Published online Sep 28, 2011. doi: 10.3748/wjg.v17.i36.4099
Revised: February 19, 2011
Accepted: February 26, 2011
Published online: September 28, 2011
AIM: To evaluate whether combination therapy with anti-tumour necrosis factor α (TNFα) antibody and Zn acetate is beneficial in dextran sodium sulphate (DSS) colitis.
METHODS: Colitis was induced in CD1-Swiss mice with 5% DSS for 7 d. The experimental mice were then randomised into the following subgroups: standard diet + DSS treated (induced colitis group); standard diet + DSS + subcutaneous 25 μg anti-TNFα treated group; Zn acetate treated group + DSS + subcutaneous 25 μg anti-TNFα; standard diet + DSS + subcutaneous 6.25 μg anti-TNFα treated group and Zn acetate treated group + DSS + subcutaneous 6.25 μg anti-TNFα. Each group of mice was matched with a similar group of sham control animals. Macroscopic and histological features were scored blindly. Homogenates of the colonic mucosa were assessed for myeloperoxidase activity as a biochemical marker of inflammation and DNA adducts (8OH-dG) as a measure of oxidative damage.
RESULTS: DSS produced submucosal erosions, ulcers, inflammatory cell infiltration and cryptic abscesses which were reduced in both groups of mice receiving either anti-TNFα alone or combined with zinc. The effect was more pronounced in the latter group (vs Zn diet, P < 0.02). Myeloperoxidase activity (vs controls, P < 0.02) and DNA adducts, greatly elevated in the DSS fed colitis group (vs controls, P < 0.05), were significantly reduced in the treated groups, with a more remarkable effect in the group receiving combined therapy (vs standard diet, P < 0.04).
CONCLUSION: DSS induces colonic inflammation which is modulated by the administration of anti-TNFα. Combining anti-TNFα with Zn acetate offers marginal benefit in colitis severity.
- Citation: Barollo M, Medici V, D’Incà R, Banerjee A, Ingravallo G, Scarpa M, Patak S, Ruffolo C, Cardin R, Sturniolo GC. Antioxidative potential of a combined therapy of anti TNFα and Zn acetate in experimental colitis. World J Gastroenterol 2011; 17(36): 4099-4103
- URL: https://www.wjgnet.com/1007-9327/full/v17/i36/4099.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i36.4099
Ulcerative colitis and Crohn’s disease are chronic diseases of the gastrointestinal tract characterized by activation of the immune system with production of several inflammatory cytokines[1,2]. Altered T cell apoptosis[3,4] and abnormal production of the pro-inflammatory cytokine tumour necrosis factor α (TNFα) play a central role in intestinal inflammation of inflammatory bowel disease patients[5].
Novel treatment strategies based on the inhibition of TNFα have shown to be effective both in experimental models of colitis[6] and in inducing and maintaining remission in humans affected with inflammatory bowel disease[7]. However, as these therapies are very expensive they may represent an important and unaffordable economic burden in the near future.
Trace element metabolism is altered during inflammatory processes of the gastrointestinal tract. Zinc is essential for intestinal homeostasis, since there are several zinc-dependent antioxidant enzymes such as superoxide dismutase which converts superoxide to hydrogen peroxide and metallothionein which can neutralize free radical production. Moreover, zinc status affects gene expression of the inflammatory cytokines TNF, IL-1B and IL-8. Zinc deficiency causes functional defects in T cells, neutrophils and macrophages, and positive modulatory responses are produced following zinc supplementation[8]. In the model of acetic acid-induced ulcerations, zinc reduced mucosal damage[9]. In models of experimental colitis both oral and topical zinc treatment were found to decrease intestinal inflammation, to favour mucosal healing and to improve immune function[10], We therefore thought that zinc may be useful if added to conventional anti-TNFα therapy in modulating the symptoms of dextran sodium sulphate (DSS)-induced colitis in mice and in decreasing oxidative stress.
Male CD1 Swiss mice, 4 wk old, weighing 20-25 g purchased from Charles River (Calco, Italy) were used in this study. The animals were kept in plastic platform cages in a temperature controlled room (22 °C) under a 12-h light-dark cycle, with free access to water and standard chow containing 125 mg/kg zinc oxide. The experimental protocol was approved by the Veterinary and Health Committee of the University of Padua.
Mice were fed 5% DSS (5% dextran sulphate solution purchased from ICN Pharmaceuticals, SRL, Italy) dissolved in drinking water in one single cycle to induce acute colitis. The cycle consisted of administering 5% DSS for 7 d which caused loose stools in all animals and the presence of gross rectal bleeding in about 50% of the animals.
The animals were randomised into the following six groups each with 6 mice: (1) healthy untreated mice receiving standard diet; (2) induced colitis group, i.e., mice receiving standard diet + 5% DSS for 7 d; (3) mice receiving standard diet + 675 mg/kg Zn acetate supplement starting 7 d before induction of colitis; (4) mice receiving standard diet + 25 μg anti-TNFα intraperitoneally after 1 wk of DSS administration; (5) mice receiving standard diet + 675 mg/kg Zn acetate supplement + 25 μg anti-TNFα intraperitoneally after 1 wk of DSS administration; and (6) mice receiving standard diet + 675 mg/kg Zn acetate supplement + 6.25 μg anti-TNFα intraperitoneally after 1 wk of DSS administration. The three groups receiving anti-TNFα treatment were sacrificed 48 h after initiation of treatment. Anti-TNFα monoclonal antibody (rat anti-mouse TNFα) was purchased from Biosource International Inc. (United States) and Zn Acetate 675 mg/kg diet, from Mucedola SRL, (Milano, Italy).
Damage was assessed macroscopically by scoring the number and extent of ulcers, adhesions, and thickness of the colonic wall[11] and histologically by scoring cryptitis, crypt abscesses and epithelial injury. Colonic tissue samples were obtained and processed for myeloperoxidase and 8-hydroxydeoxyguanosine (8-OHdG) in order to quantify inflammation and DNA damage.
Colonic samples were immediately fixed in buffered formalin (10%). After fixation, the specimens were routinely processed and embedded in paraffin. Serial histology sections of 4 μm thickness were obtained from each paraffin block and mounted on poly-L-lysine coated slides. Sections were stained with haematoxylin-eosin and examined blindly.
Cryptitis was defined as the presence of polymorphonuclear cells within crypt epithelium, while crypt abscesses were defined as the presence of polymorphonuclear cells within the crypt lumens. Epithelial injury included changes such as crypt regeneration, mucodepletion, cuboidal shape, nuclear enlargement, loss of surface cells, erosion, and ulceration. Each of the features, defined above, was scored on a 0 to 3+ scale based on the severity and degree of involvement[12,13].
Mean colonic activity scores for cryptitis, crypt abscesses and epithelial injury were marked for each slide on the following basis: 0 (no activity); 1-2 (mild activity); 3-4 (moderate activity); 5-6 (severe activity).
Assessment of myeloperoxidase (MPO) activity was assessed according to the method previously described[14]. Briefly, colonic tissue samples were minced in 1 mL of 50 mmol/L potassium phosphate buffer (pH = 6.0) containing 14 mmol/L hexadecyltrimethylammonium bromide (Fluka), homogenized and sonicated. The lysates were frozen and thawed thrice, then centrifuged for 2 min in cold at 1 5000 g. Aliquots of the supernatants were mixed with potassium phosphate buffer containing o-dianisidine-HCl (Sigma-Aldrich, St. Louis, MO, United States) and 0.0005% H2O2. MPO activity was expressed as units/g of wet tissue. The enzyme unit was defined as the conversion of 1 mol of H2O2 per min at 25.
Oxidative DNA damage was assessed following previously described methods[15]. Briefly, colonic biopsy specimens were thawed, homogenized in a separation buffer and approximately 20 μg of purified DNA per sample was injected into the HPLC system (Shimadzu, Kyoto, Japan). The 8-OHdG was detected using an electrochemical detector (ESA Coulochem II 5200A, Bedford, MA, Untied States). The levels of 8-OHdG were expressed as the number of 8-OHdG adducts per 105 dG bases. The coefficient of variation was < 10%; 100 μg of DNA were required for the determination.
Data are expressed as median (interquartile range). Statistical data were analyzed with Mann-Whitney U test for comparison of the groups and Spearman’s rank correlation test. P values less than 0.05 were considered significant.
The macroscopic score was increased significantly in untreated colitic mice. Groups treated with anti-TNFα or anti-TNFα and zinc acetate showed a decreased macroscopic score which was more evident in the combined diet. Chronic feeding of DSS significantly increased the colonic activity score. The administration of anti-TNFα alone or combined with zinc acetate significantly reduced this index. The effect appeared to be significantly more evident in the group receiving anti-TNFα and zinc acetate than in the group receiving anti-TNFα alone. The administration of a reduced dose of anti-TNFα (6.25 μg) was effective only if combined with zinc acetate (Table 1).
| Macroscopic score | Colonic activity index | Myeloperoxidase activity (U/g) | |
| Controls | 0 (0-0) | 0 (0-0) | 1.9 (1.34-1.1) |
| Colitis | 1 (1-1)b | 4 (1-1)a | 5.69 (0.04-0.21)b |
| Colitis + Zinc | 2 (2-2)b | 5 (0-0)a | 7.8 (0-0.8)e |
| Colitis + anti-TNFα (25 μg) | 0.5 (0-1) | 4 (1-3)a | 4.85 (1.81-3)d |
| Colitis + Zinc + anti-TNFα (25 μg) | 0 (0-0)c | 1 (2-0)ad | 3.88 (2.9-2.87) |
| Colitis + anti-TNFα (6.25 μg) | 1 (0.25-1) | 5 (0-2) | 5.44 (0.63-0) |
| Colitis + Zinc + anti-TNFα (6.25 μg) | 0.5 (0-1) | 3 (2-0) | 4.42 (0.34-0.33) |
Myeloperoxidase activity was increased in all colitic mice. However, there was a significant reduction in this activity in the groups treated with anti-TNFα alone and anti-TNFα + Zn supplementation, with a slightly better effect in the group receiving the combination therapy. A lower dose of anti-TNFα was associated with reduced MPO activity only in the group receiving both zinc and anti-TNFα (Table 1).
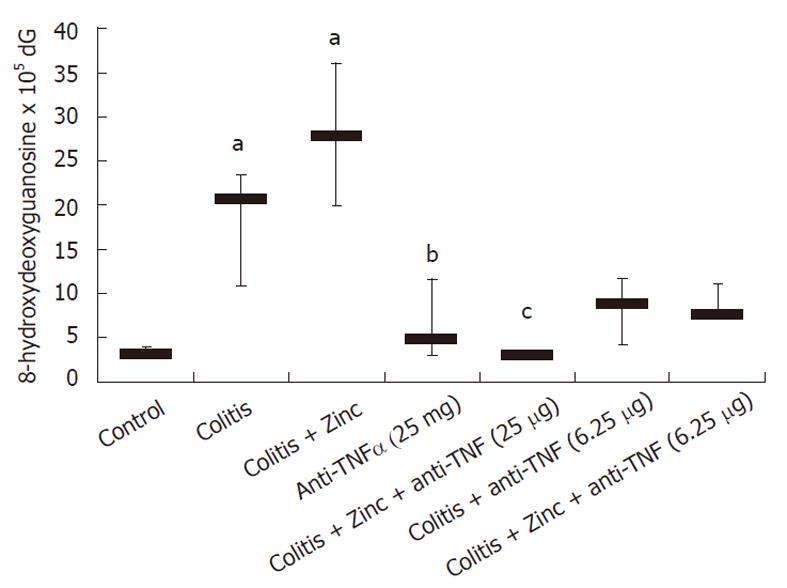
Oxidative damage was significantly increased in colitic mice. Anti-TNFα significantly reduced DNA adducts, OH-dG levels were similar in the group receiving both anti-TNFα and zinc acetate (Figure 1). Anti-TNFα treatment significantly reduced DNA adducts at both doses used. In both groups receiving the combination therapy, DNA adducts were reduced compared to anti-TNFα therapy alone, but no significant effect was demonstrated with respect to the groups receiving anti-TNFα alone (Figure 1).
Chemically induced models of intestinal inflammation are widely used as surrogate models of chronic inflammatory bowel disease and oral DSS administration effectively resembles human inflammatory bowel disease with similar clinical features (bloody diarrhoea) and endoscopic/histological findings (ulcerations and neutrophil infiltration). DSS is believed to be directly toxic to gut epithelial cells of the basal crypts and affects the integrity of the mucosal barrier.
Zinc metabolism has been reported to be reduced in about 65% of patients with Crohn’s disease. In an experimental model of colitis we also reported that zinc supplementation induced metallothionein expression, while having little effect on the short-term course of colitis[16]. Zinc has several potential mechanisms of action which can benefit the inflammatory process. It regulated tight junction permeability in an experimental model of colitis[17] and in Crohn disease[18]. Sturniolo et al[19] reported that zinc sulphate enemas exert an anti-inflammatory action on experimental colitis.
In the last few years, biological therapies have changed the pharmacological armamentarium of inflammatory bowel disease therapy: the first and still most widely used drug is the anti-TNFα monoclonal antibody, infliximab[20]. Even in experimental models of colitis, the subcutaneous administration of infliximab reduced the inflammatory activity as well as tissue TNFα concentration[21]. Our experimental approach which added zinc acetate to the diet while administering anti-TNFα monoclonal antibody, aimed to examine the effects on DSS-induced colitis in mice.
The mucosa did not show complete healing probably because the treatment effects were evaluated 48 h after treatment. Nevertheless, therapy with anti-TNFα ameliorated the macroscopic and histological aspects and decreased myeloperoxidase concentration. Similar results were reported by Videla et al[22] who found that anti-TNFα significantly reduced the release of inflammatory mediators and induced histopathological remission in a model of experimental colitis. Zinc alone had little effect in ameliorating the severity of acute colitis induced by intra-rectal instillation of dinitrobenzene-sulphonic acid in rats, even though Tran et al[23] and Luk et al[24] recently reported some therapeutic effects of zinc supplementation in DSS-induced colitis in mice.
Zinc supplementation alone worsened the histopathological and biochemical aspects of colitis compared to colitis alone and this can be explained by the fact that superoxide dismutase by itself is a pro-oxidant enzyme by virtue of its ability to generate hydrogen peroxide[25,26]. This may explain why a worsening of colitis was recorded when zinc was added alone. However, in our study zinc allowed us to reduce the dose of anti-TNFα maintaining the same biochemical and morphological effects.
Acute colitis is characterised by an increased production of free radicals which contribute to protein, DNA chain and lipid damage. As the antioxidant potential of colonic epithelial cells is quite low, this results in tissue injury[27]. The administration of antioxidants thus has the potential to improve the outcome of experimental colitis by scavenging free radicals. In our experimental conditions, the oxidative damage, expressed by DNA adducts was significantly reduced in the groups treated with anti-TNFα confirming the findings of Popivanova et al[28]. In our study, the effect of anti-TNFα on oxidative stress appeared to be dose-dependent with the highest dose having the strongest effect in reducing oxidative damage, and the combination of anti-TNFα and zinc supplementation added little effect.
Obermeier et al[29] reported that excess nitric oxide formation occurs in experimental colitis and can be decreased by treatment with rat anti-mouse TNF and interferon gamma monoclonal antibodies. In several studies, zinc supplementation ameliorated antioxidant concentrations thus reducing the production of oxidative species[27-30]. In the present study, zinc supplementation allowed a reduction in the dose of anti-TNFα antibody, while maintaining the same level of reduced intestinal inflammation observed with a higher dose of anti-TNFα antibody alone, as quantified by the four parameters of tissue inflammation utilized in the study. This effect is in accordance with the described capability of zinc to increase antioxidant concentration and reduce oxidative species.
In conclusion, the combined administration of zinc acetate in the diet along with the systemic administration of anti-TNFα had a positive effect in reducing the severity of DSS-induced colitis in mice, with reduced production of DNA adducts. Moreover, the same effect was demonstrated with the reduced anti-TNFα dose combined with zinc. This experimental approach offers the advantage of reducing the potential side effects of anti-TNFα and costs, while ameliorating oxidative stress and inflammation in patients with inflammatory bowel disease.
Dextran sodium sulphate (DSS) colitis is a well-known model of inflammatory bowel disease in which the authors tested the effect of the well-known drug anti-tumour necrosis factor α (TNFα) combined with zinc with the aim of evaluating the possibility of lowering the dose of anti-TNFα.
In this article the authors explore the possibility of a combination therapy in inflammatory bowel disease (IBD) in order to reduce potential side effects and costs.
In this article, zinc was added to a biological therapy in order to evaluate the effects of this combination therapy. There are no articles in the literature exploring this combination therapy.
The potential applications include the possibility of adding zinc to anti-TNFα therapy. Moreover, future perspectives include the application of other combination therapies in inflammatory bowel disease.
This study considers the investigation of the role of combined administration of Zinc acetate in the diet with systemic administration of anti TNF alfa on the effect of the severity of experimental colitis in mice induced by DSS. The study is set up correctly. The paper is written sufficiently good, the Introduction give a good overview about the study background and the authors raised clearly the hypothesis of the study. The description of methods used is accurate. The results are presented clearly and have been discussed well, the table and figure give good overview about the results.
