Published online Nov 21, 2011. doi: 10.3748/wjg.v17.i43.4793
Revised: June 9, 2011
Accepted: June 13, 2011
Published online: November 21, 2011
AIM: To evaluate the value of ABC (D) stratification [combination of serum pepsinogen and Helicobacter pylori (H. pylori) antibody] of patients with gastric cancer.
METHODS: Ninety-five consecutive patients with gastric cancer were enrolled into the study. The serum pepsinogen I (PG I)/pepsinogen II (PG II) and H. pylori antibody levels were measured. Patients were classified into five groups of ABC (D) stratification according to their serological status. Endoscopic findings of atrophic gastritis and histological differentiation were also analyzed in relation to the ABC (D) stratification.
RESULTS: The mean patient age was (67.9 ± 8.9) years. Three patients (3.2%) were classified into group A, 7 patients (7.4%) into group A’, 27 patients (28.4%) into group B, 54 patients (56.8%) into group C, and 4 patients (4.2%) into group D, respectively. There were only three cases in group A when the patients taking acid proton pump inhibitors and those who had undergone eradication therapy for H. pylori (group A’) were excluded. These three cases had mucosal atrophy in the grey zone according to the diagnostic manual of ABC (D) stratification. Histologically, the mean age of the patients with well differentiated adenocarcinoma was significantly higher than that of the patients with poorly differentiated adenocarcinoma (P < 0.05). There were no differences in the pattern of atrophy in the endoscopies between the well differentiated and poorly differentiated groups.
CONCLUSION: ABC (D) stratification is a good method for screening patients with gastric cancers. Endoscopy is needed for grey zone cases to check the extent of mucosal atrophy.
- Citation: Kudo T, Kakizaki S, Sohara N, Onozato Y, Okamura S, Inui Y, Mori M. Analysis of ABC (D) stratification for screening patients with gastric cancer. World J Gastroenterol 2011; 17(43): 4793-4798
- URL: https://www.wjgnet.com/1007-9327/full/v17/i43/4793.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i43.4793
Gastric cancer remains the second leading cause of cancer death in Japan, although its mortality has continued to decrease for decades[1]. Screening systems or methods to detect early gastric cancers have contributed to the decrease in gastric cancer deaths[2,3]. Mass screening for gastric cancer by X-ray examination was introduced in the 1960s and has been epidemiologically confirmed to be effective for reducing gastric cancer mortality[2,3]. However, the decreases in efficiency have been thought to result from the decreased coverage rates and a tendency for the same participants to undergo gastric cancer screening repeatedly. Therefore, a more effective screening system, focusing on high-risk patients, is needed.
Helicobacter pylori (H. pylori) infection and subsequent atrophic gastritis have been regarded as risk factors for gastric cancer[4-6]. The combination of serum pepsinogen (PG) and H. pylori antibody [ABC (D) stratification] has been suggested to serve as a useful predictive marker for patients with gastric cancers[4-6]. This combination of serum markers would represent a much simpler and less invasive method than endoscopy, and therefore, would be suitable for a large general population. A large scale study by Ohata et al[4] reported that the combination of serum PG and anti-H. pylori antibody provide a good method for predicting the development of gastric cancer. Mizuno et al[6] also reported the usefulness of this screening method in a population-based cohort study.
However, the studies concerning the use of ABC (D) stratification only focused on patients with no disease or an unknown disease status. Analyses of the ABC (D) stratification of patients with confirmed gastric cancer are lacking. The aim of this study was to evaluate the ABC (D) stratification so as to provide information that might be useful for screening patients with gastric cancers.
Ninety-five consecutive patients with gastric cancer were enrolled in the study. All patients were diagnosed as having gastric cancer at Shirakawa Clinic (Maebashi, Japan) between November 2007 and October 2009. IgG antibodies to H. pylori and the levels of pepsinogen I (PG I) and II (PG II) were measured and classified by ABC (D) stratification. Group A consisted of patients with normal PG and H. pylori antibody (-); group B had normal PG and H. pylori (+); group C had atrophic PG and H. pylori (+); and group D had atrophic PG and H. pylori (-). Group A’ included patients with normal PG who were H. pylori (-) after H. pylori eradication therapy or who were being treated with proton pump inhibitors.
A data collection sheet was designed to obtain the relevant clinical information about the patients for review. All of the patients provided written informed consent before receiving the examination. Olympus XQ260 or N260 (Olympus Optical Co, Tokyo, Japan) instrument was used for endoscopic examination. When gastric cancer was suspected by routine endoscopy, chromoendoscopy with indigo carmine and a biopsy were performed. All cases were histologically confirmed to have gastric cancer. According to the endoscopic gastric mucosal findings, the patients were classified into three categories: those without atrophy, those with close-type atrophy, and those with open-type atrophy[7].
H. pylori infection was determined by enzyme-linked immunosorbent assay (ELISA) kits (E-plate EIKEN H. pylori, Eiken Chemical Co., Ltd., Tokyo, Japan). Subjects with ≥ 10 U/mL were classified as having H. pylori infection. Those with < 10 U/mL were regarded as being H. pylori negative. The sensitivity and specificity of this assay for H. pylori infection were 100% and 93.8%, respectively. The levels of PG I and PG II were measured using the E-plate EIKEN Pepsinogen I and the E-plate EIKEN Pepsinogen II (Eiken Chemical Co., Ltd., Tokyo, Japan), respectively. Atrophic gastritis was diagnosed according to the serum PG I and PG II criteria proposed by Miki and others[8-10]. Briefly, the serum PG status was defined as atrophic when the criteria of both serum PG I level ≤ 70 ng/mL and a PG I/PG II ratio ≤ 3.0 were simultaneously fulfilled. All other cases were classified as normal. These criteria have a sensitivity of 70.5% and a specificity of 97%.
Differences between the groups were analyzed by Fischer’s exact probability test and Mann-Whitney’s U test when a significant difference was obtained by the Kruskal-Wallis test. A P value less than 0.05 was considered to be significant.
Ninety-five patients were diagnosed as having gastric cancer at our institution between November 2007 and October 2009. The mean age of the patients was (67.9 ± 8.9) years (range, 38-83, median 69). There were 72 male and 23 females. According to ABC (D) stratification, there were 3 (3.2%) patients in group A, 7 patients (7.4%) in group A’, 27 (28.4%) in group B, 54 (56.8%) in group C, and 4 (4.2%) in group D, respectively (Table 1). There were no significant differences in the mean age, sex ratio, location of the tumor, macroscopic findings or histological type among the five groups. According to endoscopic findings, two cases (2.1%) had no atrophy, 21 (22.1%) had closed-type atrophy, and 72 (75.8%) had open-type atrophy. The relationship between ABC (D) stratification and the endoscopic atrophic border are shown in Table 2. There were no significant differences in the endoscopic atrophic border pattern among the five groups. There were 35 patients with closed-type or open-type atrophy in the PG negative group.
| Characteristics | All patients | A | B | C | D | |
