Published online Oct 28, 2011. doi: 10.3748/wjg.v17.i40.4503
Revised: October 28, 2010
Accepted: November 5, 2010
Published online: October 28, 2011
AIM: To investigate whether systemic lupus erythematosus (SLE) is associated with benign focal liver lesions and vascular liver diseases, since these have been occasionally reported in SLE patients.
METHODS: Thirty-five consecutive adult patients with SLE and 35 age- and sex-matched healthy controls were evaluated. Hepatic and portal vein patency and presence of focal liver lesions were studied by colour-Doppler ultrasound, computerized tomography and magnetic resonance were used to refine the diagnosis, clinical data of SLE patients were reviewed.
RESULTS: Benign hepatic lesions were common in SLE patients (54% vs 14% controls, P < 0.0001), with hemangioma being the most commonly observed lesion in the two groups. SLE was associated with the presence of single hemangioma [odds ratios (OR) 5.05; 95% confidence interval (CI) 1.91-13.38] and multiple hemangiomas (OR 4.13; 95% CI 1.03-16.55). Multiple hemangiomas were associated with a longer duration of SLE (9.9 ± 6.5 vs 5.5 ± 6.4 years; P = 0.04). Imaging prior to SLE onset was available in 9 patients with SLE and hemangioma, showing absence of lesions in 7/9. The clinical data of our patients suggest that SLE possibly plays a role in the development of hemangioma. In addition, a Budd-Chiari syndrome associated with nodular regenerative hyperplasia (NRH), and a NRH associated with hepatic hemangioma were observed, both in patients hospitalized for abdominal symptoms, suggesting that vascular liver diseases should be specifically investigated in this population.
CONCLUSION: SLE is associated with 5-fold increased odds of liver hemangiomas, suggesting that these might be considered among the hepatic manifestations of SLE.
- Citation: Berzigotti A, Frigato M, Manfredini E, Pierpaoli L, Mulè R, Tiani C, Zappoli P, Magalotti D, Malavolta N, Zoli M. Liver hemangioma and vascular liver diseases in patients with systemic lupus erythematosus. World J Gastroenterol 2011; 17(40): 4503-4508
- URL: https://www.wjgnet.com/1007-9327/full/v17/i40/4503.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i40.4503
The spectrum of liver disease in systemic lupus erythematosus (SLE) patients is wide, ranging from benign conditions (such as benign liver lesions and mild chronic hepatitis), to aggressive and potentially lethal disorders (such as vascular liver diseases, including portal and hepatic veins thrombosis, nodular regenerative hyperplasia and idiopathic portal hypertension)[1,2]. Benign focal liver lesions, such as cavernous hemangioma, have been reported in SLE[3,4], and vascular liver diseases in SLE have been frequently observed in autoptic series[5], suggesting an association between these disorders, but data in living SLE patients are scarce and rely on isolated case reports.
Color-Doppler ultrasonography (CDUS) is the initial imaging technique used to screen patients with suspected liver disease, since it allows a non-invasive, real-time evaluation of abdominal organs and vessels, which is repeatable, easy to perform and inexpensive compared with other techniques. Ultrasound allows an accurate identification and characterization of focal hepatic lesions[6], and of portal vein and hepatic veins patency; in cases of portal or hepatic vein thrombosis, CDUS accuracy is similar to that of computerized tomography (CT)[7-13].
This case-control study was aimed at investigating, via CDUS, whether SLE is associated with focal lesions and vascular liver diseases.
We prospectively included in the present study all consecutive patients with an established diagnosis of SLE, namely at least 4 criteria among those published in the guidelines by the American College of Rheumatology[14]. Exclusion criteria were the presence of previously recognized liver disease from alcohol, hepatitis B virus (HBV) or hepatitis C virus (HCV) virus or autoimmune causes, and personal history of malignancy.
Thirty subjects with SLE, normal aspartate transaminase (AST)/alanine transaminase (ALT) and no recognized liver disease observed at our outpatient Unit, and 5 patients with SLE requiring hospitalization for any cause at our Unit over 24 mo were consecutively enrolled.
