Peritoneal adhesions represent an important clinical challenge in gastrointestinal surgery. Peritoneal adhesions are a consequence of peritoneal irritation by infection or surgical trauma. They are a major cause of morbidity, resulting in multiple complications, many of which may manifest several years after the initial surgical procedure[1,2]. Development of peritoneal adhesions has been studied extensively, but to date, there has been no definitive strategy to prevent their formation, as controversies concerning the effectiveness of available preventive agents still exist. In addition, most of the available clinical literature concern gynecological patients; for patients undergoing general and/or abdominal surgery, no recommendations or guidelines exist[3]. The aim of this review is to present the epidemiology, pathogenesis and various prevention strategies of adhesion formation. We performed a literature search for this review in Medline and PubMed, using the key words: “adhesions”, “intraperitoneal adhesions”, “intra-abdominal adhesions”, “adhesion reduction”, “adhesion prevention”, “adhesion formation”, “adhesion pathophysiology”. We also reviewed the reference lists in all articles retrieved in the search, as well as those of major texts regarding peritoneal adhesion formation. Both clinical and experimental studies upon adhesion formation were retained. There was no restriction regarding publication language.
Definition, epidemiology and consequences of peritoneal adhesions
Peritoneal adhesions are pathological bonds usually between omentum, loops of bowel and the abdominal wall. These bonds may be a thin film of connective tissue, a thick fibrous bridge containing blood vessels and nerve tissue, or a direct contact between two organ surfaces[4]. According to their etiology, peritoneal adhesions may be classified as congenital or acquired, which can be postinflammatory or postoperative (the most frequent)[5]. Among postoperative adhesion formation, three processes may be distinguished: adhesion formation (adhesions formed at operative sites); de novo adhesion formation (adhesions formed at non-operative sites); and adhesion reformation (adhesions formed after the lysis of previous adhesions)[6] . Diamond et al[7] have distinguished type 1 and type 2 formation of postoperative peritoneal adhesions. Type 1 or de novo adhesion formation concerns adhesions formed at sites that did not have previous adhesions, including type 1A (no previous operative procedure at the site of adhesions) and type 1B (previous operative procedures at the site of adhesions). Type 2 involves adhesion reformation, with two separate subtypes: type 2A (no operative procedure at the site of adhesions besides adhesiolysis) and type 2B (other operative procedures at the site of adhesions besides adhesiolysis)[7].
Peritoneal adhesions are mostly induced by surgical procedures in the peritoneal cavity, and their prevalence after major abdominal procedures has been evaluated at 63%-97%[8,9]. Overall, approximately one-third of patients who underwent open abdominal or pelvic surgery were readmitted an average of two times over the subsequent 10 years for conditions directly or possibly related to adhesions, or for further surgery that could potentially be complicated by adhesions; > 20% of all such readmissions occurred during the first year after initial surgery, and 4.5% of readmissions were for adhesive small bowel obstruction (ASBO)[1,10-13]. Colorectal surgery has proved to be the most important type of surgery that may cause intra-abdominal adhesions[14]. This surgery has the highest total number of inpatient episodes, inpatient days, operating time, theater time, and costs due to peritoneal adhesion-related intestinal obstruction[14]. Among open gynecological procedures, ovarian surgery had the highest rate of readmissions directly related to adhesions (7.5/100 initial operations)[13].
Small bowel obstructions (SBO) is the most common complication of peritoneal adhesions[1,2,8,9]. At Westminster Hospital (London, United Kingdom), intestinal obstruction accounted for 0.9% of all admissions, 3.3% of major laparotomies, and 28.8% of cases of large or SBO over 24 years[5]. A 1992 British survey has reported an annual total of 12 000-14 400 cases of adhesive intestinal obstruction. Barmparas et al[15] have studied the incidence and risk factors for ASBO following laparotomy. The overall incidence of ASBO was 4.6% and the risk of ASBO was highly influenced by the type of procedure, with ileal pouch-anal anastomosis being associated with the highest incidence of SBO[15]. In 1988 in the United States, admissions for adhesiolysis accounted for nearly 950 000 d of inpatient care[5]. All these studies have demonstrated that ASBO is a significant health issue both in the developed and developing world. However, ASBO risk factors, such as the type of past surgical procedure, the site of adhesions, as well as the timing and recurrence rate of adhesive obstruction, remain unpredictable or poorly understood[5].
In addition to ASBO, peritoneal adhesions may cause pelvic or abdominal pain, and infertility[1,2,16]. Peritoneal adhesions may also prolong the time needed to gain access to the abdominal cavity at subsequent surgery[17,18], and may increase the risk of bowel injury during subsequent surgery[19]. Controversy remains on the role of peritoneal adhesions on abdominal pain. Adhesions have been implicated as a significant cause of chronic pelvic pain, and their surgical lysis has been proposed as the therapeutic modality of choice[20,21]. However, chronic pelvic pain is one of most common gynecological complaints and yet remains an enigma. A comparison of chronic pelvic pain patients and asymptomatic infertility patients has not revealed a significant difference in the density or the location of adhesions[22]. Thus, it is possible that a common mechanism for pelvic pain exists and that adhesions are only associated features. Bradykinin, histamine and other autocoids are able to stimulate pain receptors. For
Rapkin et al[22], these findings question the role of pelvic adhesions as a cause of chronic pelvic pain. According to other authors, although adhesions are thought to cause pain indirectly by restricting organ motion, thus stretching and pulling smooth muscle of adjacent viscera or the abdominal wall, adhesions themselves are capable of generating pain stimuli. Sulaiman et al[23] have studied the distribution, location, size and type of nerve fibers present in human peritoneal adhesions, associated or not with chronic pelvic pain. They have found that nerve fibers, identified histologically, ultrastructurally, and immunohistochemically, were present in all examined peritoneal adhesions. Furthermore, fibers expressing the sensory neuronal markers calcitonin gene-related protein and substance P were present in all adhesions irrespective of reports of chronic abdominopelvic pain. That study has suggested that these structures may be capable of conducting pain after appropriate stimulation, and peritoneal adhesions are implicated as a cause of chronic abdominopelvic pain. In addition, many patients are relieved of their symptoms after adhesiolysis[23].
As consequence, peritoneal adhesions have a significant economic impact. Their direct costs in Sweden can be estimated to be $13 million annually[24]. It has been estimated that in the United States, there are 117 hospitalizations for adhesion-related problems per 100 000 people, and the total cost for hospital and surgical expenditure is about $1.3 billion[25]. In some European countries, the direct medical costs for adhesion-related problems are more than the surgical expenditure for gastric cancer and almost as much as for rectal cancer[3,26,27]. Indeed, postoperative adhesions have a profound economic impact, including the surgical procedure itself, hospitalization, recuperation and lost productivity[25]. During 1988, excluding patient and indirect costs, hospitalization in the United States, accounting for 948 727 d of inpatient care, was responsible for an estimated $1179.9 million in expenditure, of which $925 million was associated with hospital costs and $254.9 million with surgeons’ fees[25]. The study of Ray et al[28] has demonstrated substantial costs associated with surgical procedures and hospitalization for adhesiolysis. During 1996, the total annual cost of adhesions management exceeded $2 billion, excluding recuperation and lost productivity[28]. Hospitalization for adhesiolysis alone cost > $700 million. Furthermore, > 300 000 patients are estimated to undergo surgery to treat adhesion-induced SBO in the United States annually[25]. Thus, developing effective strategies for adhesion prevention may help to reduce adhesions management costs and unnecessary morbidity and mortality rates.
Postoperative peritoneal adhesion pathophysiology
The first peritoneal adhesions were described at post-mortem examination of a patient with peritoneal tuberculosis in 1836. To explain this finding, it was suggested in 1849 that coagulated lymphatic vessels may turn into fibrinous adhesions[29,30]. Until now, the exact pathophysiology of peritoneal adhesions has remained elusive. Despite many clinical and experimental studies, peritoneal adhesions pathophysiology remains controversial.