Peer reviewers: Dr. Tamara Vorobjova, MD, PhD, Scimed. Senior Researcher in Immunology, Department of Immunology, Institute of General and Molecular Pathology, University of Tartu, Ravila, 19, Tartu 51014, Estonia; Jay Pravda, MD, Inflammatory Disease Research Center, West Palm Beach, FL 33420, United States
S- Editor Sun H L- Editor Webster JR E- Editor Xiong L
| 1. | Torres MI, Rios A. Current view of the immunopathogenesis in inflammatory bowel disease and its implications for therapy. World J Gastroenterol. 2008;14:1972-1980. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 66] [Cited by in RCA: 70] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 2. | Sanchez-Munoz F, Dominguez-Lopez A, Yamamoto-Furusho JK. Role of cytokines in inflammatory bowel disease. World J Gastroenterol. 2008;14:4280-4288. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 440] [Cited by in RCA: 514] [Article Influence: 30.2] [Reference Citation Analysis (2)] |
| 3. | Nielsen OH, Vainer B, Madsen SM, Seidelin JB, Heegaard NH. Established and emerging biological activity markers of inflammatory bowel disease. Am J Gastroenterol. 2000;95:359-367. [PubMed] |
| 4. | Shanahan F. Crohn’s disease. Lancet. 2000;359:62-69. [RCA] [DOI] [Full Text] [Cited by in Crossref: 358] [Cited by in RCA: 353] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 5. | Papadakis KA, Targan SR. Tumor necrosis factor: biology and therapeutic inhibitors. Gastroenterology. 2000;119:1148-1157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 255] [Cited by in RCA: 259] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 6. | Worledge KL, Godiska R, Barrett TA, Kink JA. Oral administration of avian tumor necrosis factor antibodies effectively treats experimental colitis in rats. Dig Dis Sci. 2000;45:2298-2305. [PubMed] [DOI] [Full Text] |
| 7. | Akobeng AK, Zachos M. Tumor necrosis factor-alpha antibody for induction of remission in Crohn's disease. Cochrane Database Syst Rev. 2004;CD003574. [PubMed] |
| 8. | Filippi J, Al-Jaouni R, Wiroth JB, Hébuterne X, Schneider SM. Nutritional deficiencies in patients with Crohn's disease in remission. Inflamm Bowel Dis. 2006;12:185-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 188] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 9. | Troskot B, Simicevic VN, Dodig M, Rotkvic I, Ivankovic D, Duvnjak M. The protective effect of zinc sulphate pretreatment against duodenal ulcers in the rat. Biometals. 1997;10:325-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 10. | Bucci I, Napolitano G, Giuliani C, Lio S, Minnucci A, Di Giacomo F, Calabrese G, Sabatino G, Palka G, Monaco F. Zinc sulfate supplementation improves thyroid function in hypozincemic Down children. Biol Trace Elem Res. 1999;67:257-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 36] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 11. | Morris GP, Beck PL, Herridge MS, Depew WT, Szewczuk MR, Wallace JL. Hapten-induced model of chronic inflammation and ulceration in the rat colon. Gastroenterology. 1989;96:795-803. [PubMed] |
| 12. | Jenkins D, Balsitis M, Gallivan S, Dixon MF, Gilmour HM, Shepherd NA, Theodossi A, Williams GT. Guidelines for the initial biopsy diagnosis of suspected chronic idiopathic inflammatory bowel disease. The British Society of Gastroenterology Initiative. J Clin Pathol. 1997;50:93-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 195] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 13. | Tanaka M, Riddell RH, Saito H, Soma Y, Hidaka H, Kudo H. Morphologic criteria applicable to biopsy specimens for effective distinction of inflammatory bowel disease from other forms of colitis and of Crohn's disease from ulcerative colitis. Scand J Gastroenterol. 1999;34:55-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 101] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 14. | Krawisz JE, Sharon P, Stenson WF. Quantitative assay for acute intestinal inflammation based on myeloperoxidase activity. Assessment of inflammation in rat and hamster models. Gastroenterology. 1984;87:1344-1350. [PubMed] |
| 15. | Helbock HJ, Beckman KB, Shigenaga MK, Walter PB, Woodall AA, Yeo HC, Ames BN. DNA oxidation matters: the HPLC-electrochemical detection assay of 8-oxo-deoxyguanosine and 8-oxo-guanine. Proc Natl Acad Sci USA. 1998;95:288-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 504] [Cited by in RCA: 482] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 16. | Di Leo V, D'Incà R, Barollo M, Tropea A, Fries W, Mazzon E, Irato P, Cecchetto A, Sturniolo GC. Effect of zinc supplementation on trace elements and intestinal metallothionein concentrations in experimental colitis in the rat. Dig Liver Dis. 2001;33:135-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 17. | Sturniolo GC, Di Leo V, Ferronato A, D'Odorico A, D'Incà R. Zinc supplementation tightens "leaky gut" in Crohn's disease. Inflamm Bowel Dis. 2001;7:94-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 117] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 18. | Chen BW, Wang HH, Liu JX, Liu XG. Zinc sulphate solution enema decreases inflammation in experimental colitis in rats. J Gastroenterol Hepatol. 1999;14:1088-1092. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 19. | Sturniolo GC, Fries W, Mazzon E, Di Leo V, Barollo M, D'inca R. Effect of zinc supplementation on intestinal permeability in experimental colitis. J Lab Clin Med. 2002;139:311-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 93] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 20. | Blam ME, Stein RB, Lichtenstein GR. Integrating anti-tumor necrosis factor therapy in inflammatory bowel disease: current and future perspectives. Am J Gastroenterol. 2001;96:1977-1997. [PubMed] |
| 21. | Triantafillidis JK, Papalois AE, Parasi A, Anagnostakis E, Burnazos S, Gikas A, Merikas EG, Douzinas E, Karagianni M, Sotiriou H. Favorable response to subcutaneous administration of infliximab in rats with experimental colitis. World J Gastroenterol. 2005;11:6843-6847. [PubMed] |
| 22. | Videla S, García-Lafuente A, Antolín M, Vilaseca J, Guarner F, Crespo E, González G, Salas A, Malagelada JR. Antitumor necrosis factor therapy in rat chronic granulomatous colitis: critical dose-timing effects on outcome. J Pharmacol Exp Ther. 1998;287:854-859. [PubMed] |
| 23. | Tran CD, Ball JM, Sundar S, Coyle P, Howarth GS. The role of zinc and metallothionein in the dextran sulfate sodium-induced colitis mouse model. Dig Dis Sci. 2007;52:2113-2121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 51] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 24. | Luk HH, Ko JK, Fung HS, Cho CH. Delineation of the protective action of zinc sulfate on ulcerative colitis in rats. Eur J Pharmacol. 2002;443:197-204. |
| 25. | Koningsberger JC, van Asbeck BS, van Faassen E, Wiegman LJ, van Hattum J, van Berge Henegouwen GP, Marx JJ. Copper, zinc-superoxide dismutase and hydrogen peroxide: a hydroxyl radical generating system. Clin Chim Acta. 1994;230:51-61. [PubMed] |
| 26. | Yim MB, Chock PB, Stadtman ER. Copper, zinc superoxide dismutase catalyzes hydroxyl radical production from hydrogen peroxide. Proc Natl Acad Sci USA. 1990;87:5006-5010. [PubMed] |
| 27. | Lih-Brody L, Powell SR, Collier KP, Reddy GM, Cerchia R, Kahn E, Weissman GS, Katz S, Floyd RA, McKinley MJ. Increased oxidative stress and decreased antioxidant defenses in mucosa of inflammatory bowel disease. Dig Dis Sci. 1996;41:2078-2086. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 316] [Cited by in RCA: 340] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 28. | Popivanova BK, Kitamura K, Wu Y, Kondo T, Kagaya T, Kaneko S, Oshima M, Fujii C, Mukaida N. Blocking TNF-alpha in mice reduces colorectal carcinogenesis associated with chronic colitis. J Clin Invest. 2008;118:560-570. [PubMed] |
| 29. | Obermeier F, Kojouharoff G, Hans W, Schölmerich J, Gross V, Falk W. Interferon-gamma (IFN-gamma)- and tumour necrosis factor (TNF)-induced nitric oxide as toxic effector molecule in chronic dextran sulphate sodium (DSS)-induced colitis in mice. Clin Exp Immunol. 1999;116:238-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 261] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 30. | Mulder TP, van der Sluys Veer A, Verspaget HW, Griffioen G, Peña AS, Janssens AR, Lamers CB. Effect of oral zinc supplementation on metallothionein and superoxide dismutase concentrations in patients with inflammatory bowel disease. J Gastroenterol Hepatol. 1994;9:472-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 0.8] [Reference Citation Analysis (0)] |