Thirty-five age and sex-matched healthy controls without SLE were included. Controls were consecutively recruited among subjects referred for an abdominal ultrasound examination to the Ultrasound Laboratory of our Unit for the following reasons: routine screening (n = 12), follow-up of renal or hepatic cyst (n = 11), evaluation of gallbladder lithiasis (n = 6), and abdominal discomfort (n = 6). Healthy state was ensured by specific questions on liver, heart, lung or renal diseases, history of malignancy, and chronic medication intake. Subjects with history of any of these conditions were excluded.
This study was approved by the Senior Staff Committee of University Hospital, a board which regulates non-interventional studies and is comparable to an Institutional Review Board. The nature of the study was explained to the patients and controls, and a written informed consent was obtained in each case, according to the principles of the Declaration of Helsinki (revision of Edinburgh 2000).
After an overnight fast, patients and controls were entered in the ultrasound examination room and invited to remain in the supine position for 10 min. Thereafter, an abdominal color-Doppler ultrasonography (CDUS) examination was performed by an experienced operator by using last-generation duplex equipment (Esaote Ansaldo AU Technos, Genoa, Italy) with a 4.5-7 MHz convex probe provided by a color, power and pulsed Doppler device.
Liver parenchyma and portal and hepatic veins patency were systematically evaluated. Location, number and size of the focal liver lesions were recorded. If present, they were diagnosed as[6]: (1) typical liver hemangioma: round-shaped, hyperechoic lesion with sharp margins, up to 4 cm in size. No Doppler signal inside the lesion; (2) atypical liver hemangioma: size > 4 cm; hypoechoic lesion or heterogeneous echopattern. No Doppler signal inside the lesion; and (3) focal nodular hyperplasia (FNH): hypo- iso- or slightly hyperechoic lesion < 3 cm with sharp margins and typical color-Doppler findings: central feeding artery and spoke wheel centrifugal vascular pattern.
When ultrasound (US) suggested atypical hemangioma, FNH or lesions of uncertain nature, a definite diagnosis was achieved by magnetic resonance imaging or by multislice CT scan.
Routine clinical and laboratory data were collected, and data on the duration of the disease and its treatment were recorded. Systemic lupus erythematosus disease activity index (SLEDAI)[15] and Systemic lupus international collaborating clinics/American college of rheumatology damage index for systemic lupus erythematosus (SLICC/ACR)[16] were calculated.
Statistical analysis was performed by SPSS 12.0 statistical package (SPSS Inc., Chicago, IL, United States). All results are expressed as mean ± SD. Comparisons between cases and controls were done by Student’s t test for unpaired data for continuous normally distributed variables, and by χ2 test for frequencies; Mann-Whitney test was used for non-normally distributed continuous variables. The strength of the association between SLE and the conditions in study were estimated by odds ratios (OR) and their 95% confidence interval (CI). A P value of < 0.05 was considered statistically significant.
Table 1 summarizes the main characteristics of the studied patients with SLE.
| Overall(n = 35) | Outpatients(n = 30) | Hospitalized(n = 5) | |
| Age (yr) | 50 ± 20 | 50 ± 17 | 51 ± 29 |
| Gender (M/F) | 2/33 | 1/29 | 1/4 |
| SLE duration (yr) | 6.9 ± 7.0 | 6.5 ± 6.6 | 9.6 ± 9.8 |
| LAC positivity | 8/35 | 7/30 | 1/5 |
| SLEDAI1 | 8.9 ± 4.2 | 9.1 ± 4.3 | 7.6 ± 2.8 |
| ACR/SLICC1 | 2.1 ± 1.6 | 2.1 ± 1.7 | 2.2 ± 1.3 |
| Acrocyanosis1 | 24/33 | 18/28 | 4/5 |
| Steroid treatment1 | 26/33 | 22/28 | 4/5 |
| Duration of steroid therapy (yr)1 | 6.4 ± 6.3 | 5.9 ± 5.7 | 9.5 ± 9.4 |
| AST (U/L) | 22 ± 9 | 20 ± 5 | 34 ± 18 |
| ALT (U/L) | 22 ± 9 | 18 ± 8 | 24 ± 13 |
| Bilirubin (mg/dL) | 0.6 ± 0.4 | 0.5 ± 0.2 | 1.1 ± 0.8 |
| GGT (U/L) | 32 ± 40 | 22 ± 20 | 67 ± 72 |
| ALP (U/L) | 190 ± 105 | 171 ± 78 | 247 ± 159 |
| Thrombosis of hepatic veins | 1 | 0 | 1 |
| Normal liver echopattern | 9 | 8 | 1 |
| NRH | 2 | 0 | 2 |
| Single hepatic hemangioma | 12 | 9 | 3 |
| Multiple hepatic hemangioma | 7 | 7 | 0 |
| Atypical hemangioma | 5 | 2 | 3 |
| FNH | 2 | 2 | 0 |
As shown, 19 patients (54.2%) showed one or more benign focal liver lesion. Hemangioma was the most frequent diagnosis, being observed in 19 cases (54.2%). FNH was observed in 2 cases (5.7%), and in both cases was associated with hemangioma.
Hemangiomas were observed both in outpatients and in hospitalized patients. Among hospitalized patients, a single hemangioma was seen in association with nodular regenerative hyperplasia (NRH); in two additional patients with no abdominal symptoms, admitted for fever in one case and for polyarthralgia in one case, a single hepatic hemangioma of large size in the right lobe (4.5 cm and 6 cm) was diagnosed.
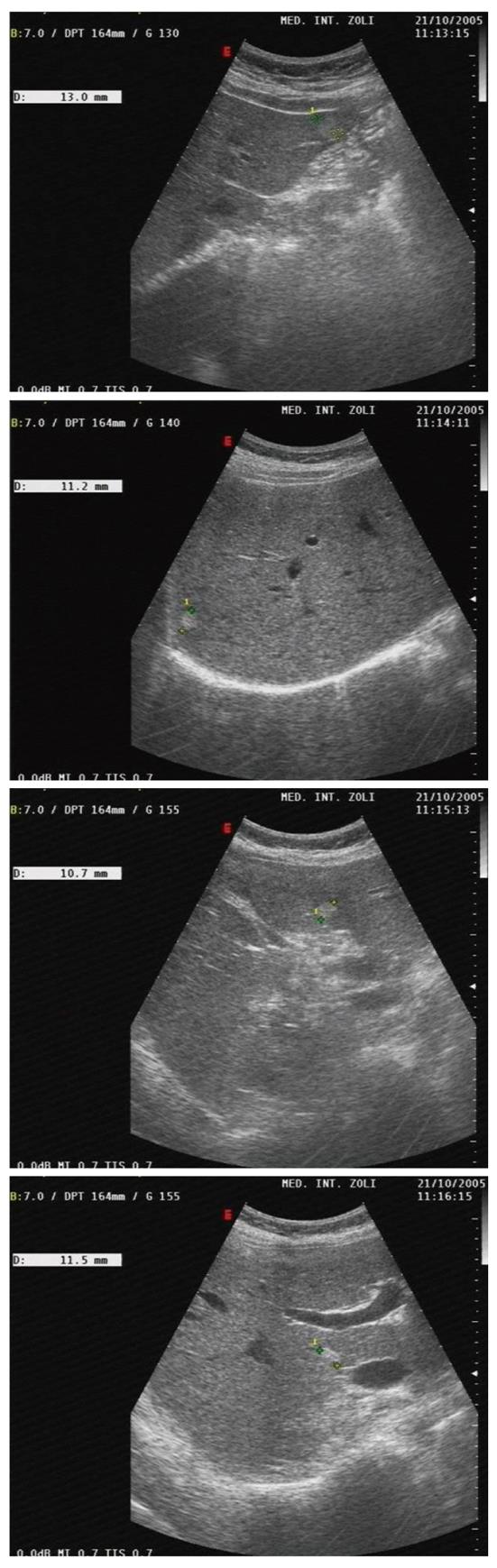
Among outpatients, 16 (53.3%) had focal liver lesions; 9 had a single hepatic hemangioma (in two cases with atypical hypoechogenic US aspect requiring CT scan; size 10-22 mm), and 7 (20% of patients of the whole series and 23.3% of outpatients) had multiple typical hepatic hemangiomas (number 2-9; size 10-22 mm) (Figure 1).
Hemangiomas did not demonstrate a preferential location inside the liver, being equally distributed in all segments.
Two patients with multiple hepatic hemangiomas had a FNH associated (confirmed by magnatic resonance imaging). Furthermore, one patient showed previously undiagnosed Caroli disease of left liver lobe associated with a single atypical hemangioma of the right lobe. These findings were confirmed by abdominal MR.
Five control subjects (14%) were diagnosed of hemangioma. Two of them had multiple lesions (2 lesions in both cases).
SLE was associated with the presence of single hemangioma (OR 5.05; 95% CI 1.91-13.38) and multiple hemangiomas (OR 4.13; 95% CI 1.03-16.55).
In order to ascertain whether hemangiomas formation was associated with SLE onset, we evaluated the clinical files of patients with liver hemangioma to identify imaging prior to SLE development. These data were found in 9/19 cases and consisted of US examination in 7 and CT scan in 2. In 7/9 cases imaging studies documented a normal liver, while in two cases a hemangioma was already present. In the two cases of pre-existent liver hemangioma, the lesion’s size at the time of the present study was stable in one, and increased from 15 to 21 mm in one.
We did not find any significant difference between patients with and without hemangiomas for laboratory parameters, age and prevalence of the main clinical manifestation of SLE (glomerulonephritis, neurological symptoms, pulmonary hypertension, and polyserositis) (Table 2).
| Patients with HH(n = 19) | SingleHH(n = 12) | Multiple HH(n = 7) | Patients without HH (n = 16) | |
| Age (yr) | 51 ± 16 | 52 ± 17 | 52 ± 17 | 49 ± 18 |
| Gender (M/F) | 2/17 | 2/10 | 0/7 | 0/16 |
| SLE duration (yr) | 7.1 ± 6.4 | 5.5 ± 6.4 | 9.9 ± 6.5a | 6.8 ± 6.1 |
| Clinical flares (n) | 3.5 ± 1.8 | 2.9 ± 1.4 | 4.2 ± 2.1 | 2.7 ± 1.4 |
| SLEDAI1 | 8.3 ± 4.3 | 8.9 ± 5.1 | 7.4 ± 2.6 | 10.0 ± 4.3 |
| ACR/SLICC1 | 2.3 ± 1.9 | 2.0 ± 1.7 | 3.0 ± 2.0 | 1.9 ± 1.4 |
| Acrocyanosis1 (%) | 94.4 | 100.0 | 85.7 | 43.8b |
| Steroid treatment (%)1 | 75.0 | 60.0 | 100.0 | 87.5 |
| Yr of steroid Rx (yr)1 | 6.8 ± 6.2 | 8.2 ± 7.1 | 5.5 ± 5.6 | 6.6 ± 6.1 |
| LAC positivity (%) | 22.2 | 18.2 | 28.6 | 25.0 |
| AST (U/L) | 23 ± 11 | 25 ± 14 | 21 ± 4 | 21 ± 7 |
| ALT (U/L) | 18 ± 6 | 17 ± 6 | 20 ± 8 | 19 ± 11 |
| Bilirubin (mg/dL) | 0.7 ± 0.5 | 0.7 ± 0.7 | 0.7 ± 0.3 | 0.5 ± 0.2 |
| GGT (U/L) | 36 ± 54 | 42 ± 65 | 23 ± 9 | 29 ± 28 |
| ALP (U/L) | 213 ± 133 | 243 ± 134 | 169 ± 138 | 168 ± 65 |
We observed a significantly higher prevalence of acrocyanosis in patients with hemangioma compared with patients without focal liver lesions: 94% vs 47% (P = 0.02).
We evaluated whether the presence of liver hemangioma was associated with duration of SLE disease, activity of the disease at onset (SLEDAI), damage index at the time of US examination (SLICC) and number of clinical flares of SLE.
Multiple hepatic hemangiomas were associated with a longer duration of SLE disease (9.9 ± 6.5 vs 5.5 ± 6.4, P = 0.049). Patients with hepatic hemangiomas also showed a higher number of clinical flares, but the difference did not reach statistical significance.
