Published online Nov 7, 2011. doi: 10.3748/wjg.v17.i41.4602
Revised: January 5, 2011
Accepted: January 12, 2011
Published online: November 7, 2011
AIM: To compare long-term results of gastric cancer patients undergoing laparoscopic and open gastrectomy in a single unit.
METHODS: From February 2000 to September 2004, all patients with adenocarcinoma of the stomach were assessed to entry in this longitudinal prospective non-randomized trial. Primary endpoint was cancer-related survival and secondary endpoints were overall survival, evaluation of surgical complications and mortality.
RESULTS: Fifty-eight patients were enrolled. Forty-seven patients were followed-up (range 11-103, median 38 mo). Four patients were lost at follow up. Twenty-two patients underwent a laparoscopic gastric surgery (LGS) and 25 had a standard open procedure (OGS). No statistical difference was found between the two groups in terms of 5 years cancer-related mortality rate (50% vs 52%, P = 1), and 5 years overall mortality rate (54.5% vs 56%, P = 1). Accordingly, cancer-related and overall survival probability by Kaplan-Meier method showed comparable results (P = 0.81 and P = 0.83, respectively). We found no differences in surgical complications in the 2 groups. There was no conversion to open surgery in this series.
CONCLUSION: LGS is as effective as OGS in the management of advanced gastric cancer. However LGS cannot be recommended routinely over OGS for the treatment of advanced gastric cancer.
-
Citation: Sica GS, Iaculli E, Biancone L, Carlo SD, Scaramuzzo R, Fiorani C, Gentileschi P, Gaspari AL. Comparative study of laparoscopic
vs open gastrectomy in gastric cancer management. World J Gastroenterol 2011; 17(41): 4602-4606 - URL: https://www.wjgnet.com/1007-9327/full/v17/i41/4602.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i41.4602
Radical surgical resection of the stomach and regional lymph nodes dissection is still the mainstream of the treatment of advanced gastric cancer (AGC). In 1994 Kitano et al[1]reported the first laparoscopic gastric resection and Billroth reconstruction in a patient with early gastric cancer (EGC). Laparoscopic gastric surgery (LGS) has been shown to improve short-term results and quality of life, compared to standard techniques[2,3,4] and has become an acceptable alternative approach in the management of EGC especially in Japan and Korea[5,6,7]. The experience of laparoscopic gastric surgery in the western world is smaller because most of the gastric cancers are seen in an advanced stage and LGS is not considered an acceptable alternative to standard open surgery as yet. Little is known about long-term results and tumour recurrence after laparoscopic gastrectomy in AGC and most of the reported series are small[8].
However, the National Institute for Clinical Excellence in the United Kingdom has recently published an overview on interventional procedures regarding LGS[9]. LGS emerges as a feasible and oncologically secure procedure with several benefits.
Until large and well-conducted trials are undertaken also in the west and also including patients with AGC, case-control studies and preliminary data are important to guide the necessary acceptance to LGS.
LGS has been performed by our group since 1999. We herein report our 5 years results of a consecutive series of patients who have undergone LGS or standard open gastric surgery mostly for AGC over a four year period (February 2000-September 2004) and followed-up accordingly. The aim of this study was to evaluate long-term results of laparoscopy-assisted gastrectomy compared to open standard procedures in terms of overall and cancer-related survival probabilities.
Between February 2000 and September 2004 all patients with preoperative diagnosis of non-metastatic gastric adenocarcinoma seen at our institution were evaluated to enter in this longitudinal prospective non-randomized trial. All patients underwent diagnostic work-up according to a standard protocol [endoscopy with biopsy, total-body computed tomography (CT) scan, endoscopic ultrasound (EUS) in selected patients]. Eligible patients were assigned either to laparoscopic or open procedure at the multidisciplinary cancer meeting, after the preoperative staging, solely on the basis of the availability of an upper gastrointestinal surgeon with experience in advanced laparoscopic surgery and accordingly to patients’ preference. Demographics and preoperative clinical data analyzed included gender, age, body mass index (BMI), the American Association of Anaesthetists (ASA) score, pathological tumor-node-metastasis (pTNM) stage, tumour location, histological differentiation. Patients were stratified into 3 groups (proximal, distal, intermediate) according to tumour location.