| No. of patients | 95 | 10 | 27 | 54 | 4 | |
| Age (yr) | Mean (range) | 67.9 ± 8.9 (38-83) | 69.2 ± 10.0 (48-79) | 65.5 ± 9.0 (43-77) | 68.4 ± 7.6 (38-83) | 70.3 ± 6.9 (63-78) |
| Median | 69 | 70.5 | 67 | 69.5 | 70 | |
| Sex | Male/female | 72/23 | 5/5 | 19/8 | 45/9 | 3/1 |
| Location | U/M/L | 19/42/34 | 2/6/2 | 2/14/11 | 15/22/17 | 0/0/4 |
| Macroscopic type | Elevated/flat/depressed | 51/4/40 | 5/0/5 | 12/0/15 | 31/3/20 | 3/1/0 |
| Differentiation | Well diff/poorly diff | 76/19 | 8/2 | 18/9 | 47/7 | 3/1 |
| Endoscopic atrophic border | ||||
| ABC (D) stratification | Non | Closed type | Open type | Total |
| A: H. pylori (-) PG (-) | 1 | 3 | 6 | 10 (10.6%) |
| B: H. pylori (+) PG (-) | 1 | 7 | 19 | 27 (28.4%) |
| C: H. pylori (+) PG (+) | 0 | 10 | 44 | 54 (56.8%) |
| D: H. pylori (-) PG (+) | 0 | 1 | 3 | 4 (4.2%) |
| Total | 2 (2.1%) | 21 (22.1%) | 72 (75.8%) | 95 (100%) |
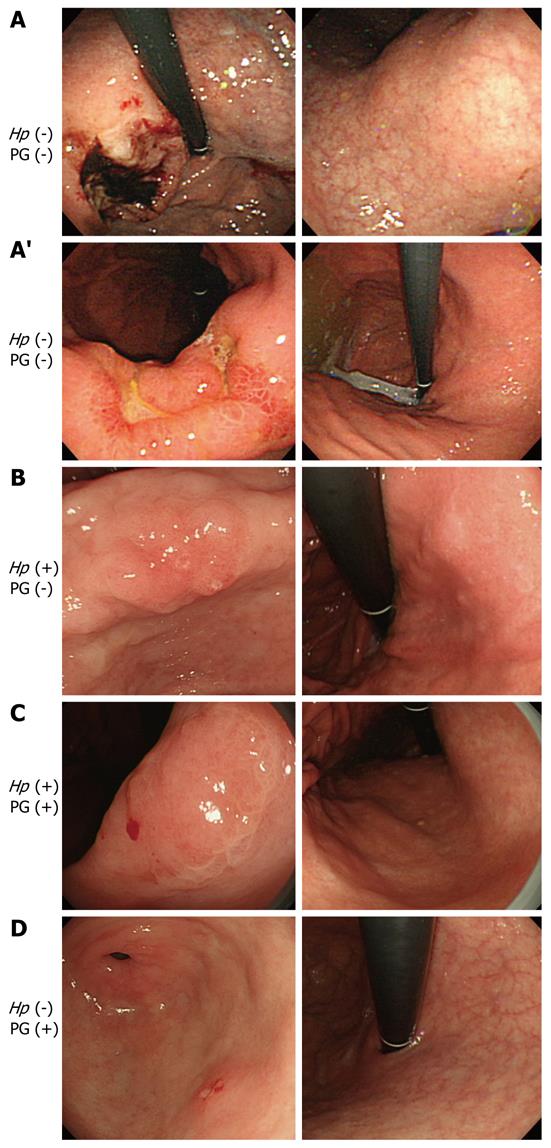
Figure 1 shows the representative cases of gastric cancer in groups A-D. Figure 1A shows a 70-year-old male with H. pylori (-) and PG (-). However, open- type atrophy was found in endoscopy. He should have been classified into group D because of the extent of mucosal atrophy. The PG I level was low (28.5 ng/mL), although PG was negative based on the PG I/PG II ratio (3.9). Figure 1A’ shows a 48-year-old female with H. pylori (-) and PG (-). She had no atrophy in the endoscopic findings. This case received post-eradiation therapy for H. pylori. The patient was H. pylori negative with the titer of the H. pylori antibody under 10 U/mL. However, the antibody titer was 7.6 U/mL, so she was not completely H. pylori negative. Figure 1B shows a 72-year-old male with H. pylori (+) and PG (-). He had closed-type atrophy in the endoscopic findings. Figure 1C shows a 71-year-old male with H. pylori (+) and PG (+). This case was positive for both H. pylori and PG. He had open-type atrophy in the endoscopic examinations. Figure 1D presents a 69-year-old male with H. pylori (-) and PG (+). He had open-type atrophy in endoscopy. Because of the progression of mucosal atrophy, we concluded that this patient was likely negative for H. pylori because the organism cannot live in the atrophic mucosa.
A summary of the patients with gastric cancer arising from group A and group A’ is shown in Table 3. Among 10 patients, there was one case without atrophy, 3 cases of closed-type atrophy, and 6 cases of open-type atrophy. There were only 3 (3.2%) cases in group A when the patients in group A’ (who was taking acid proton pump inhibitors and/or had received eradication therapy for H. pylori) were excluded. These 3 cases had mucosal atrophy and were classified into the grey zone pattern based on the diagnostic manual of ABC (D) stratification. Eight of the 10 patients were classified into group A or A’ because their serum PG I/PG II ratio was greater than 3. The serum PG I levels of the 8 patients were ≤ 70 ng/mL.
