Published online Oct 14, 2011. doi: 10.3748/wjg.v17.i38.4314
Revised: May 5, 2011
Accepted: May 12, 2011
Published online: October 14, 2011
AIM: To investigate the clinicopathological characteristics of late-stage lung cancer patients with gastrointestinal (GI)-tract metastases, focusing on therapeutic options and outcomes.
METHODS: Our institution (the National Taiwan University Hospital) diagnosed 8159 patients with lung cancer between 1987 and 2008, of which 21 developed symptomatic GI metastases. This study reviewed all of the patients’ information, including survival data, pathological reports, and surgical notes.
RESULTS: The most common histological type of lung cancer was adenocarcinoma, and 0.26% of patients with lung cancer developed GI metastases. The median duration from lung cancer diagnosis to GI metastases was three months (range, 0-108 mo), and the average time from diagnosis of GI metastasis to death was 2.8 mo. Most patients with symptomatic gastric and/or duodenal metastases exhibited GI bleeding and were diagnosed by panendoscopy. In contrast, small bowel metastases typically presented as an acute abdomen and were not diagnosed until laparotomy. All patients with small bowel or colonic metastases underwent surgical intervention, and their perioperative mortality was 22%. Our data revealed a therapeutic effect in patients with solitary GI metastasis and a favorable palliative effect on survival when metastases were diagnosed preoperatively. In patients with multiple GI metastases, the presentation varied according to the locations of the metastases.
CONCLUSION: Surgical treatment is worthwhile in a select group of patients with bowel perforation or obstruction. Physicians should be more alert to symptoms or signs indicating GI metastases.
- Citation: Lee PC, Lo C, Lin MT, Liang JT, Lin BR. Role of surgical intervention in managing gastrointestinal metastases from lung cancer. World J Gastroenterol 2011; 17(38): 4314-4320
- URL: https://www.wjgnet.com/1007-9327/full/v17/i38/4314.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i38.4314
Lung cancer is a major cause of cancer-related death worldwide. In Taiwan, it is the second leading cause of cancer death, with nearly 8700 new cases and 7500 deaths per year[1]. Approximately one-half of lung cancer patients develop metastases[2,3], the most common sites of which are the lymph nodes, liver, adrenal glands, bone, and brain[2]. Symptomatic gastrointestinal (GI) tract metastases are not uncommon[4,5], although GI metastases from lung cancer are rare. The reported incidence of GI metastasis from lung cancer varies from 0.5%-10%, and mainly depends on the evaluation method used (endoscopy, surgical specimens, or autopsy)[2,5-7]. The typical presentations of GI metastases are abdominal pain, bleeding, obstruction, and perforation, of which perforation is the most serious complication, necessitating surgical intervention to prevent a life-threatening event. GI tract involvement with lung cancer is generally considered to be associated with a late or advanced stage of the disease. Therefore, the question of how to manage patients with symptomatic GI metastases from lung cancer is important; however, management of the disease remains a controversial topic[2,3,6].
The present study describes the clinicopathological features of a large series of primary lung cancers manifesting GI metastases and discusses therapeutic options, with a particular focus on the role of surgeons in this uncommon oncological setting.
This research reviewed the surgical pathology reports of patients diagnosed with GI metastases from 1987 to 2008 at the National Taiwan University Hospital. Only those cases in which the primary source of the metastases was documented as lung cancer were included in our analysis. The diagnostic criteria were (1) radiological demonstration of a primary lung tumor and exclusion of tumors elsewhere, and (2) the morphology and immunohistochemical profiles were consistent with a primary pulmonary tumor. Of 8159 patients diagnosed with lung cancer, 21 were found to have GI metastases. Metastatic disease originating from a lung primary tumor was confirmed from biopsy specimens or from surgical resection of the GI tract in patients.
Patient information, including age, sex, pathology, initial lung cancer stage, interval between lung tumor diagnosis and discovery of GI involvement, clinical presentation, other metastatic site locations at the time of GI metastases, and patient survival, was recorded and reviewed retrospectively. Clinical and other follow-up data were obtained in all cases from patient records and referring physicians. Analysis of the survival data was performed by the Kaplan-Meier method. Differences between subgroups were compared for statistical significance using the log-rank test. P values were two-sided and the significance level was set at 0.05.