The eligibility criteria were: histological diagnosis of gastric cancer at any stage (≥ 1b), tumour location below the cardias and above the pylorus, pre-operative work-up indicating a surgical resection with an aim to perform R0 gastrectomy. Exclusion criteria were: age < 18 or > 75 years, BMI > 32, ASA score > 3, previous gastric surgery, presence of lynitis plastica, para-aortic lymph-nodes involvement, systemic metastases, contraindications to laparoscopy (severe cardiovascular disease, large abdominal wall or diaphragmatic hernias, etc). There was no selection bias during the enrollment process and inclusion criteria were the same for both groups.
Fifty-eight consecutive patients were enrolled in the study. Data from 47 patients who underwent open gastric surgery (OGS, n = 25) or LGS (n = 22) and then entered our oncologic follow-up (FU) protocol were included. Of this group of patients 91.5% had an AGC and underwent a D2 lymph nodes dissection. Four patients (8.5%) had a tumour in stage 1b and had a dissection of regional nodes (D1) + group β. The type of surgical resection and lymph nodes dissection were the same in both groups according to tumour location and disease stage. OGS required a midline xipho-umbilicus or a bilateral sub costal access. LGS was performed using a 3 ports plus liver retractor technique; at the end of the procedure the first port access was enlarged up to 5 cm to retrieve the specimen and to complete the already stapled esophago-jejunum side-to side anastomoses, if required. The same preoperative and postoperative care was provided to both groups. Adjuvant therapy, where appropriate, was similar for all patients. Patients were followed-up in the outpatient setting until cancer-related death or to the endpoint of the study, September 30, 2009. Follow-up schedule included history, clinical examination, blood tests and tumour markers every 6 mo. Upper gastrointestinal endoscopy was performed twice a year for the first 2 years then as clinically indicated. Total body CT scan was performed at 6 mo from surgery and then annually. Positron emission tomography (PET)-CT was indicated in selected cases.
Primary endpoints of the study were cancer related survival rate and disease-free interval. Secondary endpoints were overall survival rate and evaluation of number of harvested lymph nodes. Cancer related survival represents the number of patients alive at the end of the follow-up period (5 years). Disease free interval represents the period of time in which the patients were free of disease during the follow-up period.
All data were collected on a Microsoft® Excel spreadsheet and derived retrospectively from the database. Results were expressed as median and range of observed values. Statistical analyses were obtained with SPSS 17.0. Qualitative data were compared using Fisher’s exact test or Pearson’s χ2 test; quantitative data showing a normal distribution were compared using a paired t test. Survival rates were assessed by Kaplan-Meier method and analyzed by the log-rank test. Regardless of the statistical analysis used, P < 0.05 was considered statistically significant.
Eighty-three patients with diagnosis of gastric cancer were referred to our unit from February 2000 to September 2004. Of these patients, 58 were eligible to enter the study. Eight patients were excluded thereafter because of distant nodal involvement, liver metastases or peritoneal carcinosis found intra-operatively. Three patients died within 90 d of surgery, one in the OGS group from complications related to surgery and one in each group due to pre-surgical co-morbidity. According to this trial purpose, these cases were not included in the analysis of long-term results. Four patients were lost at follow-up (8.5%), two in the LGS group and 2 in the OGS group (P = 1). The median follow up was 38 mo (range 11-103) in the whole series, 39 mo in the LGS group (range 12-100) and 38 mo in the OGS group (range 11-103; P = 0.7).
Among the 47 patients left for the purpose of the study (26 males and 21 females; median age 68, range 38-75), 22 underwent LGS (46.8%) and 25 had a standard OGS (53.2%). According to tumour location and intra-operative findings, 12 patients had a total gastrectomy (5 LGS, 7 OGS) and 25 a sub-total gastrectomy (13 LGS, 13 OGS). No conversion from laparoscopic to open surgery occurred in the LGS group.
There were no significant differences between the two groups with respect to demographics and preoperative data considered, type of resection, type of lymphadenectomy and number of harvested lymph nodes. The characteristics of the study population and the type of surgical procedures are summarized in Tables 1 and 2 respectively.
