Published online Nov 21, 2011. doi: 10.3748/wjg.v17.i43.4825
Revised: June 21, 2011
Accepted: June 28, 2011
Published online: November 21, 2011
AIM: To study the effects of synthetic nonmethylated CpG-containing oligodeoxynucleotides (CpG-ODNs), either alone or combined with recombinant Hepatitis B surface antigen (HBsAg) polypeptide, on the phenotype, function, and intracellular signaling pathways of monocyte-derived dendritic cells (DCs) in patients with chronic hepatitis B (CHB).
METHODS: Peripheral blood monocytes isolated from CHB patients and healthy volunteers were induced to be dendritic cells by recombinant human granulocyte-monocyte colony stimulating factor and interleukin-4. The DCs were then treated with CpG-ODNs, CpG-ODNs/HBsAg, or tumor necrosis factor (TNF)-α for 18 h. The expression of surface molecules including HLA-DR, CD86, and CD1a in DCs were detected by flow cytometry, and the expression of signal transducers and activators of transcription (STAT1, 3, 4, 5, 6) and suppressors of cell signaling (SOCS1, 3) were determined by Western blotting assay. In addition, the capacity of DCs to stimulate allogeneic T lymphocytes and the amount of IL-12p70 released from DCs were measured.
RESULTS: In the DCs derived from patients with CHB, treatment with TNF-α, CpG-ODNs, or CpG-ODNs/HBsAg, as compared to the vector control, significantly increased the expression of HLA-DR, stimulated the release of IL-12p70, and enhanced the capacity of DCs to stimulate allogenic T lymphocytes. The expressions of STAT1/4/6 and SOCS1/3, but not STAT3/5, were upregulated by TNF-α, CpG-ODNs, and CpG-ODNs/HBsAg. In addition, the expression of CD1a was upregulated only in the presence of both CpG-ODNs and HBsAg.
CONCLUSION: The treatment with CpG-ODNs, either alone or combined with HBsAg, has a remarkable stimulatory effect on the impaired phenotype and function of DCs in CHB, possibly by regulating the expression of STAT1, 4, 6 and SOCS1, 3.
- Citation: Xiang XX, Zhou XQ, Wang JX, Xie Q, Cai X, Yu H, Zhou HJ. Effects of CpG-ODNs on phenotype and function of monocyte-derived dendritic cells in chronic hepatitis B. World J Gastroenterol 2011; 17(43): 4825-4830
- URL: https://www.wjgnet.com/1007-9327/full/v17/i43/4825.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i43.4825
Peripheral blood monocyte-derived dendritic cells (MoDCs) from patients with chronic hepatitis B (CHB) are abnormal in morphology and function[1,2]. However, the molecular mechanism of the MoDCs’ dysfunction in CHB remains unclear. This study aimed to evaluate the effect of synthetic nonmethylated CpG-containing oligodeoxynucleotides (CpG-ODNs), combined with recombinant hepatitis B surface antigen (HBsAg) polypeptide, on the phenotype and function of monocyte-derived dendritic cells (DCs) in patients with chronic hepatitis B. In addition, we attempted to shed light on the involvement of intracellular signaling molecules, including signal transducers and activators of transcription (STAT1, 3, 4, 5, 6) and suppressors of cell signaling (SOCS1, 3) in the MoDCs dysfunction under the condition of CHB[3,4,5].
Twenty-four patients with CHB [23 males and 1 females, mean age (33.4 ± 11.8) years] were enrolled in this study. Diagnosis of CHB was made by clinical findings: elevated serum alanine aminotransferase (ALT) levels for more than six months and the presence of HBsAg, HBeAg, anti-HBc, and HBV DNA (> 1 × 106 copies/mL). Among all patients, other hepatitis-related virus infection such as hepatitis A virus, hepatitis C virus, hepatitis E virus, and transfusion transmitted virus were excluded. None of the enrolled patients had received treatment with interferon, thymosin, thymus extracts, or lamivudine. Thirteen healthy volunteers were selected as control subjects with the average age of (31.7 ± 8.7) years.