| Case | Stratification | Age (yr) | Sex | Location | Macroscopic type | Differentiation | PG I/PG II levels (ng/mL) | PG I/PG II ratio | Endoscopic findings | PPI prescription | H. pylori eradication therapy |
| 1 | A | 70 | M | U | Borrmann II | Poor | 28.5/7.3 | 3.9 | Open type | No | No |
| 2 | A' | 48 | F | M | II c + III | Signet ring cell carcinoma | 55.9/8.2 | 6.8 | Closed type | No | Yes |
| 3 | A | 68 | M | M | II a + II c | Well | 64.1/11.8 | 5.4 | Open type | No | No |
| 4 | A | 78 | F | L | II c | Well | 13.6/4.2 | 3.2 | Open type | No | No |
| 5 | A' | 67 | F | L | II c | Well | 46.4/10.8 | 4.3 | Closed type | Yes | No |
| 6 | A' | 79 | F | M | II a + II c | Well | 86.2/10.9 | 7.9 | Closed type | Yes | No |
| 7 | A' | 77 | M | M | LST-G | Well | 384.9/54.3 | 7.1 | Non | Yes | No |
| 8 | A' | 77 | F | U | II c | Well | 17.3/5.2 | 3.3 | Open type | No | Yes |
| 9 | A' | 57 | M | M | II c | Well | 36.9/6.8 | 5.4 | Open type | No | Yes |
| 10 | A' | 71 | M | M | II a + II c | Well | 38.6/7.9 | 4.9 | Open type | No | Yes |
Histologically, there were 76 (80.0%) cases of well or moderately differentiated adenocarcinomas (well differentiated group) (Table 4). There were 19 (20.0%) cases of poorly differentiated adenocarcinomas or signet ring cell carcinomas (poorly differentiated group). The mean age of the patients in the well differentiated group [(70.2 ± 5.8) years] was significantly higher than that of the poorly differentiated group [(61.4 ± 12.9) years, P < 0.05]. There were no differences in the sex ratio or the pattern of atrophy in endoscopy between the well differentiated group and the poorly differentiated group. The proportion of group C patients tended to be higher in the well differentiated group, although it did not reach statistical significance. It was supposed that the patients with well differentiated adenocarcinomas would shift from group B to C as they aged.
| All patients | Well diff | Poorly diff | P value | |
| No. of patients | 95 | 76 | 19 | |
| Male:female | 72:23 | 58:18 | 14:5 | NS |
| Age (yr, mean ± SD) | 67.9 ± 8.9 | 70.2 ± 5.8 | 61.4 ± 12.9 | < 0.05 |
| ABC (D) stratification | ||||
| A: H. pylori (-) PG (-) | 10 (10.6%) | 8 (10.5%) | 2 (10.6%) | NS |
| B: H. pylori (+) PG (-) | 27 (28.4%) | 18 (23.7%) | 9 (47.3%) | |
| C: H. pylori (+) PG (+) | 54 (56.8%) | 47 (61.8%) | 7 (36.8%) | |
| D: H. pylori (-) PG (+) | 4 (4.2%) | 3 (3.9%) | 1 (5.3%) | |
| Endoscopic atrophic border | ||||
| Non | 2 (2.1%) | 2 (2.6%) | 0 (0%) | NS |
| Closed type | 21 (22.1%) | 15 (19.7%) | 6 (31.6%) | |
| Open type | 72 (75.8%) | 59 (77.6%) | 13 (68.4%) |
The relationship between ABC (D) stratification and the endoscopic atrophic border according to the histological differentiation is shown in Tables 5 and 6. There were no differences in the distribution of the endoscopic atrophic border between the well differentiated and poorly differentiated groups.
| Endoscopic atrophic border | ||||
| ABC (D) stratification | Non | Closed type | Open type | Total |
| A: H. pylori (-) PG (-) | 1 | 2 | 5 | 8 (10.5%) |
| B: H. pylori (+) PG (-) | 1 | 5 | 12 | 18 (23.7%) |
| C: H. pylori (+) PG (+) | 0 | 7 | 40 | 47 (61.8%) |
| D: H. pylori (-) PG (+) | 0 | 1 | 2 | 3 (3.9%) |
| Total | 2 (2.6%) | 15 (19.7%) | 59 (77.6%) | 76 (100%) |
| Endoscopic atrophic border | ||||
| ABC (D) stratification | Non | Closed type | Open type | Total |
| A: H. pylori (-) PG (-) | 0 | 1 | 1 | 2 (10.6%) |
| B: H. pylori (+) PG (-) | 0 | 2 | 7 | 9 (47.4%) |
| C: H. pylori (+) PG (+) | 0 | 3 | 4 | 7 (36.8%) |
| D: H. pylori (-) PG (+) | 0 | 0 | 1 | 1 (5.3%) |
| Total | 0 (0%) | 6 (31.6%) | 13 (68.4%) | 19 (100%) |
A screening program with an upper gastrointestinal series has been confirmed to be effective for reducing mortality from gastric cancer in Japan[2,3]. Since the X-ray with photofluorography was first introduced in the 1960s, it has played a key role in gastric cancer screening[11,12]. However, the existing program by the X-ray was introduced prior to the discovery of H. pylori and documentation of its carcinogenicity. Only approximately 13% of the target population participated in the program[13]. Given these drawbacks, it is necessary to establish an effective screening system, focusing on high-risk status such as H. pylori infection and atrophic gastritis. The combined use of serum anti-H. pylori antibodies and PG measurement has been reported to be a useful screening method for gastric cancers[4], and several recent reports have confirmed the usefulness of this screening method[5,6]. However, most of these reports targeted the general population during routine health check-ups. The ABC (D) stratification of patients who have already been diagnosed with gastric cancer has not been sufficiently investigated. In this study, we evaluated the ABC (D) stratification of patients with confirmed gastric cancers.
Rapid progress has been made in the studies on stomach carcinogenesis since the discovery of H. pylori. The relationship between atrophic gastritis and gastric cancer has been confirmed epidemiologically. Uemura et al[14] reported that only H. pylori-infected subjects developed gastric cancer among a group of patients with organic or functional gastroduodenal disorders. Fukase et al[15] reported a randomized controlled trial in which H. pylori eradication contributed to the reduction of metachronous gastric cancer after endoscopic resection of early gastric cancer. Not surprisingly, the combination of H. pylori infection determined by serum anti-H. pylori antibodies and atrophic gastritis determined by serum PG levels is promising for diagnosing gastric cancer[4-6].
Mizuno et al[6] reported that the atrophy-positive H. pylori-positive group (group C in ABC (D) stratification system) had a moderately high hazard ratio of 11.23, while the atrophy-positive H. pylori-negative group (group D) had a markedly higher hazard ratio of 14.81. These two groups are therefore considered the most appropriate candidates for gastric cancer screening. It is well known that anti-H. pylori antibody production may be reduced when atrophy progresses because H. pylori does not survive very well in the intestinal metaplasia mucosa[6]. As group D represents the status of severe atrophic gastritis with marked intestinal metaplasia, it is the highest-risk group for developing gastric cancer. In addition, the atrophy-negative H. pylori-positive group (group B) had a relatively high hazard ratio of 4.20[6]. This group represents the status of H. pylori-induced active gastritis without extensive atrophy, which is thought to be one of the factors that contribute to the diffuse-type gastric cancer. As diffuse-type gastric cancer grows and invades faster than the intestinal type, this group is considered to be a candidate for a gastric cancer screening program. In this study, there were only 3 cases in group A when group A’ patients were excluded. Seven patients in group A’ were receiving proton pump inhibitor therapy or had previously been treated for eradicating H. pylori infection. As a result, precise medical interviews, such as prescription of proton pump inhibitors and a past history of H. pylori eradication are needed. Three group A cases had mucosal atrophy upon endoscopic examination. Based on this mucosal atrophy, these cases should be classified into group D. The pepsinogen levels of these patients are shown in Table 3. In this study, the serum PG status was defined as atrophic when the criteria of both serum PG I level ≤ 70 ng/mL and a PG I /PG II ratio ≤ 3.0 were simultaneously fulfilled. These criteria have a sensitivity of 70.5% and a specificity of 97%[16]. The serum PG I levels of the 3 group A cases were all ≤ 70 ng/mL. They were classified into normal PG because they had a PG I/PG II ratio > 3.0. This indicates that special attention should be paid to avoiding false negative cases of atrophic gastritis. None of the gastric cancer patients in our study were both H. pylori (-) and without atrophic gastritis. Ohata et al[4] reported a study with a cohort of 4655 healthy asymptomatic subjects (average age, 49 years) who were followed up for a mean period of 7.7 years. No cancer developed in the H. pylori (-)/normal PG group during their study period[4].
Graham and Asaka[17] proposed an eradication program for gastric cancer. Under their proposal, all adults would receive non-invasive testing for H. pylori infection and atrophic gastritis. All H. pylori infected patients would have confirmed H. pylori eradication[17]. Those with atrophic gastritis would be considered for further evaluation and possible surveillance[17]. The cases that were H. pylori (-) and had no atrophy would be excluded from the follow-up program. However, additional attention should be paid to mucosal atrophy, because the sensitivity of PG only 70.5%. Since the ultrathin transnasal endoscopy can be used for health check-ups, gastric cancer screening with ABC (D) stratification in combination with endoscopy may represent a useful screening system. In conclusion, our findings suggest that a combination screening for the H. pylori antibody titer and serum PG status may therefore be useful for predicting the development of gastric cancer. However, additional attention should be paid to avoiding false negatives for the patients who are taking acid proton pump inhibitors and those who have received prior eradication therapy for H. pylori. Endoscopy is needed for grey zone cases to accurately determine the mucosal atrophy status.
Gastric cancer remains the second leading cause of cancer death in Japan, although its mortality has continued to decrease for decades. Screening systems or methods to detect early gastric cancers have contributed to the decrease in gastric cancer deaths. The combination of serum pepsinogen (PG) and Helicobacter pylori (H. pylori) antibody [ABC (D) stratification] can serve as a useful predictive marker for diagnosing patients with gastric cancers.
Recent studies concerning the use of ABC (D) stratification have focused on patients with either no disease or an unknown disease. There have so far been few analyses of ABC (D) stratification of patients confirmed to have gastric cancer.
ABC (D) stratification may be useful for predicting the development of gastric cancer. However, additional attention should be paid to avoiding false negatives for patients who are taking acid proton pump inhibitors and those who have received prior eradication therapy for H. pylori. Endoscopy is needed to evaluate grey zone cases to accurately determine the mucosal atrophy status.
Combination screening for the H. pylori antibody titer and serum PG status [ABC (D) stratification] is a good method for screening patients with gastric cancers.
ABC (D) stratification is a screening method for patients with gastric cancer using a combination of the H. pylori antibody titer and serum PG status.
This manuscript describes the evaluation of ABC (D) stratification in a group of patients confirmed to have gastric cancer. The authors found that ABC (D) stratification correlated closely with the disease status, but noted possible false negatives due to the fact that some patients have previously received treatments aimed at eradicating H. pylori. Overall, the study is well designed and the analysis approach is sound. Although the findings were somewhat expected based on previous studies, the study does provide further support for the use of the ABC (D) stratification system. The findings of this study would therefore be of interest to other researchers in this field if published.
Peer reviewer: Hai-Yong Han, PhD, MS, BS, Division of Clinical Translational Research, The Translational Genomics Research Institute, 445 N Fifth Street, Phoenix, AZ 85004, United States
S- Editor Zhang SJ L- Editor Ma JY E- Editor Zhang DN
| 1. | The Editorial Board of the Cancer Statistics in Japan. Cancer Statistics in Japan 2008. Tokyo: Foundation for Promotion Cancer Research 2008; . |
| 2. | Oshima A, Hirata N, Ubukata T, Umeda K, Fujimoto I. Evaluation of a mass screening program for stomach cancer with a case-control study design. Int J Cancer. 1986;38:829-833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 107] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 3. | Fukao A, Tsubono Y, Tsuji I, HIsamichi S, Sugahara N, Takano A. The evaluation of screening for gastric cancer in Miyagi Prefecture, Japan: a population-based case-control study. Int J Cancer. 1995;60:45-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 117] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 4. | Ohata H, Kitauchi S, Yoshimura N, Mugitani K, Iwane M, Nakamura H, Yoshikawa A, Yanaoka K, Arii K, Tamai H. Progression of chronic atrophic gastritis associated with Helicobacter pylori infection increases risk of gastric cancer. Int J Cancer. 2004;109:138-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 354] [Cited by in RCA: 373] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 5. | Watabe H, Mitsushima T, Yamaji Y, Okamoto M, Wada R, Kokubo T, Doi H, Yoshida H, Kawabe T, Omata M. Predicting the development of gastric cancer from combining Helicobacter pylori antibodies and serum pepsinogen status: a prospective endoscopic cohort study. Gut. 2005;54:764-768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 290] [Cited by in RCA: 310] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 6. | Mizuno S, Miki I, Ishida T, Yoshida M, Onoyama M, Azuma T, Habu Y, Inokuchi H, Ozasa K, Miki K. Prescreening of a high-risk group for gastric cancer by serologically determined Helicobacter pylori infection and atrophic gastritis. Dig Dis Sci. 2010;55:3132-3137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 74] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 7. | Kimura K, Takemoto T. An endoscopic recognition of the atrophic border and its significance in chronic gastritis. Endoscopy. 1969;1:87-97. [RCA] [DOI] [Full Text] [Cited by in Crossref: 612] [Cited by in RCA: 740] [Article Influence: 43.5] [Reference Citation Analysis (3)] |
| 8. | Miki K, Ichinose M, Kakei N, Yahagi N, Matsushima M, Tsukada S, Ishihama S, Shimizu Y, Suzuki T, Kurokawa K. The clinical application of the serum pepsinogen I and II levels as mass screening method for gastric cancer. Aspartic Proteinase: Structure, Function, Biology and Biomedical Implications. New York: Plenum Press 1995; 139-143. |
| 9. | Ichinose M, Miki K, Furihata C, Kageyama T, Hayashi R, Niwa H, Oka H, Matsushima T, Takahashi K. Radioimmunoassay of serum group I and group II pepsinogens in normal controls and patients with various disorders. Clin Chim Acta. 1982;126:183-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 101] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 10. | Miki K, Ichinose M, Shimizu A, Huang SC, Oka H, Furihata C, Matsushima T, Takahashi K. Serum pepsinogens as a screening test of extensive chronic gastritis. Gastroenterol Jpn. 1987;22:133-141. [PubMed] |
| 11. | Hamashima C, Shibuya D, Yamazaki H, Inoue K, Fukao A, Saito H, Sobue T. The Japanese guidelines for gastric cancer screening. Jpn J Clin Oncol. 2008;38:259-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 275] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 12. | Miles A, Cockburn J, Smith RA, Wardle J. A perspective from countries using organized screening programs. Cancer. 2004;101:1201-1213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 148] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 13. | Statistics and Information Department, Ministry of Health, Labour, and Welfare. National Reports on Cancer Screening Programs 2004. Tokyo: Health and Welfare Statistics Association 2006; . |
| 14. | Uemura N, Okamoto S, Yamamoto S, Matsumura N, Yamaguchi S, Yamakido M, Taniyama K, Sasaki N, Schlemper RJ. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med. 2001;345:784-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3126] [Cited by in RCA: 3177] [Article Influence: 132.4] [Reference Citation Analysis (0)] |
| 15. | Fukase K, Kato M, Kikuchi S, Inoue K, Uemura N, Okamoto S, Terao S, Amagai K, Hayashi S, Asaka M. Effect of eradication of Helicobacter pylori on incidence of metachronous gastric carcinoma after endoscopic resection of early gastric cancer: an open-label, randomised controlled trial. Lancet. 2008;372:392-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 876] [Cited by in RCA: 932] [Article Influence: 54.8] [Reference Citation Analysis (0)] |
| 16. | Watanabe Y, Kurata JH, Mizuno S, Mukai M, Inokuchi H, Miki K, Ozasa K, Kawai K. Helicobacter pylori infection and gastric cancer. A nested case-control study in a rural area of Japan. Dig Dis Sci. 1997;42:1383-1387. [PubMed] |
| 17. | Graham DY, Asaka M. Eradication of gastric cancer and more efficient gastric cancer surveillance in Japan: two peas in a pod. J Gastroenterol. 2010;45:1-8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 64] [Article Influence: 4.0] [Reference Citation Analysis (0)] |