CHROMOENDOSCOPY
Chromoendoscopy involves topical application of various dyes during endoscopy which improves the visualisation of mucosal surfaces. The stains can be divided into three main classes: contrast, absorptive and reactive. Contrast stains, for example indigo carmine (IC), accumulate in the mucosal fissures thereby accentuating surface topology. In contrast, absorptive stains such as Lugol’s iodine (LI), crystal violet and methylene blue (MB) are absorbed into components of the cellular structure in the mucosa. Differences in the uptake of these stains can therefore be used to elucidate different types of mucosa. Reactive stains such as Congo red and Phenol red are pH-dependant. Congo red turns dark blue or black in acidic conditions, while phenol is yellow in an acidic environment and turns red in the presence of alkaline substances. These stains are, however, not used routinely in the oesophagus. There are two essential steps in chromoendoscopy - firstly, removal of mucous which is then followed by dye application. The former is achieved by using water, or occasionally some centres have advocated the use of a mucolytic agent; N-Acetylcysteine[1,2]. This can be achieved by flushing the agent through the working channel, using a spray catheter or even administering it as an oral solution before the endoscopic procedure[3]. Once the mucous is cleared, the dye can then be applied. The volume, concentration and the dye contact time varies considerably. Canto’s group[4,5] used 10-20 mL of 0.5% MB for every 5 cm of Barrett’s mucosa, while Ragunath et al[6] used 4 mL of 0.5% MB for every 1 cm.
LUGOL’S IODINE
LI is a compound iodine solution that is absorbed by the glycogen containing squamous epithelium and stains it brown. The demarcation between squamous epithelium of the oesophagus and columnar epithelium of the stomach can therefore be clearly delineated. Damaged mucosa due to oesophagitis or malignant infiltration does not stain as well as normal mucosa. In BE, specialised intestinal metaplasia does not stain after application of LI. Woolf et al[7] used LI to improve demarcation of squamous from columnar mucosa in patients with BE.
Toluidine blue
This is an absorptive stain taken up by the nuclei of columnar cells. Chobanian and colleagues used 1% toluidine blue to aid in the endoscopic detection of BE and reported improved sensitivity compared to standard endoscopy alone[8]. The main limitation of this technique is that the dye stains all columnar cells, hence distinguishing between the intestinal and non intestinal epithelial subtypes of BE is not feasible especially since intestinal metaplasia can be patchy in BE.
Methylene blue
MB, an absorptive dye, is probably the most investigated stain for evaluation of BE and also the most controversial. It is a vital stain taken up by actively absorbing epithelial cells after topical application at a concentration of 0.5%-1.0%[9,10]. The dye is absorbed by goblet cells present in specialised intestinal metaplastic epithelium. Initial work done by Canto’s group revealed that MB can distinguish IM and dysplasia in BE with high precision. However, these results were not reproducible. Various other subsequent studies have revealed mixed findings. The main contention with MB in BE is that dysplastic areas do not stain, but the problem with that is that even areas which do not harbour IM do not absorb the dye. This makes it difficult for the endoscopist to decide on which areas to target the biopsies during the procedure. There were also some issues with the uniformity of the dye and recently even toxicity with MB. It has been examined in both long and short segment BE[11,12,13]. Two patterns of staining have been documented - diffuse and focal. Canto et al[5,11] found that most patients with long segment BE exhibited diffuse staining, whereas Wo et al[14] observed focal staining in their cohort of patients with long segment BE. Similar discrepancies have been reported in short segment BE. Sharma et al[13] found that the majority of their patients with short segment BE stained diffusely. In contrast, in 30 patients with short segment BE assessed by Kiesslich’s group[12], 80% demonstrated staining in a focal pattern.
The published data for biopsy related sensitivity of MB in detecting specialised intestinal metaplasia (SIM) vary considerably. Some studies reported high sensitivities ranging from 81% to 98%[3,11,12], while others show markedly less favourable sensitivities ranging from 37% to 61%[6,13-16]. The reasons for this variation in results are not clear. Differences in stain concentration[4,11,12] and the volume used[4,6,14] may have influenced results. The biopsy protocol used in specific studies has also varied. Some investigators performed random 4 quadrant biopsies irrespective of the staining pattern[4,6,15], while others obtained equal numbers of stained and unstained mucosa[11] or biopsied stained mucosa only[3]. Another possible explanation for the inconsistent published data is the discrepancy in operator skill and experience. Most procedures were performed by a single expert endoscopist in a “tertiary centre”[4,5,11,15], hence the generalisability of the procedure itself has to be questioned. The role of MB in the detection of foci of dysplasia in BE is even more unclear. Early studies by Canto et al[4,11] showed that dysplastic tissue did stain with MB, although histology revealed predominantly low grade dysplasia. Subsequent work suggested that lack of staining was more predictive of dysplasia, attributed to the loss of goblet cells with progression of dysplasia[5]. A focal non-staining area in a sea of blue has been found to be highly predictive of dysplastic change. However, investigators continue to report dysplasia and carcinoma occurring in stained biopsies[12,14]. The inter-observer variability in differentiating between shades of blue has not been determined, and the interpretation of deeply vs lightly stained mucosa is largely subjective. As a result of all these controversies and confusion, MB has hence not really gained widespread acceptance in the gastrointestinal fraternity.
A recent meta-analysis assessing the diagnostic yield of MB in detecting SIM and dysplasia in BE looked at 9 published studies that included 450 patients. Despite controlling for differences in technique and quality of published data, the meta-analysis showed no significant benefit of MB chromoendoscopy compared with random biopsies in detecting SIM, dysplasia or early oesophageal cancer[17].
Crystal violet
Crystal violet has been used as an absorptive stain to evaluate colonic polyps since it is preferentially taken up by the crypts of Lieberkuhn[18,19]. Its role in the assessment of BE is less clear. A case report using a combination of crystal violet and Methylene blue has been described to be useful for the detection of a minute focus of adenocarcinoma in BE[20]. These investigators found that 0.05% crystal violet directly dyes the surface of BE, thereby enhancing MB stained mucosa.
Indigo carmine
This dye is not absorbed when applied topically to the mucosa. Instead it augments mucosal details and is therefore used as a contrast stain to delineate irregularities of the mucosal surface. Since it provides a clearer definition of the mucosal pattern in BE, evaluation of IC is best considered in conjunction with magnification endoscopy. Sharma et al[21] showed that IC magnification endoscopy may improve mucosal imaging and the detection of dysplasia in BE. However, Kara et al[22] showed that when a high resolution endoscope is used, the adjunctive use of IC chromoendoscopy is of limited use for the primary detection of lesions.
High resolution magnification endoscopy
Standard video endoscopes are tailored to view the mucosa from a focal distance of 1-2 cm from the endoscope tip. With a pixel density of 200 000, detailed inspection is limited especially if the tip of the scope is advanced closer to the area of interest. The focused area tends to exhibit a blurred view. Coupled with low resolution monitors, the quality of images obtained in real time can be compromised. As technology improves, the pixel density and resolution of monitors has increased tremendously, and this has resulted in improved image quality with high resolution (> 850 000 pixel density) and high magnification (115X) systems. This phenomenon is especially crucial in BE surveillance as early, subtle lesions harbouring dysplasia or cancer should not be missed.
Recent advancements in endoscopic technology have produced high magnification endoscopes with electronically moveable lenses which allow real time visualisation of mucosal morphology in greater detail. Magnification enlarges the endoscopic image, while better resolution improves the ability to discriminate detail by enabling two closely approximated points to be better appreciated. The clinical utility of this modality had been limited by the size of the endoscope in the past. However, improvement in the design of the charged-couple device, an electronic light sensing apparatus located at the tip of the endoscope, has given rise to less bulky and more manageable instruments. High resolution magnification endoscopy (HRME) has been evaluated in coeliac disease where it was found to be valuable in assessing the degree of villous atrophy[23]. Inoue’s group used HRME to characterise the blood vessel morphology, hence facilitate the diagnosis of superficial oesophageal cancer[24]. The morphology of intrapapillary loops became progressively more tortuous and disorganised with the evolution of dysplasia to cancer. HRME has also been assessed in the stomach, where the authors have shown that it can reliably identify normal gastric mucosa, Helicobacter pylori-associated gastritis and gastric atrophy[25]. In the colon, magnification endoscopy has been used to assess colonic polyps[26,27] and colon cancer[28,29].
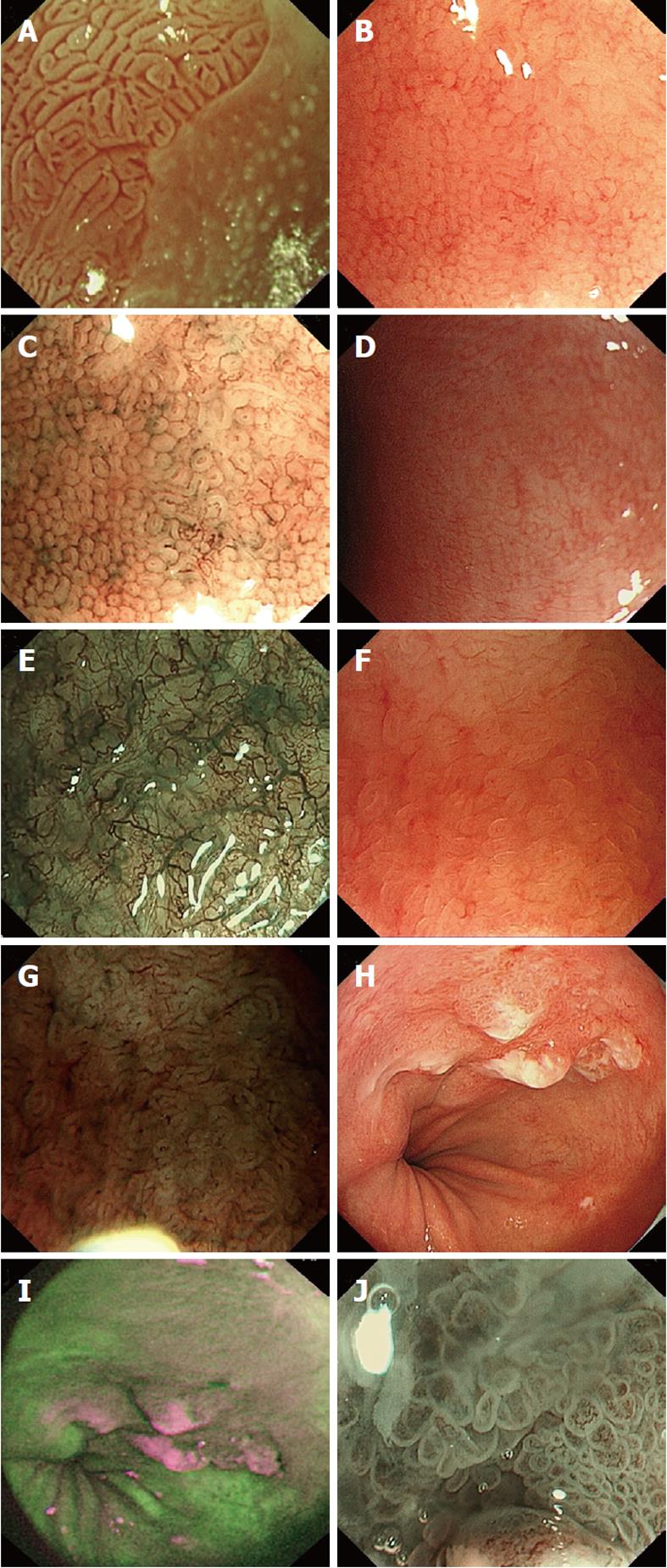
Magnification endoscopy has been proposed as a diagnostic tool to improve the sensitivity of standard endoscopy in the detection of specialised intestinal metaplasia and dysplasia. Stevens et al[30] used IC as a contrast stain to assess BE using magnification endoscopy and noted a villiform appearance correlated with the histological finding of specialised intestinal metaplasia. Endo and colleagues[31] characterised the pit pattern of BE using magnification endoscopy and MB staining and found that specialised intestinal metaplasia was detected in patients who exhibited a tubular/villous pattern in their BE segment. Similarly, Sharma’s group found that 97% of their cohort of patients with a ridged/villous pattern on magnification chromoendoscopy using IC had specialised intestinal metaplasia, and 100% with an irregular and distorted pattern exhibited high grade dysplasia[21]. Fortun and colleagues reported that enhanced magnification endoscopy with acetic acid (Figure 1A) allows clear visualisation of the epithelial pit patterns within BE, and targeted biopsy resulted in a high yield of specialised intestinal metaplasia and dysplasia[32]. However, despite the increasing availability of high resolution magnification endoscopes, there is a lack of diagnostic criteria for magnified endoscopic images.
Figure 1 Images of various advanced imaging modalities in Barrett’s oesophagus.
A: Acetic acid used to visualise Barrett’s oesophagus, ridge pattern signifying Intestinal metaplasia; B: High magnification white light endoscopy-round pits in keeping with columnar mucosa without intestinal metaplasia; C: Corresponding area on image B seen with narrow band imaging (NBI) and magnification; D: High magnification white light endoscopy - absent pits in keeping with columnar mucosa with intestinal metaplasia; E: Corresponding area on image D seen with NBI and magnification; F: High magnification white light endoscopy - villous/ridge pits in keeping with columnar mucosa with intestinal metaplasia; G: Corresponding area on image F seen with NBI and magnification; H: White light endoscopy of Barrett’s cancer; I: Corresponding area on autoflourescence imaging; J: Abnormal area on NBI with magnification showing total distortion of the pit pattern.
Autofluorescence imaging
When tissues are exposed to short wave length light, endogenous biological substances (i.e., fluorophores) are excited, leading to emission of fluorescent light of a longer wavelength. This phenomenon is known as autofluorescence[33]. Autofluorescence imaging (AFI) is a technique that can potentially differentiate tissue types based on their differences in fluorescence emission. Normal and neoplastic tissue have different autofluorescence spectra which may enable their distinction. This is due to the various different compositions of the endogenous fluorophores which includes collagen, NADH, aromatic amino acids and porphyrins in these tissues. Until recently, AFI has been restricted to either autofluorescence spectroscopy or autofluorescence endoscopy using the older generation fibre optic endoscopes[34-36]. The main limitation of AFI using this modality is that the quality of the images produced was inferior. Recently, video AFI which incorporates high resolution endoscopy has been evaluated[37]. In an uncontrolled feasibility study, AFI led to the detection of a significant number of patients with high grade dysplasia/early cancer in Barrett’s oesophagus (BE). There was, however, a very high false positive rate (51%) using this modality.
Narrow band imaging
The quest for a simpler technique which would obviate the complexity of chromoendoscopy led to the development of narrow band imaging (NBI) (Figure 1B-G: HRME and corresponding images on NBI). Termed “electronic chromoendoscopy” by some quarters, this unique technology was first described by Gono et al[38]. Standard white light endoscopy consists of 3 light waves: blue, green and red. The principles behind NBI technology are that the bandwidths of blue (440-460 nm) and green (540-560 nm) wave light are narrowed whilst the contribution of red wave light is totally negated out of the emitted light. This is achieved by a special filter which is electronically activated once the endoscopist presses a switch on the endoscope. The whole process takes less than 1 s and is practical during any endoscopy procedure provided the system is equipped with NBI. The narrowed bandwidths of green and blue light lead to superficial penetration of the mucosa accentuating the microvasculature pattern as haemoglobin has a peak absorption spectrum towards both these wave lengths. The quality of the surface pit pattern morphology is also clearly enhanced by this technology. It enables the endoscopist to switch between conventional white light and NBI views easily and quickly during the procedure, thus making the procedure itself less messy and cumbersome compared to chromoendoscopy. By depressing a lever on the endoscope, the focal distance of the lens at the tip of the endoscope can be adjusted electronically thus enabling the endoscopist to achieve a maximal magnification of 115X in real time. NBI has been evaluated in BE with very promising results[39-43]. A recent meta-analysis of 8 published studies which included 446 patients with 2194 lesions showed that NBI-Z has high diagnostic precision in detecting high grade dysplasia with a sensitivity and specificity of 96% and 94%, respectively[44]. However, the results of NBI-Z in characterising SIM were inferior with a sensitivity of 95% and a specificity of 65%.
Trimodal imaging
With various new technologies available, it was inevitable that combining them into a single system was the next step forward, hence the introduction of the novel concept of trimodal imaging. This modality incorporates three advanced endoscopy imaging techniques into a single endoscope: HRME (Figure 1H), AFI (Figure 1I) and NBI (Figure 1J), thereby enabling the endoscopist to use all 3 modalities during a single procedure. Promising early results have been reported in a multicentre feasibility study[45] and more recently in a multicentre randomised cross-over study[46].
CONCLUSION
Although chromoendoscopy has been available for more than 20 years, the lack of standardisation of the technique is one major reason for the indifference towards it. The dearth of well controlled studies that determine its clinical utility, cost efficacy, patient acceptance and tolerability in terms of the additional time needed are amongst the other reasons why chromoendoscopy has not truly caught on. With the rapid development of various novel technologies, it seems that the ideal endoscopy system could very well be on the horizon. It would incorporate a “red flag” technique similar to the AFI system but with hopefully a lower rate of false positives followed on by further detailed interrogation of the suspicious area detected by the technique with either NBI or a confocal probe to obtain “optical biopsies”. This may enable the endoscopist to ascertain the histopathological diagnosis in real time. There are, however, numerous issues which would need to be overcome. Standardisation of the various classification systems as well as incorporation of all these techniques into a single easily managed, less bulky unit which is financially viable and less time consuming could eventually lead to widespread availability of a technique in the community.