Table 1 summarizes the patient data, including clinical manifestations, diagnosis, treatment, and follow-up. The patients ranged in age from 40 to 81 years (median, 69 years), and of the five women and 16 men, 14 had a smoking history. Nine patients (one woman, eight men) developed symptomatic gastric and/or duodenal metastases. Six patients (all male) had symptomatic small bowel metastases. Three patients (two women, one man) had symptomatic colonic metastases. The other three patients (two women, one man) had multiple GI tract metastases.
| Patient number | Age (yr) /sex | Histology | Initial pTNM staging | Time period (mo) from diagnosis of lung tumor to major GI metastasis | Clinical presentation | Diagnostic procedure | Other extra-thoracic metastatic sites | Surgery | Survival |
| 1 | 58/F | Adenocarcinoma | IV | 1/stomach | Abdominal pain | PES | Brain, bone, liver, adrenal gland | Nil | 115 mo |
| 2 | 79/M | Pleomorphic carcinoma | IV | Simultaneous/stomach | Hemorrhage | PES | Bone, liver, gall bladder | Nil | 1 mo |
| 3 | 81/M | Adenocarcinoma | IV | 5/stomach | Hemorrhage | PES | Bone | Nil | 1 mo |
| 4 | 73/M | Adenocarcinoma | IIIb | 5/stomach | Hemorrhage | PES | Brain | Nil | 1 mo |
| 5 | 71/M | Squamous cell carcinoma | Ib | 9/stomach | Hemorrhage | PES, CT | Spleen, liver | Proximal gastrectomy | 16 d |
| 6 | 59/M | Adenocarcinoma | IV | 17/stomach | Hemorrhage | PES | Bone, liver | Nil | 2 mo |
| 7 | 71/M | Squamous cell carcinoma | IIIa | 108/stomach and duodenum | Hemorrhage | Laparotomy | Liver | Wedge resection of stomach | 15 d |
| 8 | 70/M | Small cell carcinoma | IIIb | 7/stomach and duodenum | Abdominal pain hemorrhage | PES | Bone, pancreas | Nil | 3 mo |
| 9 | 66/M | Adenocarcinoma | IV | Simultaneous/duodenum | Hemorrhage | PES | Adrenal gland | Nil | 1 mo |
| 10 | 72/M | Undifferentiated | IV | Simultaneous/small bowel | Bowel obstruction | CT | Brain | Segmental resection | 1 mo |
| 11 | 63/M | Adenocarcinoma | IIIb | 3/small bowel | Perforation | Laparotomy | Peritoneal seeding | Primary repair | 3 d |
| 12 | 47/M | Adenocarcinoma | IV | 2/small bowel | Bowel obstruction | Laparotomy | Nil | Segmental resection | 2 mo |
| 13 | 66/M | Adenosquamous carcinoma | IV | 3/small bowel | Bowel obstruction | Echo | Brain | Segmental resection | 3 mo |
| 14 | 73/M | Adenocarcinoma | IV | 14/small bowel | Perforation | Laparotomy | Liver | Segmental resection | 1 d |
| 15 | 71/M | Squamous cell carcinoma | IIIb | 2/small bowel | Perforation | Laparotomy | Peritoneal seeding | Segmental resection | 3 mo |
| 16 | 61/F | Adenocarcinoma | IIIb | 52/colon | Abdominal pain | CT | Nil | Right hemicolectomy | 8 mo |
| 17 | 66/M | Squamous cell carcinoma | IV | 4/colon | Hemorrhage | Colonoscopy | Liver, brain | Right hemicolectomy | 5 mo |
| 18 | 54/F | Adenocarcinoma | IIIb | 32/colon | Ileus | CT, Colonoscopy | Bone, brain | Right hemicolectomy | 4 mo |
| 19 | 40/F | Adenocarcinoma | IV | 1/multiple GI involvement | Ileus, Hemorrhage | PES, CT | Ovary, adrenal gland, pancreas | Nil | 3 mo |
| 20 | 69/M | Squamous cell carcinoma | IV | Simultaneous/multiple GI involvement | Hemorrhage | PES, CT | Bone | Nil | 2 mo |
| 21 | 80/F | Adenocarcinoma | IV | Simultaneous/multiple GI involvement | Tenesmus | CT | Ovary, uterus | Nil | 3 mo |
Studies have reported GI tract metastasis to occur in the later stages of lung cancer[1]. In our study, the median duration from lung cancer diagnosis to GI tract metastasis in the eight patients with initial stage I to III disease was 8 mo (ranging from 2 to 108 mo). In contrast, 5 of the 13 patients with stage IV lung cancer developed simultaneous GI metastases, and the medium duration of the other eight patients with GI metastases was 3.5 mo (ranging from 1 to 17 mo). The average time from GI metastasis diagnosis to death was 2.8 mo, similar to the 130.3 d reported by Yang et al[8].
Gastrointestinal hemorrhage was the most common symptom of GI metastasis (11 of 21, 52.4%). Panendoscopic biopsy easily diagnosed nine patients with gastric or duodenal metastases. Three of the six patients with small bowel metastases exhibited intestinal perforations and the others exhibited GI obstructions. Of these patients, 67% (4/6) were diagnosed by laparotomy, including three cases of intestinal perforation, which could reflect the fact that small bowel metastases are more difficult to diagnose before surgery, and the typical clinical manifestations are an acute abdomen or peritonitis. In contrast to patients with colonic metastases, the diagnosis of patients with GI metastasis could be made before surgery by computed tomography (CT) or colonoscopy. This result is possibly attributable to the slow progression of clinical manifestations and physician awareness. Clinical presentations of multiple GI involvements were variable, and depended on the major location of the metastatic GI tumors. For example, patient #19 had stage IV lung cancer and experienced mild abdominal pain for weeks. She did not pay attention to the pain, thinking it was an adverse effect of chemotherapy. Her symptoms progressed rapidly to poor appetite and little oral intake; therefore, she was admitted for further management. A CT scan revealed multiple intra-abdominal tumors involving the ovaries, pancreas, and stomach. After discussing the options with the patient and her family, they all agreed to hospice care, and the patient passed away three months later.
Half of the patients in this series (12 of 21) had primary lung adenocarcinoma and five had squamous cell carcinoma; the other four included one pleomorphic carcinoma, one small cell carcinoma, one undifferentiated carcinoma, and one adenosquamous carcinoma.
All but two of the patients (19/21, 90.5%) had metastases to other locations at the time of GI metastasis diagnosis. The most common extrathoracic metastatic sites were the liver, brain, bone, adrenal glands, spleen, and pancreas. The two cases (patients #12 and #16) with only one GI metastasis underwent surgical resection with therapeutic intent and one was still alive at the time of writing.
Upon diagnosis of stomach or duodenal involvement, conservative treatment was prescribed for symptoms, and imaging studies were performed to evaluate other possible metastatic sites. All nine patients in this subgroup had other simultaneous metastases that were considered to represent the late stage of lung cancer. If the symptoms (GI bleeding or abdominal pain) could be controlled by medication, supportive treatment was continued. Two patients underwent surgical intervention due to massive hemorrhage; patient #5 received a proximal gastrectomy for a 6.7-cm ulcerative mass over the body of the stomach with direct invasion of the spleen, and patient #7 received a wedge resection for an ulcerative lesion over the cardia of the stomach. Unfortunately, these two patients died within one month of surgery owing to postoperative complications. The other seven patients received only supportive treatment, and the median survival was one month (range, 1 to 15 mo). We observed that patients treated with conservative therapy exhibited a significant survival benefit in comparison with those who received surgical treatment (P = 0.005).
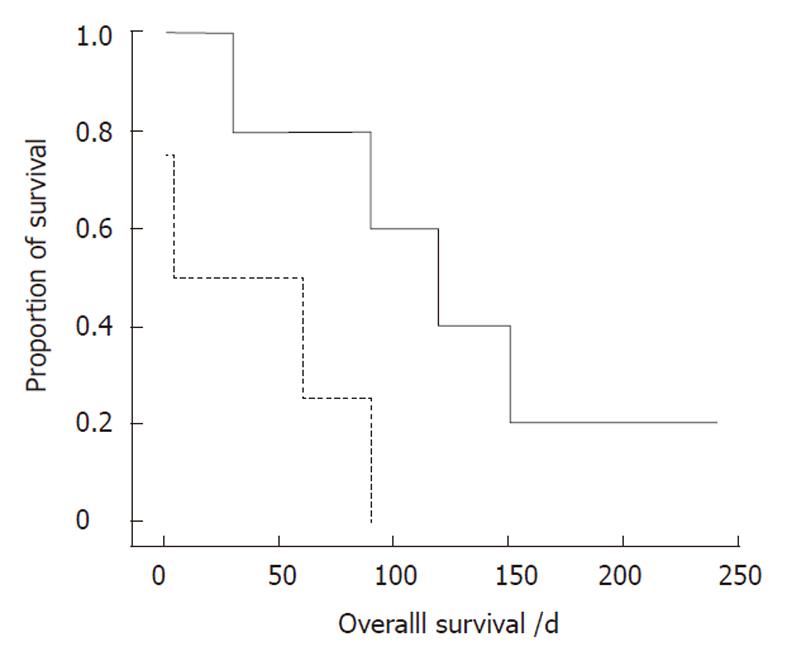
Nine patients with small bowel or colonic metastases underwent laparotomy. As mentioned above, four patients were not diagnosed until laparotomy. Patients who required immediate surgical intervention exhibited an acute abdomen, such as perforation or peritonitis. Survival comparisons of these patients, based on the timing of diagnosis, showed that the survival benefit was significantly increased in the subgroup with GI metastases diagnosed before surgery (P = 0.032, Figure 1). Accordingly, early GI metastasis detection and timely surgical intervention are important for lung cancer patients. Of the three patients with multiple GI tract metastases, all received palliative treatment and died of the disease.
