Published online Oct 14, 2011. doi: 10.3748/wjg.v17.i38.4283
Revised: March 21, 2011
Accepted: March 28, 2011
Published online: October 14, 2011
Heme oxygenase-1 (HO-1) system catalyzes heme to biologically active products: carbon monoxide, biliverdin/bilirubin and free iron. It is involved in maintaining cellular homeostasis and many physiological and pathophysiological processes. A growing body of evidence indicates that HO-1 activation may play an important protective role in acute and chronic inflammation of gastrointestinal tract. This review focuses on the current understanding of the physiological significance of HO-1 induction and its possible roles in the gastrointestinal inflammation studied to date. The ability to upregulate HO-1 by pharmacological means or using gene therapy may offer therapeutic strategies for gastrointestinal inflammation in the future.
- Citation: Zhu X, Fan WG, Li DP, Kung H, Lin MC. Heme oxygenase-1 system and gastrointestinal inflammation: A short review. World J Gastroenterol 2011; 17(38): 4283-4288
- URL: https://www.wjgnet.com/1007-9327/full/v17/i38/4283.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i38.4283
Heme oxygenase (HO) is the rate-limiting enzyme in heme catabolism, a process which leads to the generation of equimolar quantities of carbon monoxide (CO), Fe2+ and biliverdin. Three distinct HO isoforms (HO-1, HO-2 and HO-3) have been identified to date, which are the products of different genes. HO-2 is constitutively and most highly expressed in neuronal tissues contributing to cell homeostasis, whereas HO-1, also referred to as heat shock protein-32 (Hsp32), is an inducible enzyme and expressed at a relatively low level in most tissues[1]. HO-3 has been found only in the rat brain, but no activity in humans[2].
Unlike the constitutively expressed HO-2, HO-1 is exquisitely sensitive, not only to heavy metals[3], but also to all kinds of stimuli and agents that cause oxidative stress and pathological conditions. Induction of the HO-1 protein has been reported to protect against a variety of stress conditions such as ischemia[4], hemorrhagic shock[5], heat shock[6], hypoxia[7], and reactive oxygen species (ROS)[8].
In fact, there has been no other enzyme described to date that is affected by so many stimuli of diverse nature as HO-1[1]. The strong adaptive response of HO-1 to various stimuli suggests that pharmacologic modulation of HO-1 system may represent an effective and cooperative strategy to intervene in protection against inflammatory processes and oxidative tissue injury. HO-1 is expressed constitutively in normal gastric, intestinal and colonic mucosa[9,10] and up-regulated in their inflamed tissues[10]. What implications of HO-1 are in gastrointestinal inflammation and injury? In this review, we focus on this subject, and elucidated the mechanisms and some potential clinical applications to gastrointestinal inflammation.
Interestingly, expression of HO-1 is usually increased in gastrointestinal inflammation and injury. This was shown in gastric ulcers[11], colitis[12,13], radiation enteritis[14], inflammatory bowel disease (IBD)[15] of animal models or patients. Moreover, HO-1 is expressed constitutively in normal gastrointestinal tract (GIT)[9,10].
The GIT is lined by a simple epithelium that separates the hostile processes of digestion and absorption that occur in the intestinal lumen from the aseptic environment of the internal milieu by defensive mechanisms.[16] GIT undergoes constant oxidative stress, inflammation and cell cycle/apoptosis. The normal expression and up-regulation of HO-1 indicate that activation of HO-1 could act as a natural defensive mechanism to alleviate inflammation and tissue injury in the GIT[13,17,18].
HO-1 is commonly regarded as a potent anti-inflammatory enzyme and has anti-inflammatory properties. For example, HO-1 upregulated by hemin[19], heme[20] and cobalt-protoporphyrin[21] can ameliorate experimental colitis. Conversely, administration with HO inhibitor (tin mesoporphyrin, SnMP) results in exacerbation of experimental colitis along with a reduction in HO-1 activity[12].
In addition, the mechanism of action of 5-aminosalicylic acid (5-ASA, an anti-colitis agent used clinically) is attributed in part to the up-regulation of HO-1 enzyme expression and activity[22]. Moreover, some agents including glutamine[9,23], tranilast[24], RDP58[25], Octreotide[26,27], lansoprazole[28-30], Ketamine[31] Polaprezinc (PZ, an anti-ulcer drug)[32] and gliotoxin[33]may contribute to the preservation of gastrointestinal mucosa in some experimental models, such as colitis, radiation enteritis, and acute gastric mucosal lesions. This protective effect is partly mediated by the induction of HO-1 expression.
Nuclear factor-erythroid 2-related factor 2 (Nrf2) has been known to be a transcriptional factor which plays a crucial role in cytoprotection against inflammation. The severity of colitis induced by dextran sulphate sodium (DSS) in Nrf2-deficient mice is found to be associated with decreased expression of HO-1[34].
These results demonstrate that HO-1 may be implicated in cytoprotection and may be an effective agent for the treatment of diseases characterized by mucosal inflammation in GIT.
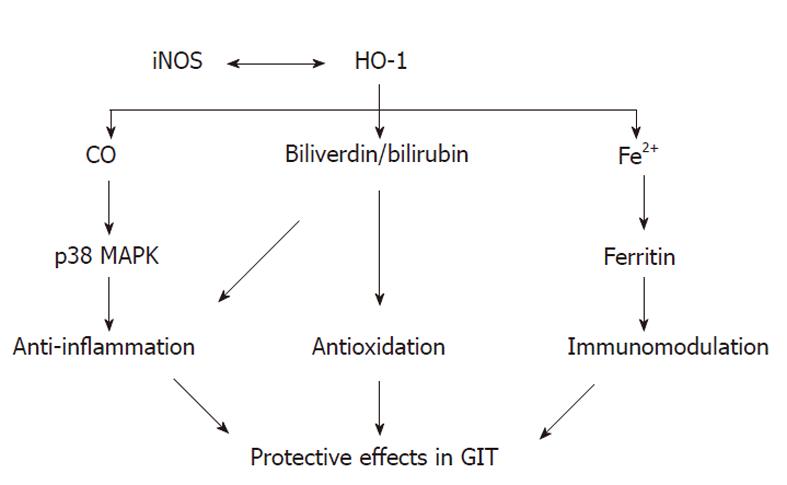
HO-1 seems to have an important protective role in ac-ute and chronic inflammation of GIT. HO-1 is the key enzyme in heme degradation and plays a key role in regulating the intracellular heme level. HO-1 activity means rapid removal of free heme, which is shown to be cytotoxic. Thus, HO-1 is associated with a protective response and contributes to the preservation of GIT mucosa (Figure 1).
Almost all CO produced in vivo comes from the degradation of heme by HO. Evidences indicate that CO mediates many of the biological actions of HO-1[35]. Otterbein et al[36] demonstrate that CO can inhibit the production of proinflammatory cytokines [tumor necrosis factor (TNF)-α, interleukin (IL)-1β, and macrophage inflammatory protein-1β] and stimulate the synthesis of the anti-inflammatory cytokine interleukin-10. Other studies also suggest that CO implicates in mediating the anti-inflammatory actions[37,38].
Hegazi et al[39] have shown that CO at a low concentration mitigates chronic intestinal inflammation in a T helper-type-1 cell-mediated mouse model of murine colitis in IL 10-deficient mice and protect against the development of postoperative ileus (POI) and necrotising enterocolitis in rodents and swine[40-42]. Moreover, Scott et al[43] demonstrate that low-dose inhaled CO selectively attenuates the remote intestinal inflammatory response elicited by hindlimb ischemia-reperfusion. And pre-treatment with CO-releasing molecules (CO-RMs) markedly reduced intestinal muscularis inflammation induced by surgical manipulation of the small intestine[44]. The anti-inflammatory actions of CO can be in large measure mediated through p38 mitogen-activated protein kinase (MAPK) pathway[36,37].
The knowledge of the role of CO in gastrointestinal inflammation is limited, but such a mechanism could be operative in GIT. Recently, Chin et al[45] pointed out that CO has been ascribed an additional novel role as a host defense molecule agent against microbes (bactericidal agent).