| LGS (n = 22) | OGS (n = 25) | P value | |
| Median age, yr (range) | 67 (38-75) | 68 (54-75) | 0.07 |
| Gender (M/F) | 13/9 | 13/12 | 0.4 |
| BMI, kg/m2 (range) | 23 (18-25) | 22 (18-26) | 0.9 |
| ASA score, median (range) | 2 (0-3) | 2 (0-3) | 0.3 |
| 0 | 5 (23%) | 2 (8%) | |
| 1 | 3 (14%) | 8 (32%) | |
| 2 | 10 (45%) | 10 (40%) | |
| 3 | 4 (18%) | 5 (20%) | |
| Tumor location (%) | 0.9 | ||
| Upper | 5 (23%) | 6 (24%) | |
| Middle | 7 (32%) | 7 (28%) | |
| Lower | 10 (45%) | 12 (48%) | |
| Lauren histology (%) | 0.7 | ||
| Intestinal | 12 (54%) | 12 (48%) | |
| Diffuse | 10 (46%) | 13 (52%) | |
| pTNM [1997] (%) | 0.6 | ||
| Ib | 2 (9%) | 2 (8%) | |
| II | 9 (41%) | 13 (52%) | |
| IIIa/b | 10 (45%) | 7 (28%) | |
| IV | 1 (5%) | 3 (12%) |
| LGS (n = 22) | OGS (n = 25) | P value | |
| Type of gastric resection (%) | 0.7 | ||
| Subtotal | 17 (77%) | 18 (72%) | |
| Total | 5 (23%) | 7 (28%) | |
| Reconstruction (%) | 0.6 | ||
| Billroth 2 | 3 (14%) | 2 (8%) | |
| Roux-en-Y | 19 (86%) | 23 (92%) | |
| Lymphadenectomy (%) | 1 | ||
| D1 | 2 (9%) | 2 (8%) | |
| D2 | 20 (91%) | 23 (92%) | |
| Lymph-nodes retrived | 29 ± 7 | 30 ± 9 | 0.5 |
A radical D2 lymph node dissection was performed in all 43 cases (91% in LGS and 92% in OGS) with a preoperative stage of T ≥ 2 or for any N+. Two patients in the LGS group (9%) and 2 in the OGS group (8%) had a preoperative T1\N0 diagnosis confirmed by EUS and underwent D1 + β lymphadenectomy according to our institution practice guidelines. The numbers of harvested lymph-nodes was 29 ± 7 in the LGS group and 30 ± 9 in the OGS group, thus showing no differences between the two techniques (P = 0.46). Three patients (1 in LGS, 4.5% and 2 in OGS, 8%) were found intra-operatively to have tumour invasion of the adjacent organs underwent an additional procedure, namely liver wedge resection, splenectomy and transverse colon resection.
In the whole study group, 24 patients (51%) died of tumour recurrence. In the LGS group recurrence was observed in 11 patients (50%), the median disease free interval was 28 mo (range 12-60) and the median survival 39 mo (range 12-60). There was no port-site recurrence. In the OGS group recurrence was observed in 13 patients (52%) after a median of 26 mo (range 10-60) (P = 0.69). Median survival of the OGS group was 38 mo (range 11-60) (P = 0.73). There was no difference in the type of recurrence in the 2 study groups. Loco-regional recurrence (defined as tumor found by TC or TC-PET involving nodes or peritoneum in the region of the previous gastrectomy, including nodes around porta hepatis) or carcinosis occurred in 6 patients, liver metastases and jaundice in 12 patients and diffuse/systemic metastases in 6 patients
Cancer related mortality rate was comparable between the two groups (P = 1).
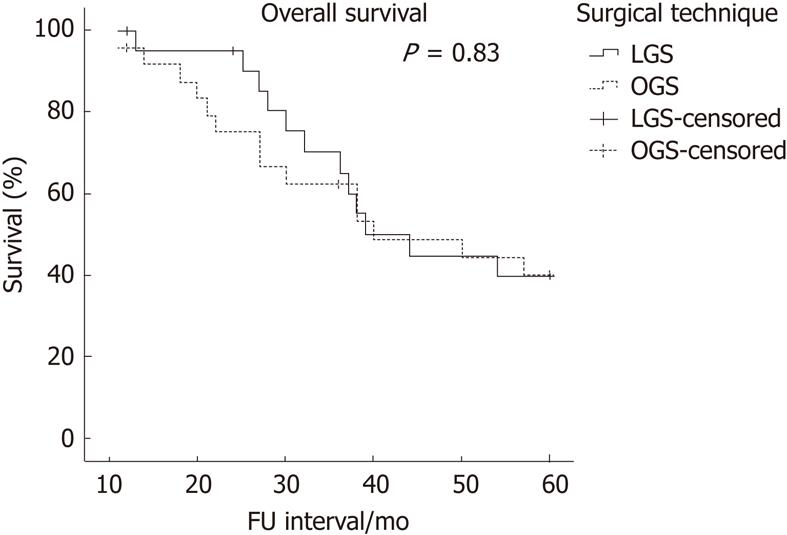
There was no difference in overall survival rate between the two groups (P = 0.9). Twelve patients (54.5%) in the LGS group and 14 patients (56%) in the OGS group died for any cause during the surveillance period.