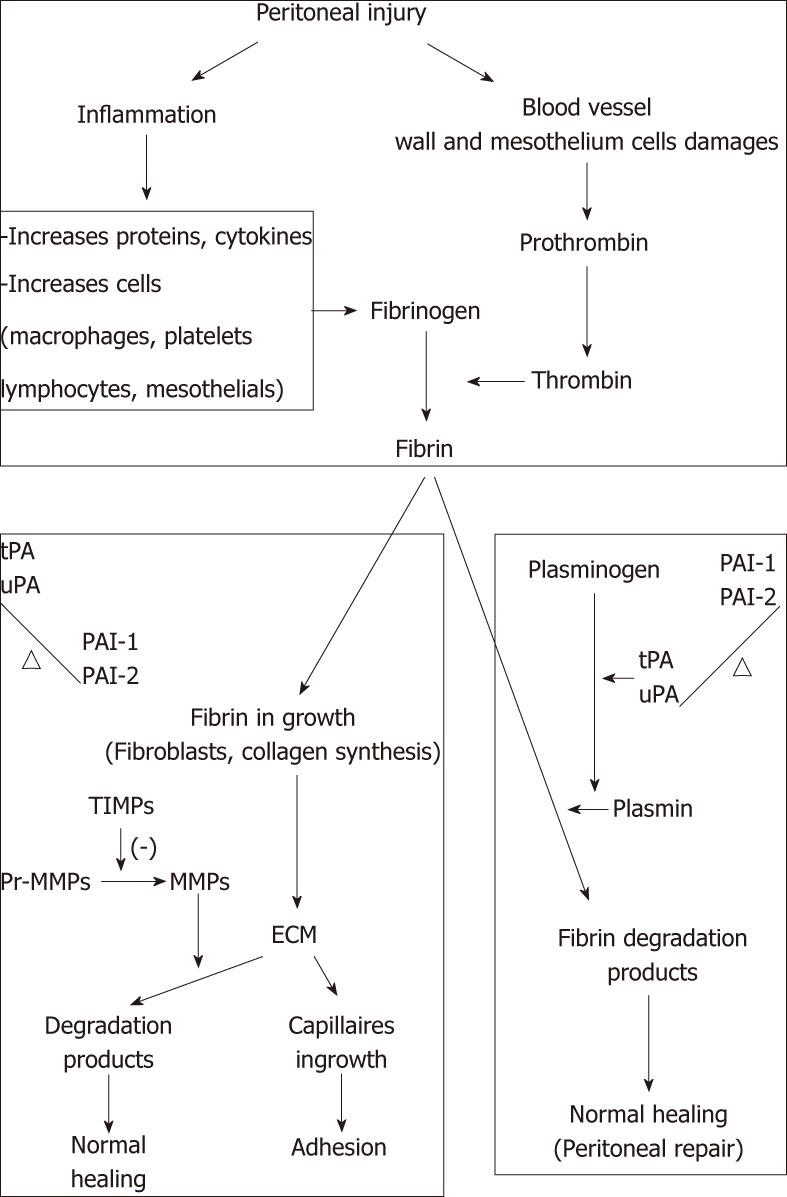
Aside from the normal peritoneal regeneration, the process of postoperative peritoneal adhesion formation may be considered as the pathological part of healing following any peritoneal injury, particularly due to abdominal surgery[5,31]. The balance between fibrin deposition and degradation is crucial in determining normal peritoneal healing or adhesion formation. If fibrin is completely degraded, normal peritoneal healing may occur. In contrast, incompletely degraded fibrin may serve as a scaffold for fibroblasts and capillary in growth to form peritoneal adhesions.
Peritoneal injury, due to surgery, infection or irritation, initiates inflammation with fibrinous exudate and fibrin formation[32]. Fibrin results from coagulation cascade activation that is activated in the peritoneal cavity, resulting in the formation of thrombin that triggers conversion of fibrinogen into fibrin. However, owing to activation of the fibrinolytic system, any intra-abdominal fibrin deposits must be lysed. After abdominal surgery, however, the equilibrium between coagulation and fibrinolysis is disturbed, in favor of the coagulation system. Thus, fibrin forms deposits are a matrix for ingrowth of fibrocollagenous tissue. Indeed, fibroblasts invade the fibrin matrix and the extracellular matrix (ECM) is produced and deposited. This ECM can still be completely degraded by the proenzymes of matrix metalloprotease (MMP), leading to normal healing. However, if this process is inhibited by tissue inhibitors of MMPs, peritoneal adhesions may be formed[33]. Generally, if fibrinolysis does not occur within 5-7 d of the peritoneal injury, the temporary fibrin matrix persists and gradually becomes organized with collagen-secreting fibroblasts. This process leads to peritoneal adhesion formation[34,35] and growth of new blood vessels mediated by angiogenic factors[13].
Activation of the fibrinolytic system results in the conversion of plasminogen into plasmin that is highly effective in the degradation of fibrin into fibrin degradation products. Tissue-type plasminogen activator (tPA) and urokinase-type plasminogen (uPA) are both plasminogen activators. They are expressed in endothelial cells, mesothelial cells and macrophages. tPA, a serine protease, is the main plasminogen activator and has a high affinity for fibrin. It binds to a specific receptor, which exposes a strong plasminogen-binding site on the surface of the fibrin molecule. Therefore, in the presence of fibrin, the activation rate of plasminogen is strikingly enhanced, whereas in the absence of fibrin, tPA is a poor activator of plasminogen[36,37]. This results in higher plasminogen activation at the sites where it is required, whereas systemic activation is prevented. In the peritoneal cavity, tPA is responsible for 95% of plasminogen-activating activity[38]. uPA is equally effective in the degradation of fibrin[39], but its much lower affinity for fibrin results in a significantly lower plasminogen-activating activity. Besides activation of plasminogen, uPA may play an important role in tissue remodeling[40].
Plasminogen activation is hampered by plasminogen-activating inhibitor (PAI)-1 and 2 throughformation of inactive complexes. The most potent inhibitor of tPA and uPA is the glycoprotein PAI-1. PAI-2 is less effective in counteracting plasminogen activators. It probably plays a role in peritoneal tissue repair[41]. Both PA-1 and PAI-2 are produced by endothelial cells, mesothelial cells, monocytes, macrophages and fibroblasts. Other plasminogen activator inhibitors have been identified: PAI-3 and protease nexin 1. Several protease inhibitors, such as α2-macroglobulin, α1-antitrypsin and α2-antiplasmin, inhibit plasmin directly. However, their roles in peritoneal fibrinolysis are not well defined[42]. The balance between plasminogen activators and plasminogen inhibitors is crucial in determining normal healing or adhesion formation (Figure 1). Therefore, PAI-1 is considered to be an important factor in the development of adhesions and high PAI concentrations are found in adhesions and peritoneal tissue of patients with extensive adhesions[43,44].
Figure 1 Balance between plasminogen activators and plasminogen inhibitors.
TIMP: Tissue inhibitors of metalloproteinases; MMP: Matrix metalloprotease; ECM: Extracellular matrix; tPA: Tissue-type plasminogen activator; uPA: Urokinase-type plasminogen; PAI: Plasminogen-activating inhibitor.
Prevention
Several preventive agents against postoperative peritoneal adhesions have been investigated. Their roles are in activating fibrinolysis, hampering coagulation, diminishing the inflammatory response, inhibiting collagen synthesis, or creating a barrier between adjacent wound surfaces. These prevention strategies can be grouped into four categories: general principles, surgical techniques, mechanical barriers, and chemical agents[3].
General principles and surgical techniques: Some basic principles should be respected during all abdominal surgical procedures. These principles are close to the “Halstedian principles” (W.S. Halsted 1852-1922), the first surgeon who recognized the importance of these measures[45]. Peritoneal damage should be avoided by careful tissue handling, meticulous hemostasis, continuous irrigation and avoiding unnecessary drying, ineffective use of foreign bodies, and suturing or clamping of tissue. The use of fine and biocompatible suture materials, atraumatic instruments and starch-free gloves is also recommended. Starched gloves are a significant risk factor for postoperative adhesions. Several experimental studies have shown that the use of starch-powdered gloves during laparotomy is associated with an increased risk of extensive postoperative peritoneal adhesions[46]. Foreign bodies most frequently found in postoperative adhesions are: surface powders from surgical gloves; lint from packs, drapes, or gowns; wood fibers from disposable paper items; and suture materials. However, recent data have suggested that, in the absence of an additional peritoneal injury, foreign bodies are an infrequent cause of adhesion induction[9,47]. Ordonez et al[48] have evaluated the effect of training on postoperative adhesion formation in a rabbit model. The training effect was evaluated by duration of surgery and amount of bleeding. This study has shown that there is a significant effect of experience on duration of surgery. With experience, duration of surgery progressively decreases, and postoperative adhesions also decrease in extent, tenacity, type and total score. According to these findings, surgical training and the respect of some basic principles (“Halstedian principles”) are important for adhesion prevention.
Some intraoperative techniques, such as avoiding unnecessary peritoneal dissection or avoiding closure of the peritoneum, should be applied. Many experimental studies have shown that non-closure of the peritoneum is associated with decreased peritoneal adhesion formation[49-51]. However, some studies have reported no difference[52,53] or even decreased peritoneal adhesion[54] with peritoneal closure. However, grafting or suturing peritoneal defects may increase peritoneal ischemia, devascularization, and necrosis, predisposing the site to decreased fibrinolytic activity and increased adhesion formation[55].
Furthermore, surgical trauma should be reduced as much as possible. The surgical approach (open vs laparoscopic) could play an important role in the development of adhesions. In most abdominal procedures, the laparoscopic approach is associated with a significantly lower incidence of postoperative peritoneal adhesions or adhesion-related re-admissions. Brokelman et al[56] have shown in a prospective trial that there is no difference in tPA antigen, tPA-activity, uPA antigen, or PAI-1 antigen concentrations in peritoneal biopsies taken at the beginning compared to the end of the laparoscopic procedure, irrespective of the intra-abdominal pressure or light activity. In contrast, some studies have reported no difference between both surgical approaches. A role for CO2 pneumoperitoneum in adhesion formation after laparoscopic surgery has been reported[48,57].
During laparoscopic surgery, CO2 pneumoperitoneum by itself has a real impact on abdominal adhesions. It has been demonstrated that adhesion formation increases with the duration of CO2 pneumoperitoneum and insufflation pressure[48,57]. Indeed, prolonged laparoscopic surgery requires long duration and large volume gas insufflations, which raise concerns about the adverse effects of prolonged gas insufflations[58]. The standard CO2 used in current laparoscopic practice is cold dry CO2, which is not physiological to the normal conditions of the peritoneal cavity[57]. Many studies have shown that short-duration laparoscopy, < 3 h, with cold dry CO2 insufflation can cause peritoneal alterations and result in numerous detrimental outcomes, including postoperative peritoneal adhesion formation[48,58]. The benefits of heated humidified CO2 insufflation (37 °C and 95% relative humidity, physiological conditions) have been reported to include less hypothermia, less postoperative pains, shortened recovery room stay, better convalescence, less tumor spread and growth[48,58], and less adhesion formation[35]. Furthermore, Molinas et al[59] have demonstrated that CO2 pneumoperitoneum increases postoperative peritoneal adhesions in a time- and pressure-dependent relationship, and that this increase is reduced by the addition of 2%-4% oxygen, suggesting peritoneal hypoxia as the driving mechanism. It supposes that when fibrinolytic activity decreases, the process of adhesion formation does not depend anymore on the surgical approach, but evolves on its own account.
Mechanical barriers: Liquid or solid mechanical barriers may prevent postoperative peritoneal adhesion formation by keeping peritoneal surfaces separate during the 5-7 d required for peritoneal re-epithelialization. They prevent contact between the damaged serosal surfaces for the first few critical days. An ideal barrier should be biodegradable, safe, non-inflammatory, non-immunogenic, persist during the critical re- mesothelialization phase, stay in place without sutures or staples, remain active in the presence of blood, and be rapidly and easily applied[60,61]. Also, it should not interfere with healing, promote infection, or cause adhesions. Barriers are currently considered the most useful adjuncts that may reduce postoperative peritoneal adhesion formation. Various solid or fluid barrier agents have been tested experimentally and in clinical trials.
Liquids such as crystalloids, dextran, hyaluronic acid, cross-linked hyaluronic acid and icodextrin have been used to prevent adhesion. They separate injured surfaces by “hydroflatation” but their effectiveness is controversial. Crystalloids, such as saline and Ringer’s lactate, are used in large amounts but they are rapidly absorbed. The most commonly used hypertonic solution was 32% dextran 70, but it was abandoned because of serious complications[61]. Other liquid barriers that have the advantage of a longer residence time in the abdominal cavity, such as hyaluronic acid (Sepracoat®, Genzyme Corporation, Cambridge, MA, United States), cross-linked hyaluronic acid (Intergel® Hyalobarrier gel; Baxter, Pisa, Italy), and icodextrin (Adept®, Baxter Healthcare Corporation, Deerfield, IL, United States) have shown promising results in experimental and clinical studies[61]. Brown et al[62] have demonstrated that Adept is a safe and effective adhesion reduction agent in laparoscopy.
There are non-absorbable and bio-absorbable films, gels or solid membranes. The most commonly used mechanical barriers are oxidized regenerated cellulose (Interceed®; Johnson & Johnson Medical, Arlington, TX, United States), expanded polytetrafluoroethylene (Preclude Peritoneal Membrane®; W.L. Gore and Associates Inc., Flagstaff, AZ, United States), hyaluronic acid-carboxymethylcellulose (Seprafilm®; Genzyme Biosurgery, Cambridge, MA, United States) and polyethylenglycol (SprayGel®; Confluent Surgical Inc., Waltham, MA, United States). Preclude is non-degradable and requires a second operation for removal. The most extensively studied bioabsorbable films are Seprafilm and Interceed. Seprafilm is absorbed within 7 d and excreted from the body within 28 d[63,64]. Prospective randomized controlled trials have shown the efficacy of Seprafilm in reducing the incidence and extent of postoperative adhesions[65-68]. However, Seprafilm may cause a significant impairment of anastomoses, and should not be applied to anastomosis cases[69]. Other experimental studies have demonstrated that covering lesions of the parietal peritoneum with microsurgically applied autologous peritoneal transplants can completely prevent severe peritoneal adhesion formation. However, the advantage of a synthetic barrier is that the material does not need to be obtained surgically and can be cut to size outside of the abdomen and then applied without sutures[70].
Chemical agents: Chemical agents generally prevent the organization of the persisting fibrin, by fibroblastic proliferation inhibition. Many agents are used to inhibit this proliferation such as, non-steroidal anti-inflammatory drugs (NSAIDs), corticosteroids, calcium channel blockers, histamine antagonists, antibiotics, fibrinolytic agents, anticoagulants, antioxidants, hormones, vitamins, colchicines and selective immunosuppressors[60].
NSAIDs reduce peritoneal adhesions in some animal models by prostaglandin and thromboxane synthesis inhibition[9]. They decrease vascular permeability, plasmin inhibitors, platelet aggregation, and coagulation and also enhance macrophage function[9]. Rodgers et al[71] have shown that postoperative administration of anti-inflammatory drugs to the site of injury reduced the formation of postoperative adhesions in two animal models. A rat model has been used to investigate the efficacy of nimesulide, a selective cyclooxygenase-2 inhibitor, in the prevention of adhesion formation. This study has shown that preoperative intramuscular or postoperative intraperitoneal administration of nimesulide to the site of injury reduced the formation of postoperative adhesion in this rat model[72]. Generally, some anti-inflammatory drugs may be effective in preventing adhesions, but there is no clinical significant evidence from any published study to recommend their use in humans for this purpose, and several side effects still have to be ascertained[73].
Corticosteroid therapy reduces vascular permeability and liberation of cytokines and chemotactic factors and has reduced peritoneal adhesion formation in some animal models[70]. However, corticosteroids have side effects, such as immunosuppression and delayed wound healing[60,74]. Kirdak et al[75] have investigated the effectiveness of different doses of methylprednisolone in preventing experimentally induced peritoneal adhesions in rats. They have found that there was no difference in the effectiveness of different methylprednisolone doses, administered topically, in preventing peritoneal adhesion formation, and furthermore, steroids did not prevent peritoneal adhesion development[75].
In animal models, these hormones may prevent adhesion formation, but some studies have not confirmed this effectiveness in humans[74]. Progesterone has been reported to have an anti-inflammatory as well as immunosuppressive effect, and may prevent adhesion formation[73]. However, Confino et al[76] have shown that there was no significant difference overall in the incidence of adhesion formation between progesterone-treated and control rabbits. They have revealed a beneficial effect of progesterone in the reduction of only minor adhesion formation formed after minor peritoneal damage[76]. Furthermore, it has been shown that neither estrogen nor gonadotropin-releasing hormone prevented adhesion formation, but there were fewer adhesions formed in estrogen-treated than untreated animals[77].
The use of anticoagulants to prevent the formation of peritoneal adhesions has been enthusiastically reported in the literature[78]. Many molecules have been used, such as heparin or dicumarol, which prevents adhesion by increasing the fibrinolysis due to serine esterase activity[79]. Heparin is the most widely investigated anticoagulant used for prevention of adhesions. However, its efficacy in reducing adhesion formation whether administered alone or in combination with interceed barrier has not been demonstrated in clinical trials[78].