HO-1 catalyzes the rate-limiting step in heme degradation to biliverdin. Biliverdin is, in turn, converted into bilirubin by biliverdin reductase at the expense of nicotinamide adenine dinucleotide phosphate (NADPH). Biliverdin and bilirubin are reducing species and hence potential antioxidants[46,47]. Several studies have demonstrated that the administration of biliverdin and/or bilirubin is potently cytoprotective in a variety of pathophysiological events, including ischemia-reperfusion injury, and transplant rejection[48,49]. In addition, bilirubin is also known to modulate immune effector functions and suppress inflammatory response[50].
Treatment with biliverdin can significantly decrease mRNA expression of inducible nitric oxide synthase (iNOS), cyclooxygenase 2, and intercellular adhesion molecule-1 as well as the inflammatory cytokines IL-6 and IL-1β, and decreased neutrophil infiltration into the jejunal muscularis in rat syngeneic small intestinal transplants[51]. Hayashi et al[52] demonstrate that the effects of HO-1 induction on leukocyte adhesion could be mimicked by bilirubin. In addition, the study of Lee et al[53] show that bilirubin exerts anti-inflammatory effects in vitro.
The data indicate that this product of HO reaction play an important role in the anti-inflammatory effects of HO-1. However, there has been no report about the measurement of tissue levels of biliverdin/bilirubin in human GIT, and even the role of the biliverdin/bilirubin pathway has not been clarified in experimental model of gastrointestinal inflammation.
Fe2+, the third product of heme decomposition, can be potentially toxic, but it can upregulate an iron-transporter pump that removes intracellular Fe2+ from the cell[54] and induces the expression of ferritin, an iron storing protein[55]. Expression of ferritin is originally reported to protect endothelial cells against oxidant damage in vitro[55]. In addition, over-expression of H-ferritin (heavy chain ferritin) has also been shown to protect cultured endothelial cells from undergoing apoptosis and protect livers from transplant-associated ischemia-reperfusion injury[56]. Increased ferritin protein levels induced by lansoprazole in endothelial cells and macrophages can reduce NADPH-dependent ROS formation, indicating that ferritin may account for the gastric protection of lansoprazole[30].
Although the roles of the iron and ferritin in the overall cytoprotective effect of HO-1 are not clear, presumably both contribute in a crucial manner to the overall antioxidant effect following increased HO-1 expression in a variety of situations[57]. Further work is clearly needed in this area.
The exact mechanisms underlying the anti-inflammatory functions of the HO-1 in gastrointestinal inflammation have not been fully elucidated. However, the signaling action of CO combined/or complemented by the antioxidant properties of biliverdin/bilirubin and the sequestration of iron by ferritin could all contribute to suppression of inflammation[58]. It becomes clear that upregulation of HO-1 and/or exogenous administration of one or more of its products would be therapeutic strategies for gastrointestinal inflammation.
The inducible isoform of nitric oxide synthase (iNOS) can produce sustained high quantities of nitric oxide (NO), which may be involved in the mucosal injury associated with IBD. Indeed, upregulation of iNOS or NO release has been demonstrated in both ulcerative colitis and Crohn’s disease[59,60]. HO-1 inducers, cadmium and bismuth salts, heme, and nitric oxide (NO) donors, act at the transcriptional level inhibiting iNOS mRNA expression in vitro[61]. Wang et al[12] investigated the possible role of HO-1 in experimental colitis in rats. Their data show that HO-1 plays a protective role in the colonic damage, and this effect probably result in part from inhibition of iNOS expression in colonic tissues. Moreover, Dijkstra et al[62] demonstrate opposite regulation of iNOS and HO-1 in intestinal epithelial cells in response to cytokine exposure and oxidative stress. These findings suggest that HO-1/CO and iNOS/NO system may act together in a complex, dynamic, and adaptable association in gastrointestinal inflammation, which remain to be elucidated further.
HO-1 is known as an oxidative stress responsive protein that is upregulated by multiple stimuli, which has been proposed to provide an important cellular response that protects cells against oxidative damage. However, humans differ quantitatively in their ability to mount an HO-1 response.
An HO-1 gene promoter microsatellite (GT)(n) dinucleotide repeat polymorphism is associated with regulation of HO-1 in response to inflammatory stimuli. Short GT repeats (< 25) are associated with highly significant up-regulation of HO-1 in response to inflammatory stimuli[63,64]. The investigators have studied the association between the HO-1 genotype and gastrointestinal inflammation. They investigated the variants of the HO-1 promotor region in 179 patients with Crohn’s disease, 110 with ulcerative colitis and 56 control patients without inflammation. The data show that (GT)(n) dinucleotide repeats of the HO-1 promotor region have no significance for the pathophysiology and disease course of IBD[65]. In gastrointestinal tumors, a potential impact of the (GT)(n) repeat polymorphism has been demonstrated[66]. But in gastrointestinal inflammation diseases which usually associate tumors, it remains to be verified.
Chronic inflammatory disorders in GIT have been linked with an increased risk of the development of gastrointestinal tumors[66]. It is well known that HO-1 is involved in inflammation and have protective effects in GIT against inflammation and oxidative injury; thus, the modulation of HO-1 through pharmacological means or the use of gene therapy may offer therapeutic strategies for gastrointestinal inflammation and more importantly, to prevent gastrointestinal cancer. A comprehensive understanding of the underlying mechanisms for the observed effects of HO-1 in gastrointestinal inflammation will be necessary in the future.
Peer reviewer: Chi Hin Cho, PhD, Chair Professor, School of Biomedical Sciences, Faculty of Medicine, The Chinese University of Hong Kong, Shatin, Hong Kong, China
S- Editor Tian L L- Editor Ma JY E- Editor Zhang DN
| 1. | Maines MD. The heme oxygenase system: a regulator of second messenger gases. Annu Rev Pharmacol Toxicol. 1997;37:517-554. [PubMed] |
| 2. | McCoubrey WK, Huang TJ, Maines MD. Isolation and characterization of a cDNA from the rat brain that encodes hemoprotein heme oxygenase-3. Eur J Biochem. 1997;247:725-732. [PubMed] [DOI] [Full Text] |
| 3. | Miura N, Shinohara Y. Cytotoxic effect and apoptosis induction by silver nanoparticles in HeLa cells. Biochem Biophys Res Commun. 2009;390:733-737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 183] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 4. | Burger D, Xiang F, Hammoud L, Lu X, Feng Q. Role of heme oxygenase-1 in the cardioprotective effects of erythropoietin during myocardial ischemia and reperfusion. Am J Physiol Heart Circ Physiol. 2009;296:H84-H93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 40] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 5. | Umeda K, Takahashi T, Inoue K, Shimizu H, Maeda S, Morimatsu H, Omori E, Akagi R, Katayama H, Morita K. Prevention of hemorrhagic shock-induced intestinal tissue injury by glutamine via heme oxygenase-1 induction. Shock. 2009;31:40-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 35] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 6. | Tsuji T, Kato A, Yasuda H, Miyaji T, Luo J, Sakao Y, Ito H, Fujigaki Y, Hishida A. The dimethylthiourea-induced attenuation of cisplatin nephrotoxicity is associated with the augmented induction of heat shock proteins. Toxicol Appl Pharmacol. 2009;234:202-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 7. | Chang AY, Chan JY, Cheng HL, Tsai CY, Chan SH. Hypoxia-inducible factor 1/heme oxygenase 1 cascade as upstream signals in the prolife role of heat shock protein 70 at rostral ventrolateral medulla during experimental brain stem death. Shock. 2009;32:651-658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 8. | Cooper KL, Liu KJ, Hudson LG. Enhanced ROS production and redox signaling with combined arsenite and UVA exposure: contribution of NADPH oxidase. Free Radic Biol Med. 2009;47:381-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 22] [Reference Citation Analysis (0)] |
| 9. | Coëffier M, Le Pessot F, Leplingard A, Marion R, Lerebours E, Ducrotté P, Déchelotte P. Acute enteral glutamine infusion enhances heme oxygenase-1 expression in human duodenal mucosa. J Nutr. 2002;132:2570-2573. [PubMed] |
| 10. | Barton SG, Rampton DS, Winrow VR, Domizio P, Feakins RM. Expression of heat shock protein 32 (hemoxygenase-1) in the normal and inflamed human stomach and colon: an immunohistochemical study. Cell Stress Chaperones. 2003;8:329-334. [RCA] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 11. | Guo JS, Cho CH, Wang JY, Koo MW. Expression and immunolocalization of heat shock proteins in the healing of gastric ulcers in rats. Scand J Gastroenterol. 2002;37:17-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 12. | Wang WP, Guo X, Koo MW, Wong BC, Lam SK, Ye YN, Cho CH. Protective role of heme oxygenase-1 on trinitrobenzene sulfonic acid-induced colitis in rats. Am J Physiol Gastrointest Liver Physiol. 2001;281:G586-G594. |
| 13. | Yun KJ, Choi SC, Oh JM. [Expression of heme oxygenase-1 in ischemic colitis]. Korean J Gastroenterol. 2005;45:335-339. [PubMed] |
| 14. | Giriş M, Erbil Y, Oztezcan S, Olgaç V, Barbaros U, Deveci U, Kirgiz B, Uysal M, Toker GA. The effect of heme oxygenase-1 induction by glutamine on radiation-induced intestinal damage: the effect of heme oxygenase-1 on radiation enteritis. Am J Surg. 2006;191:503-509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 43] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 15. | Paul G, Bataille F, Obermeier F, Bock J, Klebl F, Strauch U, Lochbaum D, Rummele P, Farkas S, Scholmerich J. Analysis of intestinal haem-oxygenase-1 (HO-1) in clinical and experimental colitis. Clin Exp Immunol. 2005;140:547-555. [DOI] [Full Text] |
| 16. | Oates PS, West AR. Heme in intestinal epithelial cell turnover, differentiation, detoxification, inflammation, carcinogenesis, absorption and motility. World J Gastroenterol. 2006;12:4281-4295. [PubMed] |
| 17. | Guo X, Shin VY, Cho CH. Modulation of heme oxygenase in tissue injury and its implication in protection against gastrointestinal diseases. Life Sci. 2001;69:3113-3119. [RCA] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 72] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 18. | Vijayan V, Mueller S, Baumgart-Vogt E, Immenschuh S. Heme oxygenase-1 as a therapeutic target in inflammatory disorders of the gastrointestinal tract. World J Gastroenterol. 2010;16:3112-3119. [PubMed] |
| 19. | Zhong W, Xia Z, Hinrichs D, Rosenbaum JT, Wegmann KW, Meyrowitz J, Zhang Z. Hemin exerts multiple protective mechanisms and attenuates dextran sulfate sodium-induced colitis. J Pediatr Gastroenterol Nutr. 2010;50:132-139. [RCA] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 48] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 20. | Varga C, Laszlo F, Fritz P, Cavicchi M, Lamarque D, Horvath K, Posa A, Berko A, Whittle BJ. Modulation by heme and zinc protoporphyrin of colonic heme oxygenase-1 and experimental inflammatory bowel disease in the rat. Eur J Pharmacol. 2007;561:164-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 21. | Berberat PO, YI AR, Yamashita K, Warny MM, Csizmadia E, Robson SC, Bach FH. Heme oxygenase-1-generated biliverdin ameliorates experimental murine colitis. Inflamm Bowel Dis. 2005;11:350-359. [RCA] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 88] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 22. | Horváth K, Varga C, Berkó A, Pósa A, László F, Whittle BJ. The involvement of heme oxygenase-1 activity in the therapeutic actions of 5-aminosalicylic acid in rat colitis. Eur J Pharmacol. 2008;581:315-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 58] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 23. | Giriş M, Erbil Y, Doğru-Abbasoğlu S, Yanik BT, Aliş H, Olgaç V, Toker GA. The effect of heme oxygenase-1 induction by glutamine on TNBS-induced colitis. The effect of glutamine on TNBS colitis. Int J Colorectal Dis. 2007;22:591-599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 42] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 24. | Sun X, Suzuki K, Nagata M, Kawauchi Y, Yano M, Ohkoshi S, Matsuda Y, Kawachi H, Watanabe K, Asakura H. Rectal administration of tranilast ameliorated acute colitis in mice through increased expression of heme oxygenase-1. Pathol Int. 2010;60:93-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 25. | Murthy S, Flanigan A, Coppola D, Buelow R. RDP58, a locally active TNF inhibitor, is effective in the dextran sulphate mouse model of chronic colitis. Inflamm Res. 2002;51:522-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 26. | Erbil Y, Giriş M, Abbasoğlu SD, Barbaros U, Yanik BT, Necefli A, Olgaç V, Toker GA. Effect of heme oxygenase-1 induction by octreotide on TNBS-induced colitis. J Gastroenterol Hepatol. 2007;22:1852-1858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 27. | Abbasoğlu SD, Erbil Y, Eren T, Giriş M, Barbaros U, Yücel R, Olgaç V, Uysal M, Toker G. The effect of heme oxygenase-1 induction by octreotide on radiation enteritis. Peptides. 2006;27:1570-1576. [PubMed] [DOI] [Full Text] |
| 28. | Takagi T, Naito Y, Yoshikawa T. The expression of heme oxygenase-1 induced by lansoprazole. J Clin Biochem Nutr. 2009;45:9-13. [PubMed] |
| 29. | Takagi T, Naito Y, Okada H, Ishii T, Mizushima K, Akagiri S, Adachi S, Handa O, Kokura S, Ichikawa H. Lansoprazole, a proton pump inhibitor, mediates anti-inflammatory effect in gastric mucosal cells through the induction of heme oxygenase-1 via activation of NF-E2-related factor 2 and oxidation of kelch-like ECH-associating protein 1. J Pharmacol Exp Ther. 2009;331:255-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 53] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 30. | Schulz-Geske S, Erdmann K, Wong RJ, Stevenson DK, Schröder H, Grosser N. Molecular mechanism and functional consequences of lansoprazole-mediated heme oxygenase-1 induction. World J Gastroenterol. 2009;15:4392-4401. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 9] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 31. | Helmer KS, Suliburk JW, Mercer DW. Ketamine-induced gastroprotection during endotoxemia: role of heme-oxygenase-1. Dig Dis Sci. 2006;51:1571-1581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 32. | Ueda K, Ueyama T, Oka M, Ito T, Tsuruo Y, Ichinose M. Polaprezinc (Zinc L-carnosine) is a potent inducer of anti-oxidative stress enzyme, heme oxygenase (HO)-1 - a new mechanism of gastric mucosal protection. J Pharmacol Sci. 2009;110:285-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 48] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 33. | Jun CD, Kim Y, Choi EY, Kim M, Park B, Youn B, Yu K, Choi KS, Yoon KH, Choi SC. Gliotoxin reduces the severity of trinitrobenzene sulfonic acid-induced colitis in mice: evidence of the connection between heme oxygenase-1 and the nuclear factor-kappaB pathway in vitro and in vivo. Inflamm Bowel Dis. 2006;12:619-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 28] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 34. | Khor TO, Huang MT, Kwon KH, Chan JY, Reddy BS, Kong AN. Nrf2-deficient mice have an increased susceptibility to dextran sulfate sodium-induced colitis. Cancer Res. 2006;66:11580-11584. [PubMed] |
| 35. | Ryter SW, Alam J, Choi AM. Heme oxygenase-1/carbon monoxide: from basic science to therapeutic applications. Physiol Rev. 2006;86:583-650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1689] [Cited by in RCA: 1801] [Article Influence: 94.8] [Reference Citation Analysis (0)] |
| 36. | Otterbein LE, Bach FH, Alam J, Soares M, Tao Lu H, Wysk M, Davis RJ, Flavell RA, Choi AM. Carbon monoxide has anti-inflammatory effects involving the mitogen-activated protein kinase pathway. Nat Med. 2000;6:422-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1667] [Cited by in RCA: 1719] [Article Influence: 68.8] [Reference Citation Analysis (0)] |
| 37. | Lee TS, Chau LY. Heme oxygenase-1 mediates the anti-inflammatory effect of interleukin-10 in mice. Nat Med. 2002;8:240-246. [PubMed] |
| 38. | Yachie A, Niida Y, Wada T, Igarashi N, Kaneda H, Toma T, Ohta K, Kasahara Y, Koizumi S. Oxidative stress causes enhanced endothelial cell injury in human heme oxygenase-1 deficiency. J Clin Invest. 1999;103:129-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 986] [Cited by in RCA: 1007] [Article Influence: 38.7] [Reference Citation Analysis (0)] |
| 39. | Hegazi RA, Rao KN, Mayle A, Sepulveda AR, Otterbein LE, Plevy SE. Carbon monoxide ameliorates chronic murine colitis through a heme oxygenase 1-dependent pathway. J Exp Med. 2005;202:1703-1713. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 175] [Cited by in RCA: 182] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 40. | Moore BA, Otterbein LE, Türler A, Choi AM, Bauer AJ. Inhaled carbon monoxide suppresses the development of postoperative ileus in the murine small intestine. Gastroenterology. 2003;124:377-391. [PubMed] |
| 41. | Moore BA, Overhaus M, Whitcomb J, Ifedigbo E, Choi AM, Otterbein LE, Bauer AJ. Brief inhalation of low-dose carbon monoxide protects rodents and swine from postoperative ileus. Crit Care Med. 2005;33:1317-1326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 55] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 42. | Zuckerbraun BS, Otterbein LE, Boyle P, Jaffe R, Upperman J, Zamora R, Ford HR. Carbon monoxide protects against the development of experimental necrotizing enterocolitis. Am J Physiol Gastrointest Liver Physiol. 2005;289:G607-G613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 57] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 43. | Scott JR, Cukiernik MA, Ott MC, Bihari A, Badhwar A, Gray DK, Harris KA, Parry NG, Potter RF. Low-dose inhaled carbon monoxide attenuates the remote intestinal inflammatory response elicited by hindlimb ischemia-reperfusion. Am J Physiol Gastrointest Liver Physiol. 2009;296:G9-G14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 44. | De Backer O, Elinck E, Blanckaert B, Leybaert L, Motterlini R, Lefebvre RA. Water-soluble CO-releasing molecules reduce the development of postoperative ileus via modulation of MAPK/HO-1 signalling and reduction of oxidative stress. Gut. 2009;58:347-356. [PubMed] |
| 45. | Chin BY, Otterbein LE. Carbon monoxide is a poison... to microbes! CO as a bactericidal molecule. Curr Opin Pharmacol. 2009;9:490-500. [PubMed] |
| 46. | Stocker R, Yamamoto Y, McDonagh AF, Glazer AN, Ames BN. Bilirubin is an antioxidant of possible physiological importance. Science. 1987;235:1043-1046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2595] [Cited by in RCA: 2635] [Article Influence: 69.3] [Reference Citation Analysis (0)] |
| 47. | Abraham NG, Kappas A. Heme oxygenase and the cardiovascular-renal system. Free Radic Biol Med. 2005;39:1-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 61] [Reference Citation Analysis (0)] |
| 48. | Fondevila C, Shen XD, Tsuchiyashi S, Yamashita K, Csizmadia E, Lassman C, Busuttil RW, Kupiec-Weglinski JW, Bach FH. Biliverdin therapy protects rat livers from ischemia and reperfusion injury. Hepatology. 2004;40:1333-1341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 136] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 49. | Clark JE, Foresti R, Sarathchandra P, Kaur H, Green CJ, Motterlini R. Heme oxygenase-1-derived bilirubin ameliorates postischemic myocardial dysfunction. Am J Physiol Heart Circ Physiol. 2000;278:H643-H651. [PubMed] |
| 50. | Willis D, Moore AR, Frederick R, Willoughby DA. Heme oxygenase: a novel target for the modulation of the inflammatory response. Nat Med. 1996;2:87-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 618] [Cited by in RCA: 622] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 51. | Nakao A, Otterbein LE, Overhaus M, Sarady JK, Tsung A, Kimizuka K, Nalesnik MA, Kaizu T, Uchiyama T, Liu F. Biliverdin protects the functional integrity of a transplanted syngeneic small bowel. Gastroenterology. 2004;127:595-606. [PubMed] [DOI] [Full Text] |
| 52. | Hayashi S, Takamiya R, Yamaguchi T, Matsumoto K, Tojo SJ, Tamatani T, Kitajima M, Makino N, Ishimura Y, Suematsu M. Induction of heme oxygenase-1 suppresses venular leukocyte adhesion elicited by oxidative stress: role of bilirubin generated by the enzyme. Circ Res. 1999;85:663-671. [PubMed] |
| 53. | Lee SH, Sohn DH, Jin XY, Kim SW, Choi SC, Seo GS. 2',4',6'-tris(methoxymethoxy) chalcone protects against trinitrobenzene sulfonic acid-induced colitis and blocks tumor necrosis factor-alpha-induced intestinal epithelial inflammation via heme oxygenase 1-dependent and independent pathways. Biochem Pharmacol. 2007;74:870-880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 84] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 54. | Ferris CD, Jaffrey SR, Sawa A, Takahashi M, Brady SD, Barrow RK, Tysoe SA, Wolosker H, Barañano DE, Doré S. Haem oxygenase-1 prevents cell death by regulating cellular iron. Nat Cell Biol. 1999;1:152-157. [PubMed] |
| 55. | Balla G, Jacob HS, Balla J, Rosenberg M, Nath K, Apple F, Eaton JW, Vercellotti GM. Ferritin: a cytoprotective antioxidant strategem of endothelium. J Biol Chem. 1992;267:18148-18153. [PubMed] |
| 56. | Berberat PO, Katori M, Kaczmarek E, Anselmo D, Lassman C, Ke B, Shen X, Busuttil RW, Yamashita K, Csizmadia E. Heavy chain ferritin acts as an antiapoptotic gene that protects livers from ischemia reperfusion injury. FASEB J. 2003;17:1724-1726. [PubMed] |
| 57. | Otterbein LE, Soares MP, Yamashita K, Bach FH. Heme oxygenase-1: unleashing the protective properties of heme. Trends Immunol. 2003;24:449-455. [RCA] [DOI] [Full Text] [Cited by in Crossref: 913] [Cited by in RCA: 968] [Article Influence: 44.0] [Reference Citation Analysis (0)] |
| 58. | Bach FH. Carbon monoxide: from the origin of life to molecular medicine. Trends Mol Med. 2006;12:348-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 59. | Cross RK, Wilson KT. Nitric oxide in inflammatory bowel disease. Inflamm Bowel Dis. 2003;9:179-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 237] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 60. | Boughton-Smith NK, Evans SM, Hawkey CJ, Cole AT, Balsitis M, Whittle BJ, Moncada S. Nitric oxide synthase activity in ulcerative colitis and Crohn's disease. Lancet. 1993;342:338-340. [DOI] [Full Text] |
| 61. | Cavicchi M, Gibbs L, Whittle BJ. Inhibition of inducible nitric oxide synthase in the human intestinal epithelial cell line, DLD-1, by the inducers of heme oxygenase 1, bismuth salts, heme, and nitric oxide donors. Gut. 2000;47:771-778. [PubMed] |
| 62. | Dijkstra G, Blokzijl H, Bok L, Homan M, van Goor H, Faber KN, Jansen PL, Moshage H. Opposite effect of oxidative stress on inducible nitric oxide synthase and haem oxygenase-1 expression in intestinal inflammation: anti-inflammatory effect of carbon monoxide. J Pathol. 2004;204:296-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 47] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 63. | Schillinger M, Exner M, Mlekusch W, Haumer M, Sabeti S, Ahmadi R, Schwarzinger I, Wagner O, Minar E. Restenosis after femoropopliteal PTA and elective stent implantation: predictive value of monocyte counts. J Endovasc Ther. 2003;10:557-565. [DOI] [Full Text] |
| 64. | Exner M, Schillinger M, Minar E, Mlekusch W, Schlerka G, Haumer M, Mannhalter C, Wagner O. Heme oxygenase-1 gene promoter microsatellite polymorphism is associated with restenosis after percutaneous transluminal angioplasty. J Endovasc Ther. 2001;8:433-440. [DOI] [Full Text] |
| 65. | Hausmann M, Paul G, Kellermeier S, Frey I, Scholmerich J, Falk W, Menzel K, Fried M, Herfarth H, Rogler G. (GT)N dinucleotide repeat polymorphism of haem oxygenase-1 promotor region is not associated with inflammatory bowel disease risk or disease course. Clin Exp Immunol. 2008;153:81-85. [RCA] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 66. | Zhu X, Fan WG, Li DP, Lin MC, Kung H. Heme oxygenase-1 system and gastrointestinal tumors. World J Gastroenterol. 2010;16:2633-2637. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 25] [Cited by in RCA: 27] [Article Influence: 1.8] [Reference Citation Analysis (0)] |