As for treatment, the rate and duration of corticosteroid therapy for SLE were similar in patients with and without hemangioma.
We observed 2 cases of vascular liver diseases (5.7%). Both were identified in hospitalized patients with abdominal symptoms. The first patient (female, 39 year) was admitted for ascites and showed chronic Budd-Chiari syndrome with clinical and US signs of portal hypertension (namely small esophageal varices at endoscopy and enlargement of portal vein and spleen, and porto-collateral circulation at US), and NRH. A percutaneous biopsy was obtained and confirmed the radiological diagnosis. The second patient (female, 47 year) was admitted for a biliary colic, and CDUS identified a diffuse and severe alteration of liver echopattern associated with a 4 cm
hyperechoic nodule. On CT scan she was diagnosed of probable NRH associated with a 4 cm hemangioma; portal and hepatic veins were patent. The patient refused liver biopsy.
The main result of the present study is the demonstration of an association between SLE and liver hemangioma. The prevalence of liver hemangioma was 54.2% in our SLE patients; this figure is more than twice the maximum expected in the general adult population (0.4%-20%)[17], and was significantly higher than the 14% observed in healthy control subjects. Accordingly, in this study SLE was associated with increased odds (5 fold increase) of hemangioma occurrence.
This data suggests that SLE may be directly implicated in the formation of benign vascular neoplasia. Some additional findings from our study support this hypothesis: hemangioma seemed to appear after SLE onset in 7 patients; it increased in size in one; hemangiomas were multiple in 40% of patients, and patients with multiple hemangiomas had a long-lasting disease.
Hepatic hemangioma is a benign vascular neoplasia of endothelial origin. Even if the pathogenetic mechanisms leading to its formation have not been fully elucidated yet, it has been proposed that hemangiomas are caused by unregulated angiogenesis due to an imbalance between angiogenic and angiostatic factors[18]. This hypothesis is supported by the observation that hemangiomas may increase in dimension over time during pregnancy and estrogen therapy[19], as a consequence of estrogens-enhanced neoangiogenesis[20].
It has been shown that SLE patients have increased circulating levels of estrogens[21] and other angiogenic factors such as vascular endothelial growth factor (VEGF) and IL-18, which are associated with activity of disease[22,23]. It could be speculated that an activation of angiogenesis might lead to liver hemangiomas formation in the course of the disease. Regrettably we lack evidence to support this hypothesis, since VEGF and other pro-angiogenic cytokines were not dosed in our patients.
In two patients of our series, hemangiomas were associated with FNH. FNH is a benign hepatocellular lesion which has been reported in 0.6%-3.0% of the general population[17]. It has been showed that FNH represents an abnormal adaptive responsive of liver parenchyma to local hemodynamic disturbances[24]. The association of hemangioma and FNH is frequent[25,26], and several authors have speculated that both lesions may have causative factors in common, including focal disturbance of the hepatic blood supply that somehow facilitates the hyperplastic development of these benign lesions[27].
In the present series of SLE patients, we found 2 cases of vascular diseases of the liver, which were diagnosed in patients hospitalized for abdominal symptoms (ascites and increase of hepatic enzymes, and suspect of biliary colic). Vascular diseases of the liver, such as hepatic and portal vein thrombosis, are life-threatening events occurring more often in patients with congenital or acquired prothrombotic condition[28]. SLE is a well recognized prothrombotic condition and vascular liver diseases have been reported in this setting[1,2]; our experience is in line with these previous reports, and suggests that US examination should be performed in cases of abdominal symptoms in SLE patients to specifically rule out vascular liver diseases.
The present study suffers from some limitations. We lack histological confirmation for the imaging findings since most lesions were found in asymptomatic patients, in whom biopsy was not performed. Still, in patients without chronic hepatic diseases, imaging techniques are considered sufficient to diagnose benign liver lesions[29].
The clinical observations from our series suggest an association between SLE and liver hemangioma formation. Future studies are needed to assess the mechanisms leading to this association.
In conclusion, this study shows that the liver is a frequent site of abnormal findings in SLE patients. SLE is associated with an increased likelihood of liver hemangiomas and multiple hepatic hemangiomas, which can be associated with FNH and vascular liver diseases. Vascular disorders can be found in patients with SLE, and should be actively looked for in SLE patients with abdominal symptoms.
Benign focal liver lesions, such as cavernous hemangioma, and vascular liver diseases have been reported in systemic lupus erythematosus (SLE), suggesting an association between these disorders.
Only a few case reports and autoptic series have been published hitherto regarding the prevalence of benign liver lesions and vascular liver diseases in patients with SLE.
In this study, the authors found that the prevalence of hepatic hemangioma is very high in patients with SLE. Moreover, liver hemangiomas in this population were often multiple, and associated in some instance with focal nodular hyperplasia and vascular liver diseases.
The clinical observations from this study suggest an association between SLE and liver hemangioma formation. Vascular hepatic disorders can be found in patients with SLE, and should be actively looked for in SLE patients with abdominal symptoms.
Although this study on liver findings in SLE patients does not lead to direct therapeutic consequences, it evaluates an interesting aspect of this disease Histological proof would of course be desirable, but in general, biopsy cannot be justified in this context.
Peer reviewer: Dr. Herwig R Cerwenka, Professor, Department of Surgery, Medical University of Graz, Auenbruggerplatz 29, A-8036 Graz, Austria
S- Editor Tian L L- Editor Rutherford A E- Editor Xiong L
| 1. | van Hoek B. The spectrum of liver disease in systemic lupus erythematosus. Neth J Med. 1996;48:244-253. [PubMed] |
| 2. | Youssef WI, Tavill AS. Connective tissue diseases and the liver. J Clin Gastroenterol. 2002;35:345-349. [PubMed] |
| 3. | Maeshima E, Minami Y, Sato M, Matsuda K, Uchiyama K, Goda M, Ueda H, Kida Y, Mune M. A case of systemic lupus erythematosus with giant hepatic cavernous hemangioma. Lupus. 2004;13:546-548. [PubMed] |
| 4. | Suzuki T, Tsuchiya N, Ito K. Multiple cavernous hemangiomas of the liver in patients with systemic lupus erythematosus. J Rheumatol. 1997;24:810-811. [PubMed] |
| 5. | Matsumoto T, Yoshimine T, Shimouchi K, Shiotu H, Kuwabara N, Fukuda Y, Hoshi T. The liver in systemic lupus erythematosus: pathologic analysis of 52 cases and review of Japanese Autopsy Registry Data. Hum Pathol. 1992;23:1151-1158. [PubMed] |
| 6. | Harvey CJ, Albrecht T. Ultrasound of focal liver lesions. Eur Radiol. 2001;11:1578-1593. [PubMed] |
| 7. | Bargalló X, Gilabert R, Nicolau C, García-Pagán JC, Ayuso JR, Brú C. Sonography of Budd-Chiari syndrome. AJR Am J Roentgenol. 2006;187:W33-W41. [PubMed] |
| 8. | Finn JP, Kane RA, Edelman RR, Jenkins RL, Lewis WD, Muller M, Longmaid HE. Imaging of the portal venous system in patients with cirrhosis: MR angiography vs duplex Doppler sonography. AJR Am J Roentgenol. 1993;161:989-994. [PubMed] |
| 9. | Janssen HL, Garcia-Pagan JC, Elias E, Mentha G, Hadengue A, Valla DC. Budd-Chiari syndrome: a review by an expert panel. J Hepatol. 2003;38:364-371. [PubMed] |
| 10. | Ralls PW, Johnson MB, Radin DR, Boswell WD, Lee KP, Halls JM. Budd-Chiari syndrome: detection with color Doppler sonography. AJR Am J Roentgenol. 1992;159:113-116. [PubMed] |
| 11. | Tanaka K, Numata K, Okazaki H, Nakamura S, Inoue S, Takamura Y. Diagnosis of portal vein thrombosis in patients with hepatocellular carcinoma: efficacy of color Doppler sonography compared with angiography. AJR Am J Roentgenol. 1993;160:1279-1283. [PubMed] |
| 12. | Valla DC. The diagnosis and management of the Budd-Chiari syndrome: consensus and controversies. Hepatology. 2003;38:793-803. [PubMed] |
| 13. | Van Gansbeke D, Avni EF, Delcour C, Engelholm L, Struyven J. Sonographic features of portal vein thrombosis. AJR Am J Roentgenol. 1985;144:749-752. [PubMed] |
| 14. | Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40:1725. [PubMed] |
| 15. | Gladman DD, Ibañez D, Urowitz MB. Systemic lupus erythematosus disease activity index 2000. J Rheumatol. 2002;29:288-291. [PubMed] |
| 16. | Gladman D, Ginzler E, Goldsmith C, Fortin P, Liang M, Urowitz M, Bacon P, Bombardieri S, Hanly J, Hay E. The development and initial validation of the Systemic Lupus International Collaborating Clinics/American College of Rheumatology damage index for systemic lupus erythematosus. Arthritis Rheum. 1996;39:363-369. [PubMed] |
| 17. | Karhunen PJ. Benign hepatic tumours and tumour like conditions in men. J Clin Pathol. 1986;39:183-188. [PubMed] |
| 18. | González Folch M. [Erythema nodosum (author's transl)]. Rev Med Chil. 1978;106:915-922. [PubMed] |
| 19. | Glinkova V, Shevah O, Boaz M, Levine A, Shirin H. Hepatic haemangiomas: possible association with female sex hormones. Gut. 2004;53:1352-1355. [PubMed] |
| 20. | Schnaper HW, McGowan KA, Kim-Schulze S, Cid MC. Oestrogen and endothelial cell angiogenic activity. Clin Exp Pharmacol Physiol. 1996;23:247-250. [PubMed] |
| 21. | Folomeev M, Dougados M, Beaune J, Kouyoumdjian JC, Nahoul K, Amor B, Alekberova Z. Plasma sex hormones and aromatase activity in tissues of patients with systemic lupus erythematosus. Lupus. 1992;1:191-195. [PubMed] |
| 22. | Robak E, Woźniacka A, Sysa-Jedrzejowska A, Stepień H, Robak T. Serum levels of angiogenic cytokines in systemic lupus erythematosus and their correlation with disease activity. Eur Cytokine Netw. 2001;12:445-452. [PubMed] |
| 23. | Robak E, Woźniacka A, Sysa-Jedrzejowska A, Stepień H, Robak T. Circulating angiogenesis inhibitor endostatin and positive endothelial growth regulators in patients with systemic lupus erythematosus. Lupus. 2002;11:348-355. [PubMed] |
| 24. | Wanless IR, Mawdsley C, Adams R. On the pathogenesis of focal nodular hyperplasia of the liver. Hepatology. 1985;5:1194-1200. [PubMed] |
| 25. | Mathieu D, Zafrani ES, Anglade MC, Dhumeaux D. Association of focal nodular hyperplasia and hepatic hemangioma. Gastroenterology. 1989;97:154-157. [PubMed] |
| 26. | Vilgrain V, Uzan F, Brancatelli G, Federle MP, Zappa M, Menu Y. Prevalence of hepatic hemangioma in patients with focal nodular hyperplasia: MR imaging analysis. Radiology. 2003;229:75-79. [PubMed] |
| 27. | Bralet MP, Terris B, Vilgrain V, Brégeaud L, Molas G, Corbic M, Belghiti J, Fléjou JF, Degott C. Epithelioid hemangioendothelioma, multiple focal nodular hyperplasias, and cavernous hemangiomas of the liver. Arch Pathol Lab Med. 1999;123:846-849. [PubMed] |
| 28. | Denninger MH, Chaït Y, Casadevall N, Hillaire S, Guillin MC, Bezeaud A, Erlinger S, Briere J, Valla D. Cause of portal or hepatic venous thrombosis in adults: the role of multiple concurrent factors. Hepatology. 2000;31:587-591. [PubMed] |
| 29. | Caseiro-Alves F, Brito J, Araujo AE, Belo-Soares P, Rodrigues H, Cipriano A, Sousa D, Mathieu D. Liver haemangioma: common and uncommon findings and how to improve the differential diagnosis. Eur Radiol. 2007;17:1544-1554. [PubMed] |