The recombinant human granulocyte-macrophage colony-stimulating factor (hGM-CSF), recombinant human interleukin-4 (hIL-4), and recombinant human tumor necrosis factor-α (hTNF-α) were purchased from Peprotech EC Ltd. (London, United Kingdom). The recombinant HBsAg was purchased from ViroStat (Portland, United States). Fluorescent isothiocyanate (FITC) anti-human CD1a, Phycoerythrocin (PE)-Cy5 anti-human CD86, Phycoerythrocin (PE), and anti-human HLA-DR were purchased from Amersham Pharmacia Biotech Inc (United States). MACS CD14 MicroBeads and MS Separation Columns were purchased from Miltenyi Biotec (Bergisch Gladbach, Germany). Human T cell enrichment columns were purchased from R and D Systems, Inc. The human IL-12p70 ELISA kit was purchased from Jingmei Biotech (Shenzhen, China). The mouse monoclonal antibody against beta actin was purchased from Abcam Ltd (Cambridge, United Kingdom). The mouse monoclonal antibody against phosphorylated STAT1/3 and the rabbit polyclonal antibody against phosphorylated STAT4/5/6 were purchased from Cell Signaling Technology (Beverly, MA, United States). The rabbit polyclonal antibodies for SOCS1/3 were purchased from Assay Designs Ltd (Michigan, United States). NE-PER nuclear and cytoplasmic extraction reagents were purchased from Pierce Biotechnology (Rockford, United States). 3H-TdR (2 mci/mL) was purchased from Isotope Institute of Atomic Energy Academy of China. Mitomycin-C and HISTOPAQUE-1077 were purchased from SIGMA (ST. LOUIS, MO, United States). The CpG-containing phosphorothioated oligodeoxynucleotides (CpG-ODNs) was custom synthesized by Shanghai Sangon Biological Engineering Technology and Service Ltd, based on the report by Merad et al[4].
The DCs were prepared following a method described by Romani et al[6], with a few modifications. Briefly, peripheral blood mononuclear cells (PBMC) were separated from the peripheral blood of patients and volunteers by HISTOPAQUE-1.077 density gradient centrifugation, and the monocytes were further selected from PBMCs using CD14 MicroBeads. The monocytes were induced to differentiate into DCs by combined treatment with hGM-CSF (1000 U/mL), hIL-4 (500 U/mL), and hTNF-α (1000 U/mL) for five or seven days according to requirement. Dendritic cells were identified by inverted phase contrast microscopy, transmission electron microscopy and flow cytometry. The DCs were then collected for subsequent experiments.
After 5-d culture, the immature DCs were collected and dispensed into a culture plate with 24 wells (1 × 106 cells/well). CpG-ODNs (10 μg/mL), CpG-ODNs (10 μg/mL) + HBsAg (10 μg/mL) or TNF-α (1000 U/mL) in phosphate buffer solution (PBS, 50 μL/mL) were added[6,7]. The DCs derived from volunteers were only treated with CpG-ODNs (10 μg/mL). All cells were cultured at 37 °C in a 5% CO2 incubator for 18 h. The cells and culture supernatant were then separately collected for further assays.
The expression rates of surface molecules, including HLA-DR-PE, CD86-(PE)-Cy5, and CD1a-FITC in DCs were analyzed by flow cytometry (FCM), and isotope control was performed as previously described[2]. The IL-12p70 content of the culture supernatant was detected by an ELISA kit according to the manufacture’s instructions. The average optical density (OD) of three replicates was calculated.
The effect stimulation of DCs on T-cell proliferation was determined using a method described by Li et al[2]. T lymphocytes, separated by T cell separation columns, were used as response cells. The DCs exposed to different immune adjuvants for five days were further treated with mitomycin C (25 μg/mL) for 45 min and used as stimulating cells. The response cells and stimulating cells were co-cultured for 96 h at the ratio of 20:1, and 3H-TdR was added 18 h before the end of culture. The radioactivity (counts per minute, cpm), reflecting T-cell proliferation, was detected by a liquid scintillation counter and expressed as the mean value of five replicates.
Cytosolic proteins were extracted from DCs treated with different immune adjuvants, separated by 10% SDS-PAGE gel electrophoresis (30 μg protein per lane), and transferred onto a nitrocellulose membrane. The membrane was incubated in blocking buffer for 2 h at room temperature, a probed with a specific primary antibody (1:1000, 4 °C, overnight), followed by the secondary antibody conjugated with horseradish peroxidase, and then developed by enhanced chemiluminescence (ECL) staining. All experiments were repeated three times and the protein signal was quantified by densitometry with a PrecisionScanLTX scanning apparatus, and normalized to β-actin as an internal reference.
The experimental data in this study were expressed as mean ± SD and analyzed by SAS6.12 statistical software. One-way ANOVA, followed by the Student-Newman-Keuls test, was used to compare the numerical data between multiple groups. A P value of < 0.05 was considered to be statistically significant.
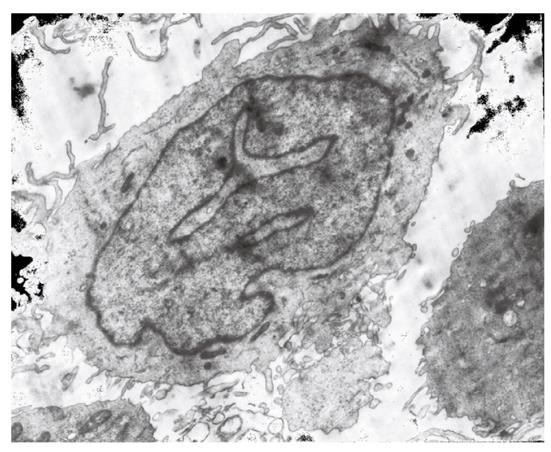
DCs were developed from CD14+ blood monocytes. Monocytes of high purity were separated from the peripheral blood using CD14 MicroBeads. After treatment with recombinant GM-CSF 1000 U/mL and IL-4500 U/mL, a large number of cell clusters appeared on day three, and most cells were suspended on day six to seven in irregular form. Abundant extended beard-like pricks and mitochondria were observed under inverted phase contrast microscope and transmission electron microscope (Figure 1). The purity of DCs was more than 85% according to the numbers of cells positive for HLA-DR, CD86 and CD1a detected by FCM. DCs treated with TNF-α (1000 U/mL) showed strong ability to stimulate the proliferation of allogeneic T lymphocytes ( Figure 1).
The number of DCs positive for HLA-DR, CD86, and CD1a after induction with GM-CSF and IL-4 were 70.4%, 81.3%, and 13.67% for CHB (n = 6), and were 83.2%, 80.6%, and 22.4% for healthy volunteers (n = 3), respectively. The number of cells positive for HLA-DR and CD1a were significantly lower in CHB than those in healthy volunteers (P < 0.05). Treatment with TNF-α, CpG-ODNs, or CpG-ODNs plus HBsAg, beginning at day 5 and continuing for 18 h, increased the numbers of cells positive for HLA-DR and CD86 by about 10%, and the rate of CD1a by about 20% in DCs from healthy volunteers (n = 3). By contrast, in DCs from the patients with CHB (n = 6), only the rates of HLA-DR were increased by those treatments (94.7%, 93.8%, and 95.14% for TNF-α, CpG-ODNs, and CpG-ODNs plus HBsAg, respectively), except that CpG-ODNs plus HBsAg significantly raised the positive rate of CD1a from 13.67% to 44.52% (Table 1).
In DCs derived from CHB patients, treatment with CpG-ODNs and TNF-α, either alone or combined with HBsAg, resulted in significant increases of supernatant IL-12p70 level, suggesting enhanced release of IL-12p70. However, the supernatant levels of IL-12p70 did not increase upon the treatment with HBsAg or PBS (Table 2).
The radioactive counts (cpm) representing allogeneic T lymphocyte proliferation in a mixed lymphocyte reaction (MLR) for normal DCs was 2290 ± 1861.1. In contrast, the cpm for CHB-derived DCs was 487.4 ± 216.3, significantly lower than that for the normal DCs (P < 0.05). Treatment with CpG-ODNs, CpG-ODNs plus HBsAg, or TNF-α greatly increased the cpm for CHB-derived DCs to 5407 ± 2359, 5831 ± 2815.4, and 9947 ± 3395.2, respectively (P < 0.05). This suggested that CpG-ODNs, either alone or combined with HBsAg, enhanced the ability of CHB-derived DCs to present antigen, similar to the effect of TNF-α.
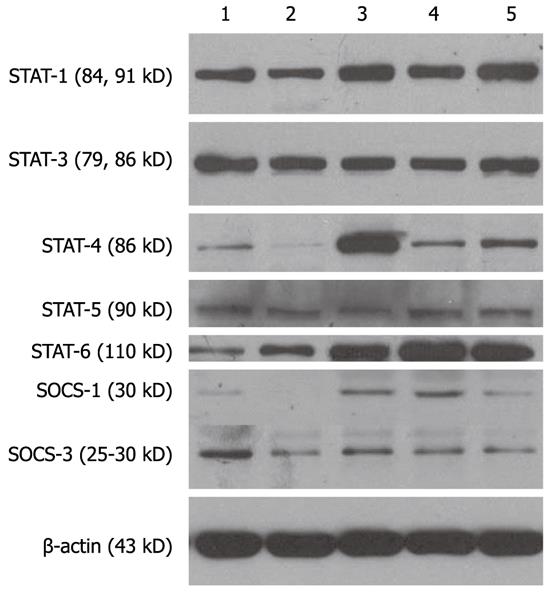
As shown by the Western blotting experiments (Figure 2), the phosphorylation signal of STAT1, STAT3, and STAT6 in mature DC cytoplasm was relatively strong, while the expression intensities of STAT4, STAT5, SOCS1, and SOCS3 were relatively weak. Compared with mature DCs, the expression intensities of STAT1, STAT3, and STAT5 in immature DC cytoplasm (Lane 2, PBS group) were weak, the expression intensity of STAT6 was strong, the expressions of STAT4 and SOCS1 was almost absent, and the expression of SOCS3 was also weak. The immune adjuvants, CpG-ODNs, CpG-ODNs plus HBsAg and TNF-α, enhanced the expression of cytosolic STAT1, STAT4, STAT6, and SOCS1 in DCs derived from CHB. In particular, the protein band intensities of STAT4 and SOCS1 in CHB-derived DCs stimulated with CpG-ODNs were increased by 12 times and five times, respectively. However, the expression of STAT3, STAT5, and SOCS3 did not change between mature DCs and immature DCs (Lane 2, PBS group). In addition, the addition of HBsAg to CpG-ODNs did not alter the effects of CpG-ODNs on the expression of the above signal proteins (Figure 2).
Recent studies indicate that DCs derived from the peripheral blood of patients with CHB have abnormal phenotypes and functions, such as downregulated expression of surface molecules (HLA-DR, CD86, CD1a, CD11c and ICAM-1), reduced ability to stimulate the proliferation of allogeneic T lymphocytes, reduced secretion of IL-12p70 and IFNγ, and disorder in the proportion of DC1/DC2[1,2]. However, the molecular immunological mechanism of DC dysfunction in CHB is unclear, and immunotherapy aimed to improve the dysfunction of DC is in the initial stages. Bacterial lipopolysaccharide (LPS), TNF-α, and phytohemagglutinin (PHA) are able to stimulate maturation of DCs; however, as inflammatory compounds, their application is limited in in vitro research and animal experiments. As reported recently, bacterial genomic DNA containing a non-methylated CpG motif is able to activate antigen-presenting cells (APC), and preferentially induce a Th1-dominating immune response[7]. An artificially synthesized non-methylated CpG-containing oligodeoxynucleotides (CpG-ODNs), similar to LPS and TNF-α, is capable of inducing maturation of DCs derived from mouse bone marrow and human peripheral blood monocyte and enhances their antigen-presenting ability[6,8-11]. CpG-ODNs administered together with a hemopoietic growth factor or an immunomodulator was shown to improve the function of DCs in experimental tumor models and reinforce the immune ability of scavenging tumor[4,5]. As a highly effective Th1-type inducing adjuvant, a phase I clinical trial of CpG-ODNs has been carried out, but little research data exists to define the effects and mechanisms of CpG-ODNs on the proliferation and maturation of peripheral blood DCs in CHB.
It is reported that the flanking sequences of non-methylated CpG-containing CpG-ODNs with highest stimulating activity differ between mice and humans. The following CpG-ODNs sequence was adopted in this study, TCCATGTCGTTCCTGTCGTT, which has the highest immune stimulating activity in humans. Our data showed that CpG-ODNs, at a dose of 10 μg/mL, significantly induced the proliferation and maturation of peripheral blood DCs from CHB over a 5-d culture period. FCM detection showed that the HLA-DR expression on DCs treated with CpG-ODNs was significantly upregulated, the amount of IL-12p70 secreted by DC was also increased, and the ability of DCs to stimulate the proliferation of allogeneic T lymphocytes was enhanced. The effect of CpG-ODNs on the proliferation of DCs was similar to that of TNF-α. However, neither TNF-α nor CpG-ODNs increased the expression of CD1a, a specific marker of mature DCs. Interestingly, the combination of CpG-ODN and the specific antigen HBsAg of HBV significantly increased the number of cells positive for CD1a among DCs from CHB. The above data indicated that combined stimulation with a Th1-type immune adjuvant and a specific antigen such as HBsAg is essential for DCs to obtain ideal antigen-presenting ability against HBV.
The STAT and SOCS signaling pathway are an important pair of positive and negative feedback systems for the intracellular signal transduction of cytokines[12,13], and play crucial roles in the development of chronic hepatitis B[14-17]. The present study showed that, under the induction with GM-CSF and IL-4, the expression levels of STAT4, SOCS1, and SOCS3 in CHB-derived DCs were very low, the expression levels of STAT3 and STAT5 were similar to those of normal mature DCs, the expression of STAT1 was somewhat lower than that of normal control DCs, and the expression of STAT6 was more enhanced than that of control mature DCs. It is reported that a variety of cytokines such as IFNα/β/γ, IL-4, IL-12, IL-10, IL-6, and GM-CSF are dependent on the STAT and SOCS pathways to initiate a cascade of phosphorylation events that lead to changed expression of target genes[18,19]. These data suggest the complexity of cytokine signaling network in DCs from CHB, which might be an important molecular immunological mechanism for the differences between individuals in terms of the short-term and long-term therapeutic effects of antiviral drugs, such as interferon and lamivudine. Our data suggested that CpG-ODNs, TNF-α, and CpG-ODNs plus HBsAg increased, to some extent, the expression levels of STAT1, STAT4, STAT6, SOCS1, and SOCS3 in the cytoplasm of DCs from CHB. The enhancement effect of CpG-ODNs on the expression of STAT4 was particularly dramatic, but the expression of STAT3 and STAT5 showed no differences between immature and mature DCs, indicating that the actions of CpG-ODNs on the STAT and SOCS pathways in DCs might be a crucial mechanism of CpG-ODNs to promote proliferation and maturation and enhance the antigen-presenting function of DCs. It is CpG-ODNs, not other nucleoside analogs, that functions as a Th1-type immune adjuvant, which has been clarified many studies[3-5,7-8,20]. Thus, it is not unexpected that CpG-ODNs improved the partial function of DCs derived from peripheral blood monocytes of CHB in vitro. What is the exact mechanism of the above effects, besides those on the STAT and SOCS pathways, and whether there are expected effects on DCs from CHB in vivo in view of complicated immunological pathogenesis of CHB, remain important questions. In the subsequent research, our study group will further investigate the clinical/therapeutic consequences of this study and will uncover the exact mechanisms of DC dysfunction in CHB, with a view to developing novel immunological treatments for CHB[16,21,22].
We are heartily thankful to Mr. Da-Kai Xiao, Mr. Dong Li and Mrs. Yi Zhang of Shanghai Institute of Hematology and Digestive Surgery for their selfless help with the experiments.
It has been demonstrated that the dendritic cells from patients with chronic hepatitis B (CHB) are abnormal in morphology and function, which is closely related to the immunologic pathogenesis of CHB. However, the molecular mechanism of dendritic cell (DC) dysfunction in CHB and how to regulate it remains unclear.
As it is known, artificially synthesized non-methylated CpG containing oligodeoxynucleotides (CpG-ODNs), similar to lipopolysaccharide and tumor necrosis factor-α, is capable of inducing DCs derived from mouse bone marrow and human peripheral blood monocyte to mature and enhancing its antigen-presenting ability. However, few research data exist to define the effects and mechanisms of CpG-ODNs on the proliferation and maturation of peripheral blood DCs in CHB.
The treatment of CpG-ODNs either alone or combined with Hepatitis B surface antigen (HBsAg) has a remarkable stimulatory effect on the impaired phenotype and function of DCs in CHB possibly by regulating the expression of signal transducer and activator of transcription (STAT) 1,4,6 and suppressor of cytokine signaling (SOCS) 1,3, which might help to develop novel immunological treatments for CHB.
In the following research, the study group will try to deepen the clinical/therapeutic consequences of this study and further study is also needed to uncover the exact mechanisms of DC dysfunction in CHB, and to develop novel immunological treatments for CHB.
Dendritic cell, Considered as a kind of professional antigen-presenting cell, is capable of initiating strong primary cellular immune response of host and determine its direction so as to play an important role in the immunologic response of anti-infection. Artificially synthesized non-methylated CpG motif containing oligodeoxynucleotides (CpG-ODNs) might directly activate antigen-presenting cells, superiorly induce Th1 type immune reaction and have wide application prospect. However, there are few research data reported about the effects of CpG-ODNs on some important cell signal transducers in cytoplasm or nucleus of DC such as STAT, SOCS.
The study is interesting, well done. The study improved the existing knowledge for DC dysfunction in chronic hepatitis B. It would be very well appreciated. Deepen the clinical/therapeutic consequences of this study possibly in the future research.
Peer reviewers: Giovanni Tarantino, MD, Professor, Department of Clinical and Experimental Medicine, Federico II University Medical School, Via S Pansini, 5, Naples 80131, Italy; Mihaela Petrova, MD, PhD, Dr., Clinic of Gastroenterology, Medical Institute, Ministry of Interior, Sofia 1606, Bulgaria
S- Editor Sun H L- Editor Stewart GJ E- Editor Zhang DN
| 1. | van der Molen RG, Sprengers D, Binda RS, de Jong EC, Niesters HG, Kusters JG, Kwekkeboom J, Janssen HL. Functional impairment of myeloid and plasmacytoid dendritic cells of patients with chronic hepatitis B. Hepatology. 2004;40:738-746. [PubMed] |
| 2. | Li RB, Chen HS, Cong X, Sun Jin, Wei L, Wang Y. Functions of cultured dendritic cells from patients with chronic hepatitis B decreased. Zhonghua Yixue Zazhi. 2002;82:887-890. |
| 3. | Gursel M, Verthelyi D, Klinman DM. CpG oligodeoxynucleotides induce human monocytes to mature into functional dendritic cells. Eur J Immunol. 2002;32:2617-2622. [PubMed] |
| 4. | Merad M, Sugie T, Engleman EG, Fong L. In vivo manipulation of dendritic cells to induce therapeutic immunity. Blood. 2002;99:1676-1682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 5. | Pilon-Thomas S, Li W, Briggs JJ, Djeu J, Mulé JJ, Riker AI. Immunostimulatory effects of CpG-ODN upon dendritic cell-based immunotherapy in a murine melanoma model. J Immunother. 2006;29:381-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 37] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 6. | Romani N, Reider D, Heuer M, Ebner S, Kämpgen E, Eibl B, Niederwieser D, Schuler G. Generation of mature dendritic cells from human blood. An improved method with special regard to clinical applicability. J Immunol Methods. 1996;196:137-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 866] [Cited by in RCA: 845] [Article Influence: 29.1] [Reference Citation Analysis (0)] |
| 7. | Kuchtey J, Chefalo PJ, Gray RC, Ramachandra L, Harding CV. Enhancement of dendritic cell antigen cross-presentation by CpG DNA involves type I IFN and stabilization of class I MHC mRNA. J Immunol. 2005;175:2244-2251. [PubMed] |