Fibrinolytic agents such as recombinant tPA, when applied locally, have reduced adhesions in animal models[73]. However, these fibrinolytic agents may cause hemorrhagic complications[73]. Three different drugs, tPA (Actilyse®; Boehringer Ingelheim International GmbH, Ingelheim am Rhein, Germany), fondaparinux (Arixtra®; GlaxoSmithKline, France), and activated drotrecogin alfa (Xigris®; Elli Lilly and Co., DSM Pharmaceuticals, Inc. Greenville, NC, United States), which affect the coagulation process at various stages, have been studied for their effectiveness in preventing intraperitoneal adhesion formation in rats[80]. All three agents were effective in preventing adhesions when compared to the control group. Nevertheless, activated drotrecogin alfa seemed the most effective except when considering clinical applicability, in which case fondaparinux seemed to offer the greatest advantage[80]. However, further studies have suggested that all these approaches may have only limited success, impeded lack of safety, efficacy and many adverse effects without eliminating the problem of postoperative peritoneal adhesion formation[81,82].
Some antibiotics are commonly used for prophylaxis against postoperative infections and adhesion formation. Less peritoneal infection may lead to less peritoneal adhesion formation. Linezolid (Zyvox®; Pfizer, New York, NY, United States) has been found to reduce intraperitoneal adhesion formation in a rat uterine horn model[83]. However, other studies have shown that intra-abdominal application itself causes adhesion formation[73]. Sortini et al[84] have shown that antibiotics led to greater adhesion formation by Zühlke score as compared to saline, whereas no difference was observed between antiseptics and saline. Indeed, antibiotics in intraperitoneal irrigation solutions have been demonstrated to increase peritoneal adhesion formation in rat models, and thus, are not recommended as a single agent for adhesion prevention[79].
Vitamin E is the most studied vitamin in adhesion prevention. In vitro studies have demonstrated that vitamin E has antioxidant, anti-inflammatory, anticoagulant and antifibroblastic effects, and decreases collagen production. It has been found to be effective for reducing adhesion formation by some authors[85]. Corrales et al[86] have shown that vitamin E, administered intraperitoneally, is as effective as carboxymethylcellulose membrane in preventing postoperative adhesions. By contrast, the same effect has not been achieved after intramuscular administration[87]. A significant difference has been found between intraperitoneal and intramuscular vitamin E administration[87]. Thus, intraperitoneal administration of vitamin E might be recommended to prevent adhesion formation. However, according to our literature review, there have been no human studies that have recommended the use of vitamin E for postoperative adhesion prevention.
One study has been carried out to elucidate the effects of different concentrations of methylene blue on the process of peritoneal adhesion formation and to define its minimum dose that can effectively prevent the formation of such adhesions in a rat model[88]. It could be concluded that 1% methylene blue had the best anti-adhesion potential[88]. If methylene blue prevents peritoneal adhesions, it can cause significant impairment of anastomotic bursting pressure during the early phase of the wound healing process by its transient inhibitory effect on the nitric oxide pathway[89].
Adhesions are a result of the inflammatory response to tissue injury in the peritoneal space. Although the mechanism is unclear, local anesthetics are reported to have some anti-inflammatory effects, as shown in some animal studies[90]. These anti-inflammatory effects are related to the inhibition of neutrophils. It has also been shown that local anesthetics activate the fibrinolytic system, reduce factor VIII, plasminogen and α2-antiplasmin concentration, and inhibit platelet aggregation[91,92]. Thus, besides the accelerative effect of a mixture of 2.5% lidocaine and 2.5% prilocaine in the wound healing process, some studies have demonstrated that intraperitoneal lidocaine and prilocaine inhibit the formation of postoperative peritoneal adhesions without compromising wound healing in a bacterial peritonitis rat model[93].
Hepatocyte growth factor (HGF) can inhibit collagen deposition and has fibrinolytic capacity[94,95]. Liu et al[96] have demonstrated that local application of recombinant adenovirus carrying the HGF gene reduced adhesion formation in a rat model. Other studies have investigated the use of gene therapy to manage postoperative adhesions. Smad7, a protein that occupies a strategic position in fibrinogenesis, inhibits transforming growth factor-β and has the potential to attenuate postoperative adhesion. Guo et al[97] have investigated in an experimental model the therapeutic potential of exogenous Smad7 to prevent fibrinogenesis in postoperative intra-abdominal adhesion. In this rat model, ultrasound-microbubble-mediated Smad7 transfection significantly decreased the incidence and severity of peritoneal adhesions, but the use of targeted gene therapy as a preventive agent against ASBO still needs extensive evaluation before any clinical trial.