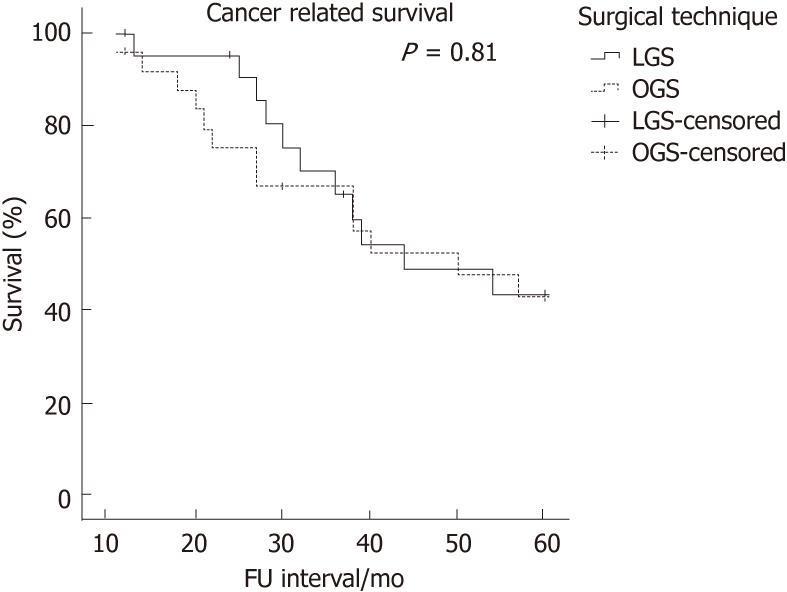
Cancer related survival and overall survival (Figures 1 and 2, respectively) show similar Kaplan-Meier curves (P = 0.81 and 0.83, respectively) for OGS and LGS patients. Survival analyses are shown in Table 3.
| LGS(n = 22) | OGS(n = 25) | P value | |
| Overall 5 yr mortality rate | 12 (54.5%) | 14 (56%) | 1 |
| Cancer-related 5 yr mortality rate | 11 (50%) | 13 (52%) | 1 |
| FU (5 yr) accomplishment | 20 (91%) | 23 (92%) | 1 |
| Type of recurrence | 0.9 | ||
| Port-site M | 0 (0%) | ||
| Locoregional/carcinosis | 2 (18%) | 4 (31%) | |
| Hepatic M | 6 (55%) | 6 (46%) | |
| Distant M | 3 (27%) | 3 (23%) | |
| Median survival, mo (range) | 39 (12-60) | 38 (11-60) | 0.7 |
| Disease free interval, mo (range) | 28 (12-60) | 26 (10-60) | 0.6 |
Oncologically adequate surgical resection is the only therapeutic modality that offers curability in patients with gastric cancer.
According to our institution policy and current international guidelines the operation of choice for gastric cancer is a total or subtotal gastrectomy, depending on tumour location, with D2 dissection in advanced gastric cancer and with D1 ±β lymphadenectomy in non infiltrating tumours.
This paper reports a consecutive series of gastrectomies for cancer performed in a single unit over a 4-year period. The number of patients included in this report is small and come after a short learning curve experience but represents the first sensible group of patients who has completed follow-up so far. Almost half of the procedures were performed laparoscopically and the patients were not randomly assigned to one or the other procedure nor selected on the basis of pre-operative criteria such as tumour extension, BMI or other. However, to have a comparable number of patients, the 2 procedures were alternated whenever possible and this may well represent a sensible bias in the present series. Furthermore patients in the laparoscopic group were slightly younger than those operated with an open approach (P = 0.07); this is possibly related to an undesired selection bias based on patients’ wishes.
Since the first report of laparoscopic gastric resection for cancer[1], various institutions around the world routinely perform LGS. However the vast majority of this experience comes from Asian medical institutions and is limited to the treatment of EGC. The standard lymphadenectomy for EGC is limited to the regional lymph nodes and even though LGS has been reported more difficult than OGS, from the 8th questionnaire survey of endoscopic surgery in Japan that recently examined 4799 cases of laparoscopic assisted distal gastrectomy, it appears clear that LGS is a safe procedure with an extremely low surgical mortality[10].
If the feasibility and safety of LGS in the treatment of EGC has been proven, it is also true that several reports have shown the efficacy of LGS in the cure of EGC with results comparable to those of an OGS series[11,12].