| 8. | Vicari AP, Chiodoni C, Vaure C, Aït-Yahia S, Dercamp C, Matsos F, Reynard O, Taverne C, Merle P, Colombo MP. Reversal of tumor-induced dendritic cell paralysis by CpG immunostimulatory oligonucleotide and anti-interleukin 10 receptor antibody. J Exp Med. 2002;196:541-549. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 279] [Cited by in RCA: 270] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 9. | Mellor AL, Baban B, Chandler PR, Manlapat A, Kahler DJ, Munn DH. Cutting edge: CpG oligonucleotides induce splenic CD19+ dendritic cells to acquire potent indoleamine 2,3-dioxygenase-dependent T cell regulatory functions via IFN Type 1 signaling. J Immunol. 2005;175:5601-5605. [PubMed] |
| 10. | Marschner A, Rothenfusser S, Hornung V, Prell D, Krug A, Kerkmann M, Wellisch D, Poeck H, Greinacher A, Giese T. CpG ODN enhance antigen-specific NKT cell activation via plasmacytoid dendritic cells. Eur J Immunol. 2005;35:2347-2357. [PubMed] |
| 11. | Latvala S, Pietila TE, Veckman V, Kekkonen RA, Tynkkynen S, Korpela R, Julkunen I. Potentially probiotic bacteria induce efficient maturation but differential cytokine production in human monocyte-derived dendritic cells. World J Gastroenterol. 2008;14:5570-583; discussion 5570-583;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 57] [Cited by in RCA: 52] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 12. | Remoli ME, Ragimbeau J, Giacomini E, Gafa V, Severa M, Lande R, Pellegrini S, Coccia EM. NF-{kappa}B is required for STAT-4 expression during dendritic cell maturation. J Leukoc Biol. 2007;81:355-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 13. | Davey GM, Heath WR, Starr R. SOCS1: a potent and multifaceted regulator of cytokines and cell-mediated inflammation. Tissue Antigens. 2006;67:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 14. | Ballas ZK, Krieg AM, Warren T, Rasmussen W, Davis HL, Waldschmidt M, Weiner GJ. Divergent therapeutic and immunologic effects of oligodeoxynucleotides with distinct CpG motifs. J Immunol. 2001;167:4878-4886. [PubMed] |
| 15. | Jackson SH, Yu CR, Mahdi RM, Ebong S, Egwuagu CE. Dendritic cell maturation requires STAT1 and is under feedback regulation by suppressors of cytokine signaling. J Immunol. 2004;172:2307-2315. [PubMed] |
| 16. | Takauji R, Iho S, Takatsuka H, Yamamoto S, Takahashi T, Kitagawa H, Iwasaki H, Iida R, Yokochi T, Matsuki T. CpG-DNA-induced IFN-alpha production involves p38 MAPK-dependent STAT1 phosphorylation in human plasmacytoid dendritic cell precursors. J Leukoc Biol. 2002;72:1011-1019. [PubMed] |
| 17. | Arbuthnot P, Capovilla A, Kew M. Putative role of hepatitis B virus X protein in hepatocarcinogenesis: effects on apoptosis, DNA repair, mitogen-activated protein kinase and JAK/STAT pathways. J Gastroenterol Hepatol. 2000;15:357-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 161] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 18. | Heim MH. Intracellular signalling and antiviral effects of interferons. Dig Liver Dis. 2000;32:257-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 19. | Sun WM. Immune signaling molecule and its signaling mechanisms. New Progress in Immunology. Beijing: the People’s Health Publishing House 2002; 453-480. |
| 20. | Flores RR, Diggs KA, Tait LM, Morel PA. IFN-gamma negatively regulates CpG-induced IL-10 in bone marrow-derived dendritic cells. J Immunol. 2007;178:211-218. [PubMed] |
| 21. | Hahm B, Trifilo MJ, Zuniga EI, Oldstone MB. Viruses evade the immune system through type I interferon-mediated STAT2-dependent, but STAT1-independent, signaling. Immunity. 2005;22:247-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 22. | Lu GF, Tang FA, Zheng PY, Yang PC, Qi YM. Entecavir up-regulates dendritic cell function in patients with chronic hepatitis B. World J Gastroenterol. 2008;14:1617-1621. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 11] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |