Published online Oct 7, 2011. doi: 10.3748/wjg.v17.i37.4206
Revised: August 1, 2011
Accepted: August 8, 2011
Published online: October 7, 2011
AIM: To assess the validity of the Milan and University of California San Francisco (UCSF) criteria and examine the long-term outcome of orthotopic liver transplantation (OLT) in patients with hepatocellular carcinoma (HCC) in a single-center study.
METHODS: This study is a retrospective review of prospectively collected data. Between 1998 and 2009, 56 of 356 OLTs were performed in patients with HCC. Based on pathological examination of liver explants, patients were retrospectively categorized into 3 grou-ps: Milan + (n = 34), Milan -/UCSF + (n = 7) and UCSF - (n = 14).
RESULTS: Median follow-up period was 39.5 (1-124) mo. The 5-year overall survival rates in the Milan +, Milan -/UCSF + and UCSF-groups were 87.7%, 53.6% and 33.3%, respectively (P < 0.000). Within these groups, tumor recurrence was determined in 5.8%, 14.3% and 40% of patients, respectively (P < 0.011). Additionally, the presence of microvascular invasion within the explanted liver had a negative effect on the 5-year disease free survival (74.7% vs 46.7%, P < 0.044).
CONCLUSION: The Milan criteria are reliable in the selection of suitable candidates for OLT for the treatment of HCC. For cases of OLT involving living donors, the UCSF criteria may be applied.
- Citation: Unek T, Karademir S, Arslan NC, Egeli T, Atasoy G, Sagol O, Obuz F, Akarsu M, Astarcioglu I. Comparison of Milan and UCSF criteria for liver transplantation to treat hepatocellular carcinoma. World J Gastroenterol 2011; 17(37): 4206-4212
- URL: https://www.wjgnet.com/1007-9327/full/v17/i37/4206.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i37.4206
Hepatocellular carcinoma (HCC) is the fifth most common malignancy in the world, and is associated with the third highest number of cancer-related deaths[1]. Moreover, for 70%-90% of HCC cases, HCC develops on a background of cirrhosis or chronic liver inflammation[2]. Currently, there are three potentially curative therapeutic options for HCC, liver resection, orthotopic liver transplantation (OLT), and local ablative therapies[3]. Although liver resection treats localized HCC, it is not optimal for treating multifocal HCC, and has no efficacy in preventing de novo HCC that can develop in the remnants of a cirrhotic liver. Alternatively, liver transplantation is an established therapy which offers the potential advantage of removing both the tumor and the organ at risk for developing future malignancies[4].
In order to identify the best candidates for OLT, a set of criteria were proposed, referred to as the “Milan” criteria. According to these guidelines, patients with cirrhosis and a solitary tumor with a diameter less than 5 cm, or patients who have up to 3 tumor nodules, each of which is smaller than 3 cm and are not characterized by vascular invasion or extrahepatic metastasis (according to preoperative radiologic findings), are patients that have a higher probability of obtaining a successful outcome following OLT. For example, the 5-year recurrence-free survival rate for a set of patients who fulfilled the Milan criteria was reported to be 83%[5]. The “Milan criteria’’ were subsequently adopted by the United Network for Organ Sharing (UNOS) in 2002 as the optimal criteria for determining the use of OLT to treat HCC[6]. However, an expanded set of criteria proposed by the University of California San Francisco (UCSF), referred to here as the “UCSF” criteria, allows patients with a solitary tumor smaller than 6.5 cm, or patients having 3 of fewer nodules, with the largest lesion being smaller than 4.5 cm or having a total tumor diameter less than 8.5 cm without vascular invasion, to undergo OLT. Based on the comparable success of this set of criteria in selecting patients for OLT, it has been suggested that the Milan criteria may be too stringent[7]. Therefore, the aim of this study was to examine the long-term outcome of patients undergoing liver transplantation to treat HCC, and to compare the use of the current criteria (both the Milan and UCSF) for the selection of HCC patients for possible OLT.
Between 1998 and 2009, 56 of 356 (15.7%) OLTs were performed in patients with HCC at the Dokuz Eylul University Hospital (Izmir, Turkey). Of these, 50 were diagnosed with HCC prior to transplantation, and 6 (10.7%) were diagnosed during OLT. According to pre-OLT imaging and post-OLT pathological evaluation, 56 patients were retrospectively classified into 3 groups: Milan +, Milan -/UCSF + and UCSF - (Table 1).
Following the pathological examination of liver explant specimens, 14 (25.0%) patients were reclassified due to underestimates of tumor size, and 7 (12.5%) patients were reclassified due to the tumor number being greater than expected (false negative rate: 25%) (Table 1). For the applied Milan and UCSF criteria, false negative rates of pre-OLT radiological evaluations were 22.7% (10/44) and 16.3% (8/49), respectively. In summary, 8 patients met the UCSF criteria prior to undergoing OLT, and exceeded the UCSF criteria following pathologic evaluation of the explants obtained.
All patients included in this study had cirrhosis due to various etiologies. A pre-operative diagnosis of HCC was based on a patient’s medical history, a physical examination, laboratory studies, α-fetoprotein (AFP) levels, and the results of one or more imaging studies [i.e., abdominal ultrasonography, contrast-enhanced computed tomography (CT), angiographic CT, or abdominal magnetic resonance imaging (MRI)]. Tumor biopsies were not performed to confirm each diagnosis. Chest CT, cranial CT, and technetium-99 m bone scintigraphy were used to detect the potential incidence of extrahepatic disease, and distant or lymph node metastases were not detected in any of the patients. Pre-OLT adjuvant therapies, including radiofrequency ablation (RFA), transarterial hepatic chemoembolization (TACE), percutaneous ethanol injection (PEI), and liver resection were performed in selected patients. In the absence of medical contraindications, patients who fulfilled the Milan criteria in pre-OLT evaluations were qualified to receive a transplant from either a living or deceased donor. Alternatively, for patients who did not fulfill the Milan criteria, these patients were qualified to receive organs from living donors only. In our series, 31 (55.3%) living and 25 (44.7%) deceased donor liver grafts were utilized. In the latter group, 3 marginal liver grafts from deceased donors were transplanted to recipients who did not fulfill the Milan criteria.
Thirteen out of 50 (26%) patients received locoregional treatment prior to OLT, which included either TACE (n = 9), liver resection (n = 2), PEI (n = 1), or RFA (n = 1). Moreover, complete radiological regression was associated with all patients who underwent TACE, PEI, or RFA. Furthermore, two patients were successfully downstaged to the Milan criteria following treatment with TACE. For the two patients who underwent curative resection for HCC, both suffered intrahepatic recurrences one year later and were scheduled to undergo OLT. Due to the use of local ablative procedures, the incidence of major morbidity was 0%.
OLTs involving deceased or living donors were performed by the same surgical team, and standard techniques were used. Briefly, patients received an immunosuppressive regimen of calcineurin inhibitors (i.e., cyclosporine A or tacrolimus), mycophenolic acid, and corticosteroids in the early post-operative period. The latter were tapered and eventually discontinued during the second month following each OLT. For patients with hepatitis B virus (HBV), peri- and post-operative hepatitis B immunoglobulin and an antiviral were administered. Lamivudine-resistant patients were treated with tenofovir. During the follow-up period, serum hepatitis B antibody levels were kept above 200 IU/L, and interferon and ribavirin treatments were initiated if hepatitis C recurred.
All explants were examined by an experienced hepatopathologist (Sagol O), and were categorized depending on the size, number, distribution, HCC histologic grade, and vascular invasion associated with each tumor.
Post-operative death was defined as death within 3 mo post-OLT. All patients underwent regular follow-up examinations in the outpatient clinic. Both the surgical team and an experienced hepatologist maintained surveillance for tumor recurrence or metastasis based on AFP levels and chest CT scans, as well as by contrast-enhanced abdominal CT scans performed once every 3 mo for the first year post-OLT, then once a year thereafter. The minimum follow-up period was 12 mo.
Statistical analyses were performed using SPSS 15.0 for Windows (SPSS, Chicago, Illinois, United States). Data are expressed as the mean ± SD, and median and range values are provided when appropriate. Quantitative variables were compared using the Kruskal-Wallis test. Comparisons between groups with regard to qualitative variables were performed using the chi-square test and Fisher’s exact test, if necessary. Survival was calculated using Kaplan-Meier estimates, with comparisons made using the log rank test. P < 0.05 was considered statistically significant.
The demographic characteristics of the patients included in this study are presented in Table 2. OLT was performed for patients who had been on a waiting list for a median of 62 d. Furthermore, the interval between when the patients were listed for transplantation and when the patients underwent transplantation was similar for both deceased and living donor transplantations (i.e., 60 d vs 68 d, respectively). The average rates of graft weight/body weight for both OLT groups were also 1.09% (range, 0.69-1.8) and 1.82% (range, 0.76-2.58), respectively.