On the other hand, the role of LGS in the treatment of AGC is still controversial and many doubts remain about its safety and oncological efficacy. However it has to be said that many case series have been recently published showing adequate resection margins and lymph nodes retrieval for LGS in AGC whilst long term results still lack of comfortable numbers[13-16].
We hereby report our 5 years follow-up of a consecutive series of patients undergoing laparoscopic vs OGS, mostly with AGC. More precisely over 90% of the patients recruited for this study underwent surgery for AGC whilst four patients (2 from each group) were in stage 1b. The survival curves were re-analyzed after the exclusion of this subgroup of patients but no differences were found (P = 0.9) as shown in Table 4.
| AGC + T1b (n = 47) | AGC (n = 43) | |
| Cancer related survival probability analyses (P) | 0.81 | 0.92 |
| Overall survival probability analyses (P) | 0.83 | 0.92 |
We demonstrate the technical feasibility of a radical lymph nodes dissection; a complete D2 dissection was always possible when appropriate, and the number of lymph-nodes dissected in all the cases of tumour > T1 complied with the Western (harvested N > 25) criteria of R0 resection for AGC. Also, no significant differences were found between the 2 groups (mean lymph nodes: LGS 29 vs OGS 30). Moreover the Kaplan-Meier survival curves show similar results when survival of the LGS group is compared to that of the OGS group (5 years cancer related survival 50% for LGS and 48% for OGS, P = 0.81).
Laparoscopic surgery may offer some advantages in oncologic patients: less surgical stress, less blood loss and complications, better cellular immunity and cytokine release pattern[7,8,17,18].
Given the little data regarding gastric cancer and considering the characteristics of these patients (fragile, elderly, often malnourished and immune-compromised) and the specific biology of the tumour itself (subserosal cancer with transcoelomic metastatic capacity), at the moment we can only speculate that LGS could potentially offer a better outcome over OGS. This preliminary experience hasn’t changed our clinical practice. However, currently we don’t offer LGS to patients with known metastatic nodes to the lymph-nodes of the second level.
A large, multicenter and randomized trial should be designed to document long term benefit of LGS in the treatment of AGC according to tumour-stage (TNM) or preoperative evaluation such as age, co-morbidity, neo-adjuvant therapy.
In conclusion, LGS seems to be as effective as OGS in the treatment of advanced gastric cancer considering the long-term survival probabilities. However, given the lack of strong evidence on a large study population and the technical expertise required to complete a R0 laparoscopic gastrectomy, the authors cannot endorse LGS over OGS for the treatment of advanced gastric cancer outside clinical trials in specialized centres.
Gastric cancer is one of the leading causes of death for solid tumours worldwide. Surgical resection is the only hope of cure. Laparoscopic gastric resection is accepted as an alternative method of cure for early gastric cancer, especially in Asia. Its role in the treatment of advanced gastric cancer is a matter of debate.
Laparoscopic surgery in cancers of the digestive tract (colon) has been demonstrated to bring some advantages, including a possible role in reducing recurrence. The authors looked at the role of laparoscopic gastric resection for advanced gastric cancer and showed that even this technically demanding procedure is safe and feasible in expert hands and that the long term results are equivalent to those of standard open surgery.
In this paper a case-control study was undertaken. Laparoscopic gastrectomy is compared to open surgery. This is one of the few papers with a long term follow-up of a consecutive series of patients with advanced gastric cancer undergoing laparoscopic surgical resection. It shows a good surgical result and reinforced the known advantages of gastrointestinal laparoscopic surgery. Case-control studies are important to guide surgical practice in cancer management.
This study could represent a step forward in minimally invasive surgery of the upper digestive tract. The long term results show that laparoscopic gastric resection is an oncologically adequate procedure that can be carried out with acceptable morbidity and mortality.
This manuscript provides a single center comparison between laparoscopic and open gastrectomy, finding no significant difference in surgical resectability or oncologic outcomes. Overall, it is well written.
Peer reviewer: Stephen M Kavic, MD, FACS, Assistant Professor of Surgery, Department of Surgery, University of Maryland School of Medicine, 22 South Greene Street, Room S4B09, Baltimore, MD 21201, United States
S- Editor Tian L L- Editor O’Neill M E- Editor Li JY
| 1. | Kitano S, Iso Y, Moriyama M, Sugimachi K. Laparoscopy-assisted Billroth I gastrectomy. Surg Laparosc Endosc. 1994;4:146-148. [PubMed] |
| 2. | Goh PM, Khan AZ, So JB, Lomanto D, Cheah WK, Muthiah R, Gandhi A. Early experience with laparoscopic radical gastrectomy for advanced gastric cancer. Surg Laparosc Endosc Percutan Tech. 2001;11:83-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 41] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 3. | Adachi Y, Suematsu T, Shiraishi N, Katsuta T, Morimoto A, Kitano S, Akazawa K. Quality of life after laparoscopy-assisted Billroth I gastrectomy. Ann Surg. 1999;229:49-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 196] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 4. | Kitano S, Adachi Y, Shiraishi N, Suematsu T, Bando T. Laparoscopic-assisted proximal gastrectomy for early gastric carcinomas. Surg Today. 1999;29:389-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 43] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 5. | Asao T, Hosouchi Y, Nakabayashi T, Haga N, Mochiki E, Kuwano H. Laparoscopically assisted total or distal gastrectomy with lymph node dissection for early gastric cancer. Br J Surg. 2001;88:128-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 94] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 6. | Dulucq JL, Wintringer P, Stabilini C, Solinas L, Perissat J, Mahajna A. Laparoscopic and open gastric resections for malignant lesions: a prospective comparative study. Surg Endosc. 2005;19:933-938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 87] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 7. | Kitano S, Shiraishi N, Uyama I, Sugihara K, Tanigawa N. A multicenter study on oncologic outcome of laparoscopic gastrectomy for early cancer in Japan. Ann Surg. 2007;245:68-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 519] [Cited by in RCA: 520] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 8. | Huscher CG, Mingoli A, Sgarzini G, Sansonetti A, Di Paola M, Recher A, Ponzano C. Laparoscopic versus open subtotal gastrectomy for distal gastric cancer: five-year results of a randomized prospective trial. Ann Surg. 2005;241:232-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 650] [Cited by in RCA: 655] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 9. | Available from: http: //www.nice.org. |
| 10. | Japanese Society for Endoscopic Surgery The 8th questionnaire survey of endoscopic surgery. J Jpn Soc Endosc Surg. 2006;5:528-628. |
| 11. | Kawamura H, Homma S, Yokota R, Yokota K, Watarai H, Hagiwara M, Sato M, Noguchi K, Ueki S, Kondo Y. Inspection of safety and accuracy of D2 lymph node dissection in laparoscopy-assisted distal gastrectomy. World J Surg. 2008;32:2366-2370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 53] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 12. | Strong VE, Devaud N, Allen PJ, Gonen M, Brennan MF, Coit D. Laparoscopic versus open subtotal gastrectomy for adenocarcinoma: a case-control study. Ann Surg Oncol. 2009;16:1507-1513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 143] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 13. | Fukunaga T, Hiki N, Tokunaga M, Nohara K, Akashi Y, Katayama H, Yoshiba H, Yamada K, Ohyama S, Yamaguchi T. Left-sided approach for suprapancreatic lymph node dissection in laparoscopy-assisted distal gastrectomy without duodenal transection. Gastric Cancer. 2009;12:106-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 14. | Song KY, Kim SN, Park CH. Laparoscopy-assisted distal gastrectomy with D2 lymph node dissection for gastric cancer: technical and oncologic aspects. Surg Endosc. 2008;22:655-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 94] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 15. | Muratore A, Zimmitti G, Lo Tesoriere R, Mellano A, Massucco P, Capussotti L. Low rates of loco-regional recurrence following extended lymph node dissection for gastric cancer. Eur J Surg Oncol. 2009;35:588-592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 16. | Lee J, Kim W. Long-term outcomes after laparoscopy-assisted gastrectomy for advanced gastric cancer: analysis of consecutive 106 experiences. J Surg Oncol. 2009;100:693-698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 63] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 17. | Kim YW, Baik YH, Yun YH, Nam BH, Kim DH, Choi IJ, Bae JM. Improved quality of life outcomes after laparoscopy-assisted distal gastrectomy for early gastric cancer: results of a prospective randomized clinical trial. Ann Surg. 2008;248:721-727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 475] [Cited by in RCA: 505] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 18. | Mochiki E, Nakabayashi T, Kamimura H, Haga N, Asao T, Kuwano H. Gastrointestinal recovery and outcome after laparoscopy-assisted versus conventional open distal gastrectomy for early gastric cancer. World J Surg. 2002;26:1145-1149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 109] [Article Influence: 4.7] [Reference Citation Analysis (0)] |