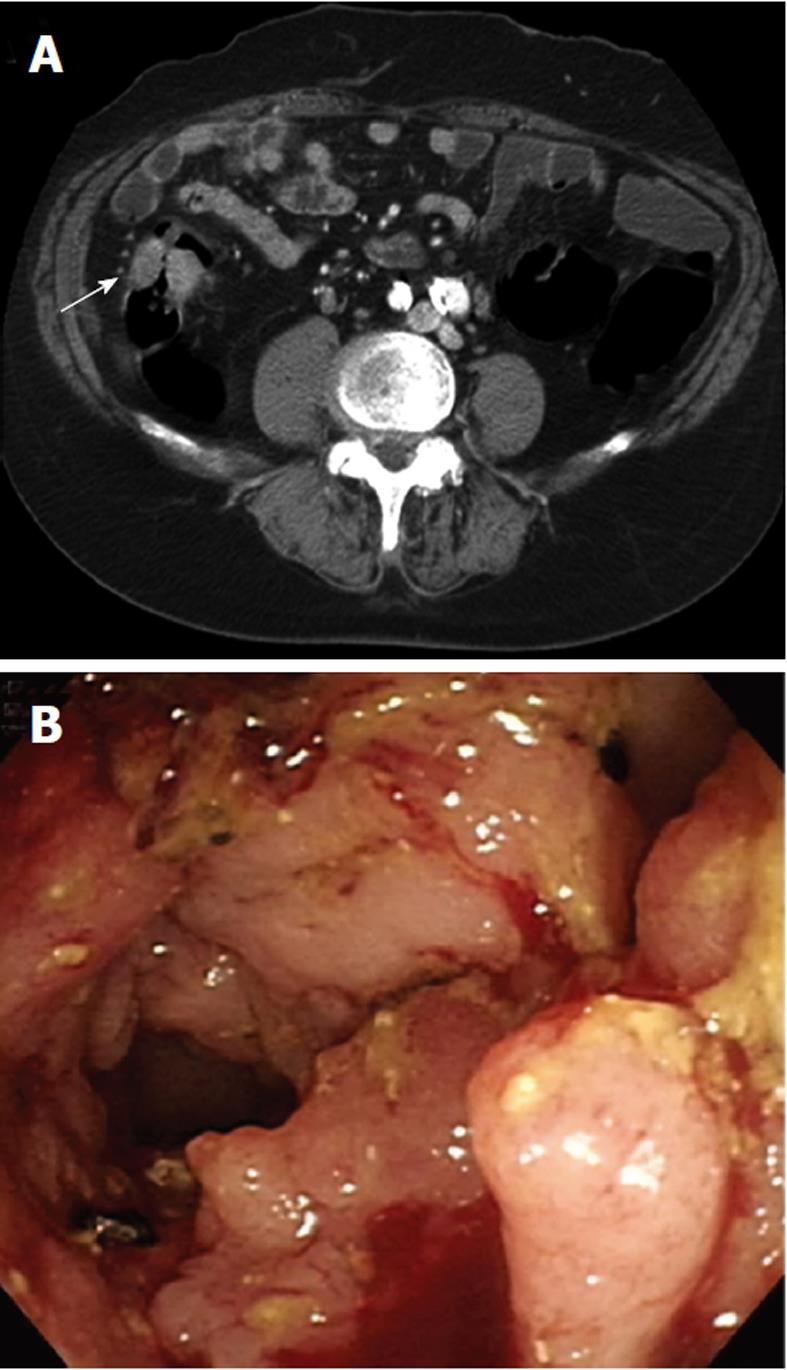
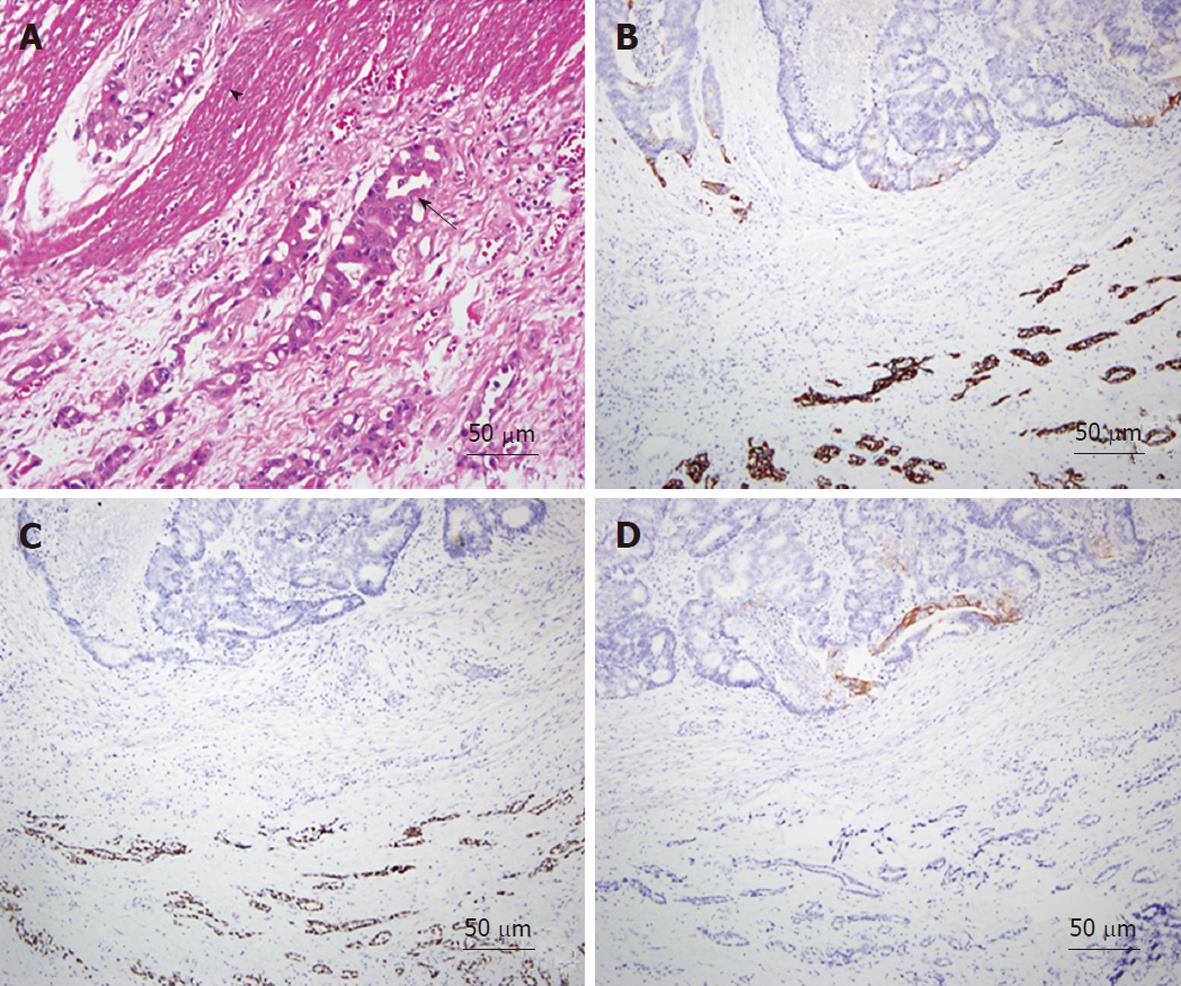
As an example, one patient (case #16 in Table 1) was diagnosed with lung adenocarcinoma in October of 2003. At that time, chest radiography showed massive left pleural effusion. A chest CT revealed a patchy consolidation with pleural thickening in the left upper lung lobe. Adenocarcinoma was confirmed by CT-guided biopsy and pleural effusion collected via aspiration for cytological analysis. After a complete staging work-up, stage IIIb lung cancer was confirmed and the patient underwent multiple courses of chemotherapy. However, the patient began experiencing abdominal pain, which was aggravated after meals, in March of 2008. A physical examination revealed diffuse abdominal tenderness without rebounding pain, and an abdominal and pelvic CT showed a single ill-defined mass with local infiltration into the ascending colon (Figure 2A). Colonoscopy revealed a circular tumor with luminal obstruction (Figure 2B), and a biopsy indicated adenoma with malignant change. Colon cancer was the tentative diagnosis; however, the actual origin of the tumor could not be confirmed. After surgical intervention, a 4-cm irregular-shaped mass invading the adjacent small bowel in the ascending colon was noted during the laparotomy, and a right hemicolectomy with a segmental resection of the small bowel was performed without complications. The pathological report revealed that the tumor involving the colon was mainly located in the pericolonic soft tissue and invaded inwards into the colonic muscular layer (Figure 3A). The morphology of the tumor was distinct, characterized by pleomorphic oval nuclei, scant cytoplasm, and an irregular glandular pattern. Immunohistochemical staining revealed the tumor cells to be positive for thyroid transcription factor-1 (TTF-1) and cytokeratin 7 (CK7), but negative for CK20 (Figure 3B-D). The diagnosis of a metastatic adenocarcinoma of pulmonary origin was confirmed.
Lung cancer is the leading cause of death from cancer worldwide, and about one-half of lung cancer patients have metastatic disease at the initial diagnosis[2,3]. The most common sites of metastatic disease are the lymph nodes (48%), liver (45%), adrenal glands (41%), bone (31%), and brain (25%)[2]. Before the 1980s, GI tract involvement was considered an unusual metastasis site from lung cancer[9,10], and the pathogenesis of bowel metastases was considered to be due to tumor-cell spreading via the hematogenous or lymphatic routes. A review of autopsy data by Antler et al[7] reported the incidence of GI metastasis to be 14%; however, 14% may be higher than the true incidence of GI metastasis, because a large number of esophageal metastases in their study were the result of direct invasion. The incidence of symptomatic small bowel metastasis has been reported to be 0.4%-0.5%[4,5]. In our series, there were 21 cases of symptomatic gastrointestinal metastases among the 8159 lung cancer patients (0.26%). The disparity between subclinical and clinical GI metastases is still high.
Gastric and/or duodenal metastases from lung cancer are very rare, and there are only a few cases of varying malignant cell types reported in the literature[11-13]. These cases exhibited symptoms of abdominal pain, chronic bleeding, anemia, or hematemesis, and the pathological results of panendoscopic biopsy provided accurate diagnoses. In contrast to the small bowel, the most common metastatic site of lung cancer in the GI tract[4,5,14,15], there have been few reports of lung cancer metastases to the colon, appendix, or anus[16-19]. Small intestine involvement often leads to an acute abdomen as a result of perforation or obstruction[4,5,15], whereas colon metastases usually result in vague symptoms. In our cases of small bowel or colon metastases, all patients required surgery to relieve the obstruction or control peritonitis. In general, the use of CT, abdominal sonography, or endoscopy plays an important role in identifying lesions.
Even with endoscopy, lung cancer involving the GI tract has no specific features, appearing as a diffuse involvement of the mucosa and multiple nodules with or without mucosa ulceration[20,21]. An experienced pathologist might be able to conclude a metastatic tumor based on morphological study of tumor tissue from surgical resection. However, in most cases histological examination with staining using cell type-specific markers is the only way to identify metastatic tumors of the GI tract. Several different CK and other protein markers are widely used to distinguish carcinomas of different origins. Rossi et al[21] concluded that lung carcinomas usually demonstrate a CK7+/CK20- immunoprofile, whereas intestinal carcinomas have a different CK7-/CK20+ pattern. Thus, CK7 is a good marker for distinguishing those cell types. In general, primary lung tumors can be identified by CK7; however, studies have demonstrated that primary adenocarcinomas of the rectum or small intestine may also express CK7 in a significant number of cases, and may even lose CK20 expression[22,23]. Therefore, to exclude a possibility like this, employing a more specific marker of lung tumor origin, such as TTF-1[24], together with CK7 and CK20 could more effectively differentiate metastatic GI tumors from lung cancer.
Every type of lung cancer can result in GI metastasis. McNeill et al[2] and Berger et al[5] reported that squamous cell carcinoma causes small bowel metastases more frequently than other lung tumor cell types. In the series of Antler et al[7], small cell carcinoma and large cell carcinoma were more likely to result in GI metastases. However, in our series, adenocarcinoma was the most frequent type resulting in GI metastases. In accordance with our results, Garwood et al[4] reported that adenocarcinoma (23.7%) and squamous cell carcinoma (22.7%) were the most common histological types causing small bowel perforations.
Our results showed that the median time from lung cancer diagnosis to GI metastasis was three months and the longest period of time was 108 mo (patient #7). Observations of previous data show that, in patients with lung metastases from colon cancer, a longer interval between colon cancer diagnosis and lung metastasis was associated with a significantly longer survival[25]. However, our findings did not show a similar survival difference when patients with an interval greater than 1 year were compared with those with an interval less than 1 year (P = 0.79). Our results also revealed no significant difference in overall survival in patients with initial stage I-III lung cancer upon GI metastasis diagnosis in comparison with those with stage IV disease. Taken together, these findings indicate that patients with GI metastases from lung cancer have a poor prognosis.
The perioperative mortality rate reported in the literature ranges from 60% to 100%[2,15]. The series of Berger et al[5] indicated no perioperative mortality in patients with small bowel metastases who underwent resection. However, in our experience, the perioperative mortality rate was 100% in the two patients with gastric and/or duodenal metastases and 22% (2/9) in patients with small bowel or colon metastases. We observed longer survival in patients with gastric and/or duodenal metastases that were managed by supportive treatment without surgery (P = 0.005). However, the relatively small sample size in this retrospective subgroup analysis and the nature of the different underlying conditions (these nine patients had other metastases at the same time) may limit the conclusion that surgical treatment is not suitable for patients with gastric or duodenal metastases from lung cancer. On the other hand, in two of our patients, radiological work-up did not show other metastases. These patients then underwent surgical resection and one is still alive. Although surgery for localized extrathoracic metastasis from lung cancer still has a palliative intent[21], some authors have reported prolonged survival in cases of surgically-resected isolated bone[26], brain[27], or small bowel[14] metastasis from lung cancer. Our results (Figure 1) are the first to show a longer survival or more favorable outcome in patients diagnosed with GI metastasis before surgery. Consequently, surgical treatment still plays an important role in lung cancer patients with GI metastases that cause bowel obstruction, perforation, or massive hemorrhage.
In summary, patients with GI metastases from lung cancer are in the latter stages of the disease. However, physicians and surgeons should be aware that surgical intervention is typically required for patients exhibiting bowel perforation or an acute abdomen. Therefore, early detection of GI metastasis in lung cancer patients and timely surgical management may provide symptom palliation in patients with a life-threatening GI event and long-term survival in those with only a solitary GI metastasis.
Lung cancer is a major cause of cancer-related death worldwide. Lung cancer patients with gastrointestinal (GI)-tract metastases are generally considered to be at a late or advanced stage of the disease. Management of the disease remains controversial. Study of the management in patients with symptomatic GI metastases from lung cancer is important.
Histological examination using cell type-specific markers is the best way to identify the origin of metastatic tumors of the GI tract. Staining using thyroid transcription factor-1 (TTF-1) as a specific marker of lung tumor origin, together with cytokeratin 7 (CK7) and CK20, could more effectively differentiate metastatic GI tumors from lung cancer.
Surgical treatment plays an important role in lung cancer patients with a GI metastasis that causes bowel obstruction, perforation, or massive hemorrhage. The results of this study were the first to show a longer survival or more favorable outcome in patients diagnosed with GI metastasis before surgery.
Surgical intervention is typically required for patients exhibiting bowel perforation or an acute abdomen. Therefore, early detection of GI metastasis in lung cancer patients and timely surgical management may provide symptom palliation in patients with a life-threatening GI event and improve long-term survival in those with only solitary GI metastasis.
CK7 and CK20 are low molecular weight cytokeratins. Their distribution is generally restricted to epithelia and neoplasms. The specific expression patterns of CKs are correlated with different pathways of epithelial differentiation, and therefore can be used to classify epithelial cells into different subtypes or origins. TTF-1 is a 38-kDa nuclear transcription protein that influences organogenesis and the maintenance of the differentiated phenotypes of various tissues, including thyroid, lung and brain.
The paper was well written and it is enhanced by the photomicrographs, radiological and endoscopic images.
Peer reviewer: Michael Leitman, MD, FACS, Chief of General Surgery, Beth Israel Medical Center, 10 Union Square East, Suite 2M, New York, NY 10003, United States
S- Editor Tian L L- Editor Stewart GJ E- Editor Xiong L
| 2. | McNeill PM, Wagman LD, Neifeld JP. Small bowel metastases from primary carcinoma of the lung. Cancer. 1987;59:1486-1489. [PubMed] |
| 3. | Woods JM, Koretz MJ. Emergency abdominal surgery for complications of metastatic lung carcinoma. Arch Surg. 1990;125:583-585. [PubMed] |
| 4. | Garwood RA, Sawyer MD, Ledesma EJ, Foley E, Claridge JA. A case and review of bowel perforation secondary to metastatic lung cancer. Am Surg. 2005;71:110-116. [PubMed] |
| 5. | Berger A, Cellier C, Daniel C, Kron C, Riquet M, Barbier JP, Cugnenc PH, Landi B. Small bowel metastases from primary carcinoma of the lung: clinical findings and outcome. Am J Gastroenterol. 1999;94:1884-1887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 133] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 6. | Goh BK, Yeo AW, Koong HN, Ooi LL, Wong WK. Laparotomy for acute complications of gastrointestinal metastases from lung cancer: is it a worthwhile or futile effort? Surg Today. 2007;37:370-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 41] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 7. | Antler AS, Ough Y, Pitchumoni CS, Davidian M, Thelmo W. Gastrointestinal metastases from malignant tumors of the lung. Cancer. 1982;49:170-172. [PubMed] |
| 8. | Yang CJ, Hwang JJ, Kang WY, Chong IW, Wang TH, Sheu CC, Tsai JR, Huang MS. Gastro-intestinal metastasis of primary lung carcinoma: clinical presentations and outcome. Lung Cancer. 2006;54:319-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 151] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 9. | Winchester DP, Merrill JR, Victor TA, Scanlon EF. Small bowel perforation secondary to metastatic carcinoma of the lung. Cancer. 1977;40:410-415. [PubMed] |
| 10. | Midell AI, Lochman DJ. An unusual metastatic manifestation of a primary bronchogenic carcinoma. Cancer. 1972;30:806-809. [PubMed] |
| 11. | Casella G, Di Bella C, Cambareri AR, Buda CA, Corti G, Magri F, Crippa S, Baldini V. Gastric metastasis by lung small cell carcinoma. World J Gastroenterol. 2006;12:4096-4097. [PubMed] |
| 12. | Suzaki N, Hiraki A, Ueoka H, Aoe M, Takigawa N, Kishino T, Kiura K, Kanehiro A, Tanimoto M, Harada M. Gastric perforation due to metastasis from adenocarcinoma of the lung. Anticancer Res. 2002;22:1209-1212. [PubMed] |
| 13. | Fletcher MS. Gastric perforation secondary to metastatic carcinoma of the lung: a case report. Cancer. 1980;46:1879-1882. [PubMed] |
| 14. | Kim MS, Kook EH, Ahn SH, Jeon SY, Yoon JH, Han MS, Kim CH, Lee JC. Gastrointestinal metastasis of lung cancer with special emphasis on a long-term survivor after operation. J Cancer Res Clin Oncol. 2009;135:297-301. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 93] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 15. | Leidich RB, Rudolf LE. Small bowel perforation secondary to metastatic lung carcinoma. Ann Surg. 1981;193:67-69. [PubMed] |
| 16. | Bastos I, Gomes D, Gouveia H, de Freitas D. Colonic metastasis of a lung carcinoma with ileocolic fistula. J Clin Gastroenterol. 1998;26:348. [PubMed] |
| 17. | Miyazaki K, Satoh H, Sekizawa K. Metastasis to appendix from lung adenocarcinoma. Int J Gastrointest Cancer. 2005;36:59-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 18. | Goldstein EB, Savel RH, Walter KL, Rankin LF, Satheesan R, Lehman HE, Steiner H. Extensive stage small cell lung cancer presenting as an acute perforated appendix: case report and review of the literature. Am Surg. 2004;70:706-709. [PubMed] |
| 19. | Kawahara K, Akamine S, Takahashi T, Nakamura A, Kusano H, Nakagoe T, Nakazaki T, Ayabe H, Tomita M. Anal metastasis from carcinoma of the lung: report of a case. Surg Today. 1994;24:1101-1103. [PubMed] |
| 20. | Hsu CC, Chen JJ, Changchien CS. Endoscopic features of metastatic tumors in the upper gastrointestinal tract. Endoscopy. 1996;28:249-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 59] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 21. | Rossi G, Marchioni A, Romagnani E, Bertolini F, Longo L, Cavazza A, Barbieri F. Primary lung cancer presenting with gastrointestinal tract involvement: clinicopathologic and immunohistochemical features in a series of 18 consecutive cases. J Thorac Oncol. 2007;2:115-120. [PubMed] |
| 22. | Saad RS, Silverman JF, Khalifa MA, Rowsell C. CDX2, cytokeratins 7 and 20 immunoreactivity in rectal adenocarcinoma. Appl Immunohistochem Mol Morphol. 2009;17:196-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 35] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 23. | Chen ZM, Wang HL. Alteration of cytokeratin 7 and cytokeratin 20 expression profile is uniquely associated with tumorigenesis of primary adenocarcinoma of the small intestine. Am J Surg Pathol. 2004;28:1352-1359. [PubMed] |
| 24. | Rossi G, Pelosi G, Graziano P, Barbareschi M, Papotti M. A reevaluation of the clinical significance of histological subtyping of non--small-cell lung carcinoma: diagnostic algorithms in the era of personalized treatments. Int J Surg Pathol. 2009;17:206-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 66] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 25. | Lin BR, Chang TC, Lee YC, Lee PH, Chang KJ, Liang JT. Pulmonary resection for colorectal cancer metastases: duration between cancer onset and lung metastasis as an important prognostic factor. Ann Surg Oncol. 2009;16:1026-1032. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 75] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 26. | Hirano Y, Oda M, Tsunezuka Y, Ishikawa N, Watanabe G. Long-term survival cases of lung cancer presented as solitary bone metastasis. Ann Thorac Cardiovasc Surg. 2005;11:401-404. [PubMed] |
| 27. | Chee RJ, Bydder S, Cameron F. Prolonged survival after resection and radiotherapy for solitary brain metastases from non-small-cell lung cancer. Australas Radiol. 2007;51:186-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |