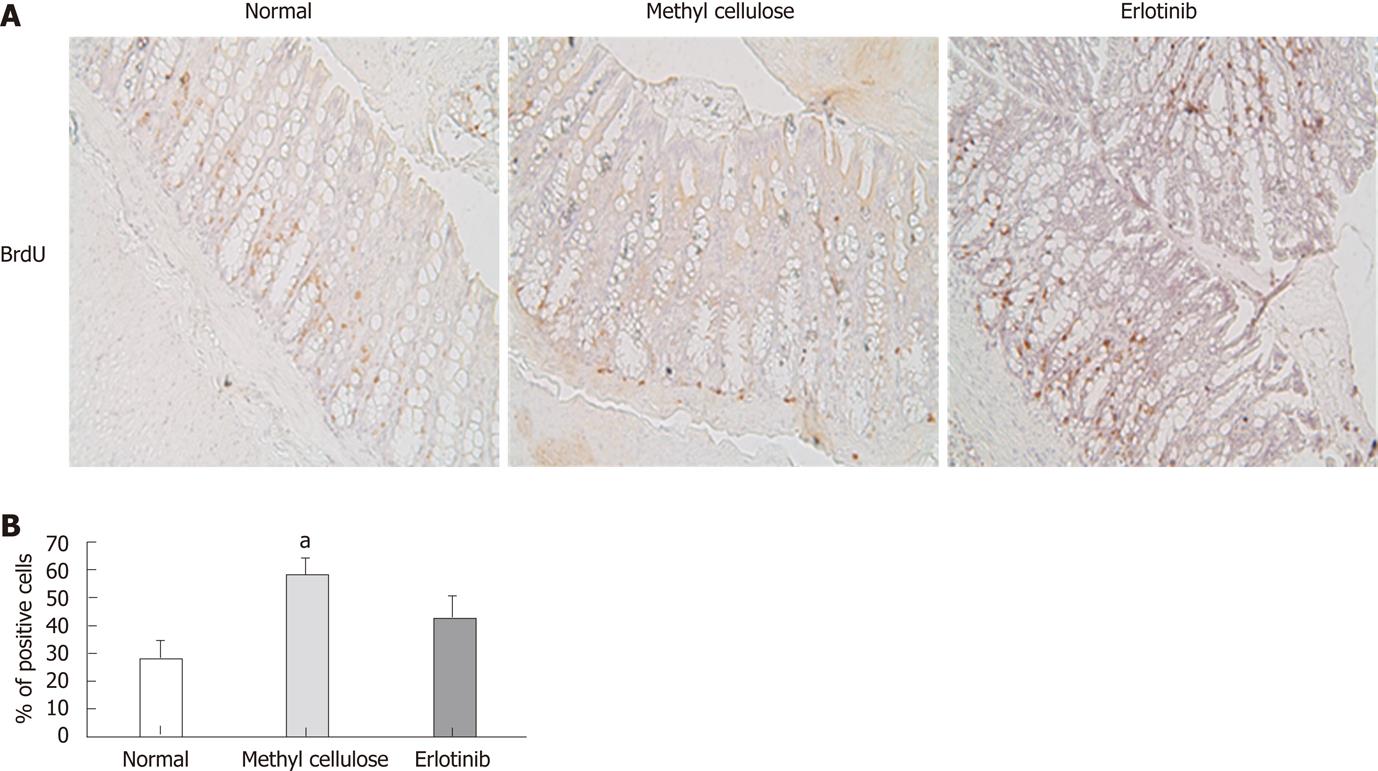
Proliferation of colon cells was examined by analyzing incorporation of BrdU. A significant increase in positively stained cells was found in animals treated with the vehicle compared with that shown in normal animals, suggesting that those animals have more cell proliferation (Figure 4). Erlotinib-treated animals had decreased cell proliferation compared to vehicle-treated animals, and this was not significantly different from that shown in the normal animals (Figure 4).
Attempts to examine EGFR Y1068 phosphorylation in rat tissue samples were not successful. The activation state of EGFR downstream signaling components Erk, Akt, and Src were then measured by Western blotting using phosphor-specific antibodies. Animals treated with erlotinib showed a tendency toward a decrease in the ratio of pErk1/2 when compared with the vehicle-treated group in the proximal, mid and distal regions, with an inhibition of the pErk1/2-to-Erk1/2 ratio of 63%, 24% and 31% in proximal, mid and distal regions, respectively, in animals treated with erlotinib compared to vehicle-treated animals (Figure 5). The ratios of pAkt/Akt and pSrc-pY416 /Src were unchanged in vehicle- and erlotinib-treated animals compared with normal animals (data not shown).
c-Myc is frequently up-regulated in colon cancer and plays an important role in the tumorigenesis of colon cancer. We examined the presence of strong c-Myc staining in the nuclei of rat colon epithelial cells. Compared with the normal group, elevated c-Myc staining was observed in methyl cellulose-treated group, although the difference did not reach statistical significance. Importantly, the c-Myc was reduced to a level similar to that found in the normal group in erlotinib-treated rats (Figure 6).
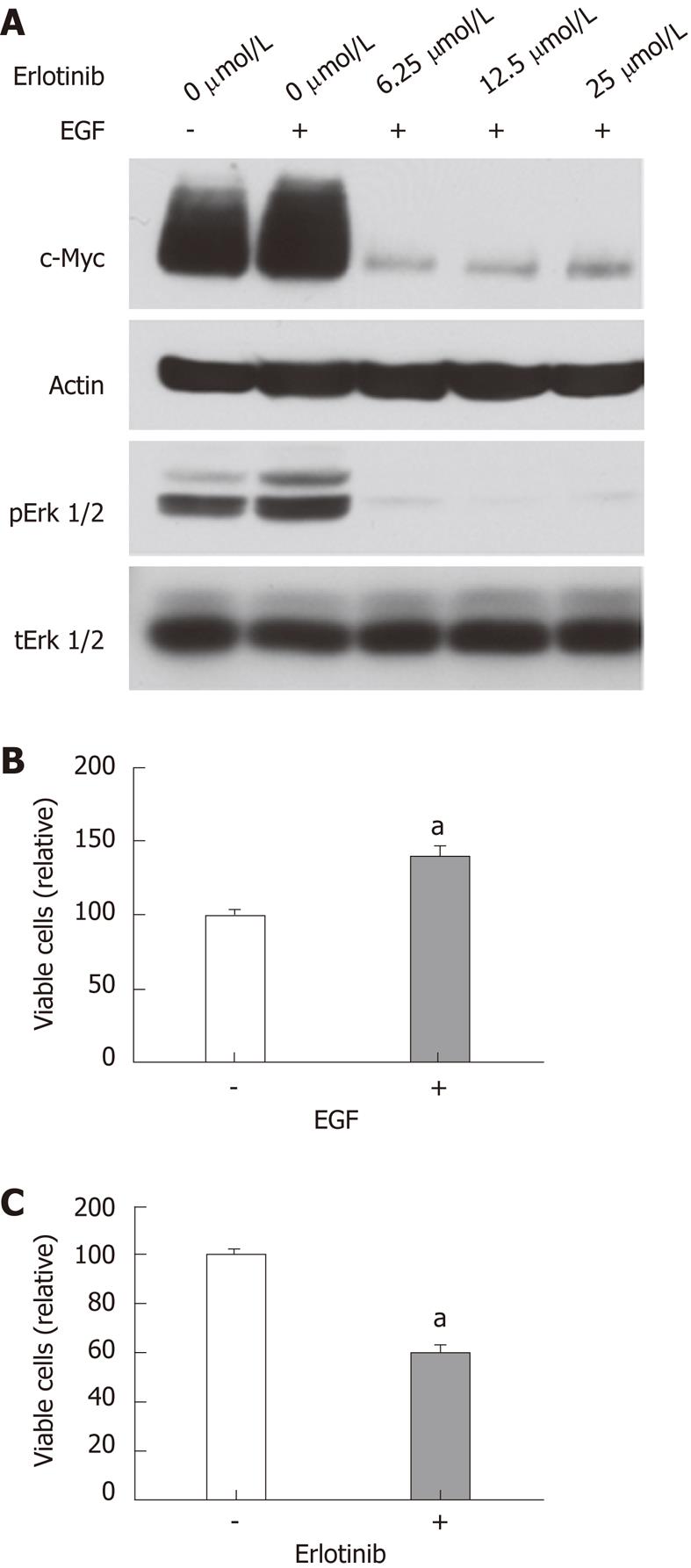
To further assess the effects of erlotinib on c-Myc expression and proliferation in rat epithelial cells, we examined the effects of erlotinib on c-Myc expression in RIE-1 cells. RIE-1 cells appeared very sensitive to serum starvation. In the presence of 5% fetal bovine serum, EGF slightly increased c-Myc and pErk1/2 levels in RIE-1 cells. Erlotinib treatment markedly reduced c-Myc and pErk1/2 levels (Figure 7A). RIE-1 cell proliferation was stimulated by EGF and inhibited by erlotinib (Figure 7B and C).
Although the underlying molecular mechanisms involved in colitis-associated cancer need to be further studied, EGFR has been implicated in the development of colonic dysplasia and CRC. The unraveling of molecules that play crucial roles in the transition from chronic inflammation to dysplasia and cancer is essential for identification of novel drug targets to develop for early intervention measures. Tumorigenesis and tumor promotion depend on cellular signaling pathways that control cell proliferation and survival, and many of these pathways are regulated by EGFR[4]. During chronic inflammation and tissue repair, EGFR activity is elevated. An over-active EGFR may promote the aberrant colonic epithelial cell proliferation and contribute to the development of dysplasia and CRC.
The investigation of the underlying events occurring in colitis-associated dysplasia is complicated by the fact that there are a limited number of animal models available to study the transition of inflammation to dysplasia. Our laboratory recently modified a well-established rat model of chronic colitis to develop an animal model that can be used to investigate ulcerative colitis-associated dysplasia[13,16]. The model uses a prolonged reactivation of inflammation with a proinflammatory drug (TNBS) to create an environment similar to that shown with long-standing colitis in humans, which progresses to cancer. This model shows a degree of dysplasia in 60%-70% of the rats[13,16], similar to what occurs in humans where not all patients develop cancer after a long period with colitis.
We show here that, in our TNBS-induced colitis-associated dysplasia model, animals that received an EGFR inhibitor (erlotinib) had significantly less dysplasia than vehicle-treated animals. This suggests that the EGFR inhibitor is effective in preventing the progression to dysplasia in this animal model. Importantly, we did not observe toxicity of erlotinib in the colon of animals treated with this EGFR inhibitor. It was reported previously that EGFR may have a protective role during acute and chronic inflammation in both the TNBS and dextran sulfate sodium animal models[6,21,22]. The signaling pathway proposed by those researchers involves substance P-NK-1R-EGFR, suggesting that the protective role of this pathway may be due to its effects on fibroblasts[6]. This cell type helps in the remodeling of damaged and/or dead cells or tissues; thus use of an EGFR inhibitor might have been expected to interfere with this process. However, our animals treated with erlotinib showed a milder expression of the disease than that shown in vehicle-treated animals (fewer animals treated with erlotinib progressed to IBD or dysplasia). Thus, erlotinib does not appear to worsen inflammation in our animal model of colitis-associated dysplasia. These data may also help to substantiate the idea of administering erlotinib in conjunction with an anti-inflammatory agent to treat the inflammation and prevent the risk of developing cancer.