| Variables | Milan + | Milan -/UCSF + | UCSF - | P value |
| No. of patients | 34 | 7 | 15 | |
| Gender (M/F) | 29/5 | 7/0 | 14/1 | |
| Age (yr) | 55.1 ± 6.6 | 51.0 ± 4.5 | 56.4 ± 5.5 | 0.222 |
| CTP (A/B/C) | 8/18/8 | 3/1/2003 | 10/4/2001 | |
| MELD | 13.3 ± 4.9 | 12.7 ± 4.3 | 13.4 ± 4.3 | 0.803 |
| AFP (ng/dL) | 4.9 | 6.1 | 11.9 | 0.953 |
| No. nodules | 1.4 ± 0.6 | 2.4 ± 1.3 | 5.7 ± 4.4 | 0.000 |
| Max diameter of largest nodule (mm) | 22.5 ± 11.6 | 45.9 ± 11.5 | 47.9 ± 23.8 | 0.000 |
| Grade | ||||
| Well | 17 (73.9) | 2 (28.6) | 4 (26.7) | |
| Moderate | 17 (53.1) | 5 (71.4) | 10 (66.7) | |
| Poor | 1 (6.6) | |||
| Microvascular invasion | 6 (17.6)a | 2 (28.6) | 7 (46.7)a | 0.034a |
The mean hospital stay for patients was 31.2 ± 21.5 d, and complications associated with surgery were experienced by 10 (17.8%) recipients. Four (7%) recipients presented with biliary leak, with two of the cases resolving and two of the cases resulting in death due to sepsis. In addition, 3 (5.3%) recipients acquired pneumonia post-operatively. Two of these patients recovered, while the other died from respiratory arrest. One recipient died due to intra-abdominal sepsis and another developed intra-abdominal hemorrhage post-operatively and underwent a second operation. Only one patient experienced a wound infection. In contrast, a total of 4 (7%) patients died due to surgical complications, while another patient died from duodenal ulcer perforation with sepsis. The overall mortality for this study was 8.9% (5/56).
Pre-operative AFP levels ranged from 1.72 to 3630 ng/dL (median, 158.7 ng/dL), with the normal range being 0.5-5 U/L. Only 6/56 (10.7%) patients had an AFP level greater than 200 ng/dL. Furthermore, the mean AFP levels during the pre-OLT period for patients with incidental HCC was 15.5 ± 26.6 ng/dL (range, 2.45-63.1).
Tumor characteristics are described in Table 2. In particular, the number of nodules per patient and the diameter of the largest nodule were significantly lower in the Milan + group compared to the Milan -/UCSF + and UCSF-groups, respectively (P < 0.000).
Adjuvant chemotherapy was an option for 13 (23.2%) patients who were medically eligible for chemotherapy and was administered post-OLT according to pathological tumors with a diameter of > 2 cm and/or microvascular invasion or post-OLT in case of recurrence. These patients received 5-fluorouracil in combination with epirubicin, mitomycin C, or cisplatin. None of the patients died as a result of chemotherapy-related complications. However, for three patients, grade 4 hematologic toxicity was reported, two patients experienced grade 3 gastrointestinal toxicity (i.e., excessive nausea and vomiting), and one patient exhibited grade 3 neurotoxicity.
Tumor recurrence was experienced by 9 (16%) patients (Table 3). Recurrence rates were 5.8%, 14.3% and 40% in the Milan +, Milan -/UCSF + and UCSF-groups, respectively. Five patients presented solely with distant metastasis in the lung (n = 3), in both the lung and bone (n = 1), and in the bone and skin (n = 1). The remaining 4 patients suffered intrahepatic tumor recurrence with (n = 2) or without (n = 2) extrahepatic metastasis (i.e., lung, adrenal gland, bone). None of the 6 recipients with incidental HCC recurred. For the treatment of tumor recurrence, chemotherapy was the only therapeutic option administered.
| Variables | Milan + | Milan -/UCSF + | UCSF - |
| No. of patients | 34 | 7 | 15 |
| Post-operative death | 2 (5.9) | - | 1 (4.5) |
| Death | 5 (14.7) | 2 (28.6) | 11 (73.3) |
| HCC recurrence | 2 (5.8) | 1 (14.3) | 6 (40.0) |
| Median follow-up | 51.5 (1:124) | 32 (1:66) | 14 (3:66) |
The median follow-up period was 39.5 mo (range, 1-124), and at this point, 19 (33.9%) patients had died. Causes of death are listed in Table 4. Correspondingly, the 1-, 3- and 5-year overall survival (OS) rates of the whole series were 80.4%, 68.9%, and 65.3%, respectively. The disease-free survival rates for the same categories were 78.6%, 67.1%, and 67.1%, respectively.
| Causes of death | n (%) |
| Sepsis (late postoperative period) | 7 (36.8) |
| Lung metastasis | 5 (26.3) |
| Sepsis (early postoperative period) | 3 (15.8) |
| Recurrent fulminant hepatitis B | 2 (10.5) |
| Duodenal ulcer perforation | 1 (5.3) |
| Intracranial hemorrhage | 1 (5.3) |
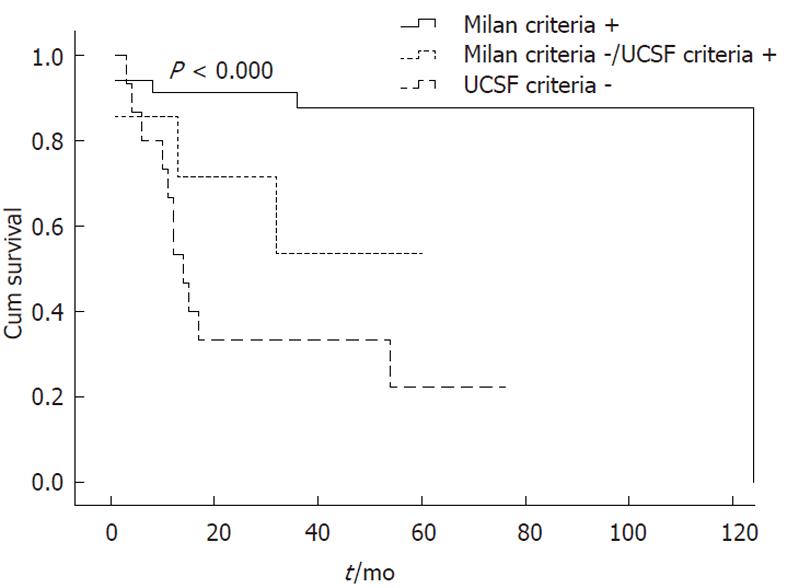
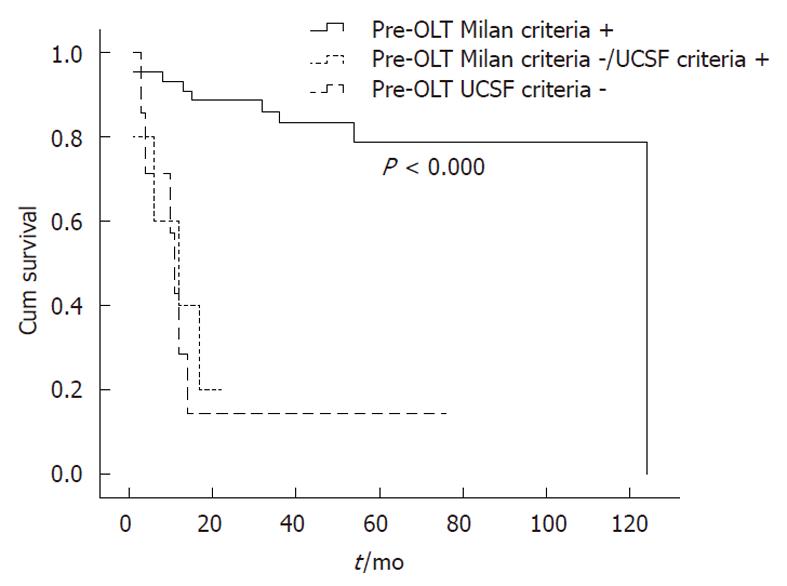
When OS rates were calculated according to the criteria used, the 1-, 3- and 5-year OS rates for the Milan + group were 91.2%, 87.7% and 87.7%, respectively. The mean survival time was 110.3 ± 7.2 mo (95% CI: 96.1-124.4) for this group. In contrast, the 1-, 3- and 5-year OS rates for Milan -/UCSF + patients were 85.7%, 53.6% and 53.6%, respectively. The mean OS period was 39.8 ± 9.1 mo (95% CI: 22.1-57.6). The OS rates for UCSF-patients were 66.7%, 33.3% and 22.2%, respectively, with a mean survival time of 29.8 ± 7.4 mo (95% CI: 15.3-44.4) (P < 0.000) (Figure 1).
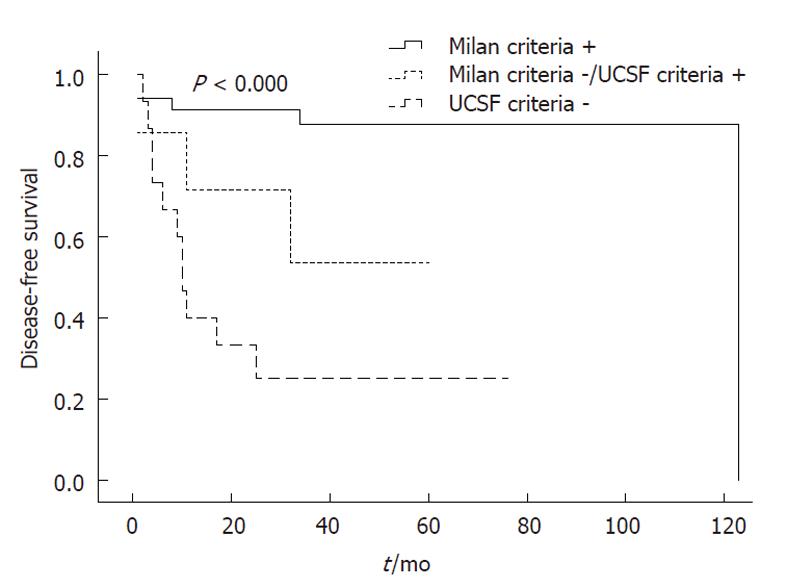
The rates of disease-free survival at 1-, 3- and 5-years post-OLT were 91.2%, 87.7% and 87.7%, respectively, for Milan + patients. Furthermore, the mean disease-free survival period was 109.3 ± 7.2 mo (95% CI: 95.2-123.1). In contrast, the 1- and 3-, and 5-year disease-free survival rates for Milan -/UCSF + patients were 71.4%, 53.6% and 53.6%, respectively, and the mean disease-free survival period was 39.6 ± 9.2 mo (95% CI: 21.6-57.5). The disease-free survival rates for the UCSF-group were 33.3%, 25.0% and 25.0%, respectively, and the mean disease-free survival period was 26.1 ± 7.8 mo (95% CI: 10.9-41.4) (P < 0.000) (Figure 2).
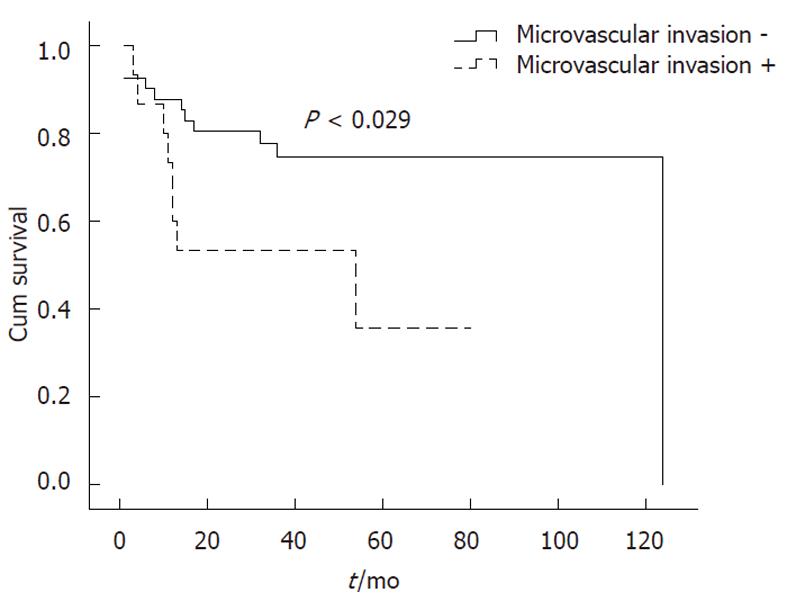
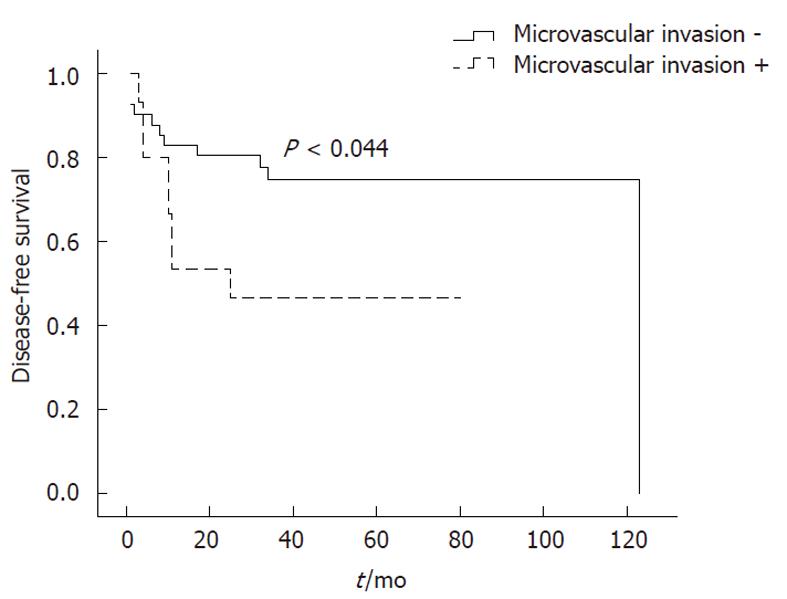
When OS rates were calculated for patients with and without microvascular invasion, the 1-, 3- and 5-year OS rates for each category were 87.8%, 74.7% and 74.7%, and 73.3%, 53.3% and 35.6%, respectively (P < 0.029) (Figure 3). Furthermore, disease-free survival rates were 82.9%, 74.7% and 74.7% for patients without microvascular invasion, and 53.3%, 46.7% and 46.7% for patients with microvascular invasion (P < 0.044) (Figure 4). Moreover, we found that the presence of microvascular invasion was significantly higher in UCSF -than Milan + patients (P < 0.034).
In the three groups which were classified based pre-OLT imaging, OS rates are shown in Figure 5. Among these, the 1-, 3- and 5-year OS rates for Milan + patients were 93.2%, 83.3%, and 78.6%, respectively. The mean survival period was 102.7 ± 7.2 mo (95% CI: 88.5-116.9 mo). In contrast, the OS rates for UCSF-patients were 28.6%, 14.3%, and 14.3%, respectively, with a mean survival period of 18.6 ± 8.9 mo (95% CI: 1.0-36.2 mo) (P < 0.000). When the same evaluations were made for Milan -/UCSF + patients (n = 5), only the 1-, and 2-year OS rates were available and were 40% and 20%, respectively, and the mean survival period was 11.6 ± 3.4 mo (95% CI: 5.0-18.2 mo) (Figure 5).
This retrospective study sought to examine the overall reliability of the Milan and UCSF criteria as clinical tools for the selection of HCC patients to be treated with OLT. Currently, the best liver transplant outcomes for HCC are obtained using the Milan criteria. For these patients, the 5-year survival rates are greater than 70% and the recurrence rate is 15%[8-10]. In 2002, UNOS adopted the “Milan criteria’’ as the optimal criteria for selecting patients for possible OLT due to HCC[6]. However, it was subsequently proposed that the Milan guidelines be expanded based on the comparable survival rates that were being achieved for patients undergoing selection based on the UCSF criteria[7]. Therefore, to investigate whether the Milan criteria are too restrictive for the selection of patients who could otherwise benefit from OLT, a series of HCC cases, who were confirmed by pathology studies of explanted liver specimens, were analyzed. In particular, Milan + patients had significantly better 5-year OS rates than both Milan -/UCSF + and UCSF-patients (87.7% vs 53.6% and 33.3%; P < 0.039 and P < 0.000, respectively). Additionally, Milan -/UCSF + patients who would be expected to obtain the maximum benefit from the proposed expanded criteria had no significant difference in survival rates compared to UCSF-patients (53.6% vs 33.3%, P < 0.239).
In most cases, patient selection criteria are based on radiological imaging performed to assess the extent of intrahepatic disease present, and to exclude extrahepatic spread. However, pre-OLT imaging studies have been shown to underestimate tumor stage in 20%-30% of cases[4,11,12]. Consistent with these results, pre-OLT imaging associated with the series of cases evaluated in this study underestimated either the size, or the number, of tumors present in 14/56 (25%) patients. As a result, 80% of patients identified as Milan -/UCSF + prior to OLT were reclassified as UCSF-following pathological evaluations of the explants obtained. In addition, the 5-year survival rate of these reclassified patients was 25%. Thus, the Milan criteria appear to provide a wider “safe” margin and reduce the negative influence of underestimates of tumor stage by pre-OLT imaging.
As the interval between imaging studies performed and the date of transplantation increases, the patient undergoing transplantation is potentially at a higher risk for tumor recurrence. This could be avoided by shortening the waiting time for a transplant by increasing the number of organ donors, or better utilization of living donors. In this series, OLT was performed a median of 62 d after the patient was placed on a waiting list. In addition, due to the limited number of deceased donors available in Turkey, transplant centers have agreed to allocate deceased donor liver grafts to HCC patients who meet the Milan criteria. Although living donors are currently utilized as a source of liver grafts for the treatment of HCC, the primary concern for transplant programs is minimization of donor morbidity and mortality. Today, living donor hepatectomies are performed safely, and for countries experiencing a shortage in deceased donors, OLTs from living donors shorten the time that patients spend on a waiting list[13]. In our study, 55.4% of the grafts used were obtained from living donors. However, despite all efforts, the morbidity and mortality of living donors following resection of the right lobe of the liver is approximately 0.5% and 35.0%, respectively[14-15]. Thus, considering the safety of living donors and the poor long-term survival rates associated with recipients exceeding the UCSF criteria, it is recommended that the UCSF criteria be followed in order to select HCC patients with the highest likelihood of survival following OLT.
Overall, the recurrence rate (16%) associated with this study was consistent with previous reports[16]. According to the patient groups, the recurrence rates were 5.8%, 14.3% and 40.0% for the Milan +, Milan -/UCSF +, and UCSF-patients, respectively. We hypothesize that these low recurrence rates are associated with the use of the Milan criteria in patient selection and especially for the allocation of deceased donor grafts.
Microvascular invasion is a key step in HCC metastasis. However, as a characteristic of tumor growth that must be determined pathologically, it is impossible to know pre-operatively if it exists. According to previous studies, the presence of microvascular invasion is considered a negative factor for OLT in the treatment of HCC. Correspondingly, in our study microvascular invasion was associated with a significant decrease in 5-year OS rates from 74.7% to 35.6% (P < 0.029). Moreover, we found that the presence of microvascular invasion was significantly higher in UCSF -than Milan + patients (P < 0.034).
There are cases where HCC is detected in explanted livers incidentally, and transplant centers worldwide have reported variable incidences of this situation. In particular, Chui et al[17] and Loinaz et al[18] reported the unexpected incidence of HCC to be 1.4% and 2.8%, respectively. In other series, slightly higher incidences of 7% and 8% have been reported[19,20]. This discrepancy could be partly due to the thickness of the liver sections used for pathologic examination. In the present series, the rate of unexpected HCC incidence was 10.7% (6/56). The mean tumor diameter associated with these cases was 14.7 mm, and the maximum nodule size was less than 20 mm. Furthermore, none of these tumors exhibited radiological features that are typically associated with HCC. Serum AFP levels for 5/6 of these patients were also within the normal range, while one patient had an AFP level of 63.1 ng/dL. However, previous studies have demonstrated that AFP levels are not a reliable indicator for the diagnosis of HCC[8], and this was consistent with our results. In addition, confirmatory biopsies were not performed for these cases since almost all of the patients had diagnostic findings identified in the imaging studies conducted. Therefore, our results indicate that the current guidelines of the American Association for the Study of Liver disease can provide a reliable diagnosis of HCC[8].
In conclusion, pre-OLT imaging continues to have a relatively high false negative rate for HCC patients considered for transplantation. The inaccuracy of imaging modalities for identification of tumor characteristics such as size and number may result in the selection of patients with unfavourable survival outcome for OLT. Based on the cases analyzed in this study, it would appear that the Milan criteria are very useful and safe in selecting recipients who will benefit from OLT. Therefore, given the limited number of deceased liver grafts available, the Milan criteria should be followed in the selection of suitable candidates for OLT for the treatment of HCC. In contrast, for cases of OLT involving living donors, the UCSF criteria may be applied. In addition, future advancement in imaging modalities may further improve the reliability and applicability of these selection criteria.
Liver transplantation offers the best long-term effective treatment for patients with hepatocellular carcinoma (HCC) in cirrhosis. Patients who fulfill the Milan criteria may have a 5-year survival of up to 88%. However, application of the Milan criteria might lead to the exclusion of patients who otherwise would benefit from orthotopic liver transplantation (OLT). Several studies have evaluated more liberal criteria for tumor staging which could be adopted without significant impairment of patient survival or tumor recurrence. However, the expansion of tumor-specific criteria for transplantation raises concerns about rational use of scarce deceased donor organs and safety issues in living donation.
The role of OLT in patients with HCC beyond the Milan criteria is a matter of debate. Several different selection criteria have been proposed to reach an optimum survival outcome after OLT. Improvements in imaging modalities and markers of aggressive tumor biology may help in selecting patients with better outcome after OLT.
In a substantial portion of HCC patients, pre-OLT imaging studies underestimate the extent of the disease. Patients who were reclassified to higher tumor stages after pathological evaluations of the explants had poor survival after OLT. The Milan criteria appear to provide a wider safe margin and reduce the negative influence of underestimates of tumor stage by pre-OLT imaging.
In the face of low deceased donor organ availability and safety issues in living donation, transplanting patients with HCC who meet the Milan criteria appears to have optimum benefit on patient survival. Better understanding of tumor behavior may help in selecting patients who may benefit from OLT even with higher tumor burden (expanded selection criteria).
Despite several studies in the literature about the Milan and University of California San Francisco (UCSF) criteria, this study for the first time suggests Milan criteria for safer preoperative radiological staging over the UCSF criteria.
Peer reviewer: Cuneyt Kayaalp, MD, Professor, Department of General Surgery, Staff Surgeon of Gastrointestinal Surgery, Turgut Ozal Medical Center, Inonu University, Malatya 44315, Turkey
S- Editor Tian L L- Editor Webster JR E- Editor Zhang DN
| 1. | Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003;362:1907-1917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3241] [Cited by in RCA: 3282] [Article Influence: 149.2] [Reference Citation Analysis (0)] |
| 2. | El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557-2576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3846] [Cited by in RCA: 4265] [Article Influence: 236.9] [Reference Citation Analysis (2)] |
| 3. | Stone HL, Meyer TC, Schilling R. Alternative medical school curriculum design: the independent study program. Med Teach. 1991;13:149-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 47] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 4. | Abrams P, Marsh JW. Current approach to hepatocellular carcinoma. Surg Clin North Am. 2010;90:803-816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 37] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 5. | Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, Montalto F, Ammatuna M, Morabito A, Gennari L. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693-699. [PubMed] |
| 6. | Jarnagin W, Chapman WC, Curley S, D'Angelica M, Rosen C, Dixon E, Nagorney D. Surgical treatment of hepatocellular carcinoma: expert consensus statement. HPB (Oxford). 2010;12:302-310. [PubMed] |
| 7. | Yao FY, Ferrell L, Bass NM, Watson JJ, Bacchetti P, Venook A, Ascher NL, Roberts JP. Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology. 2001;33:1394-1403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1594] [Cited by in RCA: 1695] [Article Influence: 70.6] [Reference Citation Analysis (0)] |
| 8. | Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208-1236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4333] [Cited by in RCA: 4507] [Article Influence: 225.4] [Reference Citation Analysis (0)] |
| 9. | Bruix J, Sherman M, Llovet JM, Beaugrand M, Lencioni R, Burroughs AK, Christensen E, Pagliaro L, Colombo M, Rodés J. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol. 2001;35:421-430. [PubMed] |
| 10. | Fuster J, Charco R, Llovet JM, Bruix J, García-Valdecasas JC. Liver transplantation in hepatocellular carcinoma. Transpl Int. 2005;18:278-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 11. | Befeler AS, Hayashi PH, Di Bisceglie AM. Liver transplantation for hepatocellular carcinoma. Gastroenterology. 2005;128:1752-1764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 95] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 12. | Yao FY, Xiao L, Bass NM, Kerlan R, Ascher NL, Roberts JP. Liver transplantation for hepatocellular carcinoma: validation of the UCSF-expanded criteria based on preoperative imaging. Am J Transplant. 2007;7:2587-2596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 382] [Cited by in RCA: 431] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 13. | Rampone B, Schiavone B, Martino A, Viviano C, Confuorto G. Current management strategy of hepatocellular carcinoma. World J Gastroenterol. 2009;15:3210-3216. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 94] [Cited by in RCA: 106] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 14. | Barr ML, Belghiti J, Villamil FG, Pomfret EA, Sutherland DS, Gruessner RW, Langnas AN, Delmonico FL. A report of the Vancouver Forum on the care of the live organ donor: lung, liver, pancreas, and intestine data and medical guidelines. Transplantation. 2006;81:1373-1385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 273] [Cited by in RCA: 263] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 15. | Ozkardesler S, Ozzeybek D, Alaygut E, Unek T, Akan M, Astarcioglu H, Karademir S, Astarcioglu I, Elar Z. Anesthesia-related complications in living liver donors: the experience from one center and the reporting of one death. Am J Transplant. 2008;8:2106-2110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 16. | Decaens T, Roudot-Thoraval F, Hadni-Bresson S, Meyer C, Gugenheim J, Durand F, Bernard PH, Boillot O, Sulpice L, Calmus Y. Impact of UCSF criteria according to pre- and post-OLT tumor features: analysis of 479 patients listed for HCC with a short waiting time. Liver Transpl. 2006;12:1761-1769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 123] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 17. | Chui AK, Rao AR, McCaughan GW, Waugh R, Verran DJ, Koorey D, Painter D, Sheil AG. An active liver transplant programme for hepatocellular carcinoma in cirrhotic patients: is it justified? Clin Transplant. 1999;13:531-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 18. | Loinaz C, Abradelo M, Gómez R, Colina F, Rey P, Ochando F, Cañete AR, González-Pinto I, Jiménez C, García I. Liver transplantation and incidental primary liver tumors. Transplant Proc. 1998;30:3301-3302. [PubMed] |
| 19. | Ferrell L, Wright T, Lake J, Roberts J, Ascher N. Incidence and diagnostic features of macroregenerative nodules vs. small hepatocellular carcinoma in cirrhotic livers. Hepatology. 1992;16:1372-1381. [PubMed] |
| 20. | Hytiroglou P, Theise ND, Schwartz M, Mor E, Miller C, Thung SN. Macroregenerative nodules in a series of adult cirrhotic liver explants: issues of classification and nomenclature. Hepatology. 1995;21:703-708. [PubMed] |