The BrdU incorporation assay was used to measure proliferation activity of the colonocytes. Normal animals incorporated BrdU by 26%; this was more than doubled in our model of colitis-associated dysplasia, where vehicle-treated animals showed a 61% incorporation of BrdU, suggesting that these animals possess a higher proliferation rate. In contrast, erlotinib-treated animals showed less BrdU incorporation (37%). Consistently, higher levels of active Erk1/2 and c-Myc were observed in the colon mucosa of vehicle-treated animals but were reduced in erlotinib-treated animals. The Ras-Erk1/2 MAP kinase pathway is known to be activated by EGFR to control cell proliferation. c-Myc overexpression is commonly observed in colon cancer. In the adenomatous polyposis coli-mutant associated CRC, c-Myc is induced by β-catenin to promote colon tumorigenesis. Our data suggest that c-Myc is also up-regulated in colitis-induced dysplasia and erlotinib can inhibit such an increase. In support of this notion, we found that erlotinib is very effective in suppressing c-Myc expression in RIE-1 cells.
In summary, we found that erlotinib is effective in preventing colitis-associated dysplasia without causing unwanted side effects in our novel rat model. Several investigations are already underway to use erlotinib for the treatment of colorectal metastasis[23-25]. In contrast, there have been no investigations into its use as a possible treatment for colitis-associated cancer. Our results suggest that EGFR plays an important role in the progression from inflammation to dysplasia and that inhibition of EGFR, possibly in combination with an anti-inflammatory agent, is a potential approach for use in IBD patients to prevent the development of CRC.
The authors would like to thank Dr. Noel RJ and Dr. Santiago P for advice in this study and the technical assistance of Benitez A. We also thank Hamilton R (Moffitt Cancer Center) for editorial assistance.
Patients with ulcerative colitis are at increased risk of developing colorectal cancer. Epidermal growth factor receptor (EGFR) up-regulation is related to the development of some cancers including colorectal cancer. Erlotinib, a potent inhibitor of the EGFR tyrosine kinase, has been shown to inhibit the EGFR signaling pathway inside the cell and block tumor cell growth in pancreatic and non-small cell lung cancer; however, its role in the transition to dysplasia is unknown.
The underlying mechanisms in ulcerative colitis-associated dysplasia are poorly understood. Understanding the mechanisms responsible for the transition from chronic inflammation to dysplasia and cancer might be helpful to diminish the risk that many patients have of developing colon cancer.
This is the first study to report that erlotinib is effective in preventing colitis-associated dysplasia without causing unwanted side effects in a rat model. Although erlotinib is currently under study for the treatment of colorectal metastasis, there have been no investigations into its use as a possible treatment for colitis-associated cancer. The results of this study demonstrated that erlotinib significantly inhibits the colitis-induced dysplasia in this animal model suggesting that EGFR plays an important role in the progression from inflammation to dysplasia.
Erlotinib may be an effective treatment for patients with long-standing colitis and with higher risk of developing cancer. In addition, it may be possible to combine an anti-inflammatory agent with erlotinib to reduce inflammation, and therefore the occurrence of dysplasia.
EGFR: EGFR is a cell surface receptor involved in several downstream signaling pathways, and is also associated with many types of cancer including colorectal cancer. EGFR expression is increased in inflamed tissues of the bowel in animal models and in patients with inflammatory bowel disease and colon cancer; Erlotinib: Erlotinib is a small molecule inhibitor which is already approved to treat non-small cell lung carcinoma and pancreatic cancer. Erlotinib exerts its biological action by reversible inhibition of tyrosine kinases on the intracellular domain.
In the present paper, the authors used ertolinib, an EGFR tyrosine kinase inhibitor, and determined its effect on the occurrence of colitis-associated dysplasia in rat. They showed that this compound significantly inhibits colitis-induced dysplasia. This is an interesting and elegant study. The model of dysplasia, validated previously by the authors, is very original. These data have potential therapeutic implications in the domain of inflammatory bowel diseases.
Peer reviewers: Ian C Lawrance, MB, BS (Hons), PhD, FRACP, Professor, Director, Centre for Inflammatory Bowel Disease, School of Medicine and Pharmacology, University of Western Australia, Centre for Inflammatory Bowel Disease, Fremantle Hospital, T Block, Alma Street, Fremantle WA 6160, Australia; Bruno Bonaz, MD, PhD, Clinique Universitaire d’Hépato-Gastroentérologie, CHU de Grenoble, BP 217, 38043 Grenoble Cedex 09, France
S- Editor Tian L L- Editor O’Neill M E- Editor Xiong L
| 1. | Farraye FA, Odze RD, Eaden J, Itzkowitz SH. AGA technical review on the diagnosis and management of colorectal neoplasia in inflammatory bowel disease. Gastroenterology. 2010;138:746-774, 774.e1-4; quiz e2-3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 367] [Cited by in RCA: 379] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 2. | Solomon MJ, Schnitzler M. Cancer and inflammatory bowel disease: bias, epidemiology, surveillance, and treatment. World J Surg. 1998;22:352-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 29] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 3. | Wheeler DL, Dunn EF, Harari PM. Understanding resistance to EGFR inhibitors-impact on future treatment strategies. Nat Rev Clin Oncol. 2010;7:493-507. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 547] [Cited by in RCA: 532] [Article Influence: 35.5] [Reference Citation Analysis (0)] |
| 4. | Lurje G, Lenz HJ. EGFR signaling and drug discovery. Oncology. 2009;77:400-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 290] [Cited by in RCA: 312] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 5. | Modjtahedi H, Essapen S. Epidermal growth factor receptor inhibitors in cancer treatment: advances, challenges and opportunities. Anticancer Drugs. 2009;20:851-855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 138] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 6. | Castagliuolo I, Morteau O, Keates AC, Valenick L, Wang CC, Zacks J, Lu B, Gerard NP, Pothoulakis C. Protective effects of neurokinin-1 receptor during colitis in mice: role of the epidermal growth factor receptor. Br J Pharmacol. 2002;136:271-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 53] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 7. | Svrcek M, El-Bchiri J, Chalastanis A, Capel E, Dumont S, Buhard O, Oliveira C, Seruca R, Bossard C, Mosnier JF. Specific clinical and biological features characterize inflammatory bowel disease associated colorectal cancers showing microsatellite instability. J Clin Oncol. 2007;25:4231-4238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 59] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 8. | Navolanic PM, Steelman LS, McCubrey JA. EGFR family signaling and its association with breast cancer development and resistance to chemotherapy (Review). Int J Oncol. 2003;22:237-252. [PubMed] |
| 9. | Trzcinski R, Bry M, Krajewska W, Kulig M, Dzyiki A. ErbB-1 expression in experimental model of inflammatory bowel disease in rats. Acta Chir Iugosl. 2004;51:85-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 10. | Malecka-Panas E, Kordek R, Biernat W, Tureaud J, Liberski PP, Majumdar AP. Differential activation of total and EGF receptor (EGF-R) tyrosine kinase (tyr-k) in the rectal mucosa in patients with adenomatous polyps, ulcerative colitis and colon cancer. Hepatogastroenterology. 1997;44:435-440. [PubMed] [DOI] [Full Text] |
| 11. | Elson CO, Sartor RB, Tennyson GS, Riddell RH. Experimental models of inflammatory bowel disease. Gastroenterology. 1995;109:1344-1367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 847] [Cited by in RCA: 843] [Article Influence: 28.1] [Reference Citation Analysis (0)] |
| 12. | Dougherty U, Sehdev A, Cerda S, Mustafi R, Little N, Yuan W, Jagadeeswaran S, Chumsangsri A, Delgado J, Tretiakova M. Epidermal growth factor receptor controls flat dysplastic aberrant crypt foci development and colon cancer progression in the rat azoxymethane model. Clin Cancer Res. 2008;14:2253-2262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 42] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 13. | Santiago C, Pagán B, Isidro AA, Appleyard CB. Prolonged chronic inflammation progresses to dysplasia in a novel rat model of colitis-associated colon cancer. Cancer Res. 2007;67:10766-10773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 14. | Appleyard CB, Wallace JL. Reactivation of hapten-induced colitis and its prevention by anti-inflammatory drugs. Am J Physiol. 1995;269:G119-G125. [PubMed] |
| 15. | Pollack VA, Savage DM, Baker DA, Tsaparikos KE, Sloan DE, Moyer JD, Barbacci EG, Pustilnik LR, Smolarek TA, Davis JA. Inhibition of epidermal growth factor receptor-associated tyrosine phosphorylation in human carcinomas with CP-358,774: dynamics of receptor inhibition in situ and antitumor effects in athymic mice. J Pharmacol Exp Ther. 1999;291:739-748. [PubMed] |
| 16. | Pagán B, Isidro AA, Coppola D, Chen Z, Ren Y, Wu J, Appleyard CB. Effect of a neurokinin-1 receptor antagonist in a rat model of colitis-associated colon cancer. Anticancer Res. 2010;30:3345-3353. [PubMed] |
| 17. | Fenoglio-Preiser CM, Noffsinger AE, Stemmermann GN, Lantz PE, Listrom MB and Rilke FO. Inflammatory bowel disease. 2nd ed. Philadelphia (PA): Lippincott Williams & Wilkins 1999; 631-716. |
| 18. | Allred DC, Clark GM, Elledge R, Fuqua SA, Brown RW, Chamness GC, Osborne CK, McGuire WL. Association of p53 protein expression with tumor cell proliferation rate and clinical outcome in node-negative breast cancer. J Natl Cancer Inst. 1993;85:200-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 536] [Cited by in RCA: 563] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 19. | Chen L, Pernazza D, Scott LM, Lawrence HR, Ren Y, Luo Y, Wu X, Sung SS, Guida WC, Sebti SM. Inhibition of cellular Shp2 activity by a methyl ester analog of SPI-112. Biochem Pharmacol. 2010;80:801-810. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 20. | Mercier S, Breuillé D, Mosoni L, Obled C, Patureau Mirand P. Chronic inflammation alters protein metabolism in several organs of adult rats. J Nutr. 2002;132:1921-1928. [PubMed] |
| 21. | Egger B, Tolmos J, Procaccino F, Sarosi I, Friess H, Büchler MW, Stamos M, Eysselein VE. Keratinocyte growth factor promotes healing of left-sided colon anastomoses. Am J Surg. 1998;176:18-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 39] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 22. | Procaccino F, Reinshagen M, Hoffmann P, Zeeh JM, Lakshmanan J, McRoberts JA, Patel A, French S, Eysselein VE. Protective effect of epidermal growth factor in an experimental model of colitis in rats. Gastroenterology. 1994;107:12-17. [PubMed] |
| 23. | Köhne CH, Lenz HJ. Chemotherapy with targeted agents for the treatment of metastatic colorectal cancer. Oncologist. 2009;14:478-488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 64] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 24. | Niederle N, Freier W, Porschen R. Erlotinib as single agent in 2nd and 3rd line treatment in patients with metastatic colorectal cancer. Results of a two-cohort multicenter phase II trial. Eur J Cancer. 2005;3 Suppl, 184 Poster 649. |
| 25. | Townsley CA, Major P, Siu LL, Dancey J, Chen E, Pond GR, Nicklee T, Ho J, Hedley D, Tsao M. Phase II study of erlotinib (OSI-774) in patients with metastatic colorectal cancer. Br J Cancer. 2006;94:1136-1143. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 104] [Cited by in RCA: 98] [Article Influence: 5.2] [Reference Citation Analysis (0)] |