Published online Sep 21, 2011. doi: 10.3748/wjg.v17.i35.4038
Revised: March 11, 2011
Accepted: March 18, 2011
Published online: September 21, 2011
AIM: To assess the diagnostic value of computed tomography (CT) imaging in screening for abdominal nonhematogenous disseminated tuberculous lymphadenopathy (TL).
METHODS: The CT scans of 12 patients with abdominal nonhematogenous disseminated TL suggestive of neoplasm were retrospectively analyzed in this review. The final diagnoses were confirmed by lymph node pathology for seven patients and by laparoscopic surgery for five patients. All of the patients were treated at our institution between April 1995 and August 2009.
RESULTS: The sites of involvement were the periportal (n = 6), peripancreatic (n = 3), periaortic (n = 3), and mesenteric (n = 2) regions. On the plain CT scan, the lymphadenopathy showed a heterogeneous isodensity or hypodensity in 11 patients and a low density in one patient. Peripheral enhancement was observed on the dynamic contrast-enhanced CT scans for all patients. In two cases, scans were more revealing during the portal venous and delayed phases.
CONCLUSION: Abdominal lymphadenopathy with predominant peripheral rim-like enhancement on the dynamic contrast-enhanced CT scan may suggest a diagnosis of TL.
- Citation: Zhang M, Li M, Xu GP, Liu HJ. Neoplasm-like abdominal nonhematogenous disseminated tuberculous lymphadenopathy: CT evaluation of 12 cases and literature review. World J Gastroenterol 2011; 17(35): 4038-4043
- URL: https://www.wjgnet.com/1007-9327/full/v17/i35/4038.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i35.4038
The global health burden of tuberculosis (TB) has risen in recent years. In 2009, an estimated 9.4 million cases and 1.7 million deaths globally could be attributed to TB. More than one-third of these cases were found in Southeast Asia[1]. Although the respiratory system remains the primary disease site, TB also increasingly affects the gastrointestinal tract, peritoneum, lymph nodes, kidneys, and other solid viscera. Intra-abdominal TB (1%-3%) constitutes up to 12% of extra-pulmonary TB, particularly in immunocompromised individuals[2,3]. Tuberculous lymphadenopathy (TL) is one of the most common findings in patients with abdominal TB. The presence of active pulmonary tuberculosis may indicate the possibility of abdominal TB involvement; however, only 15% of patients with abdominal TB have any evidence of pulmonary involvement[4]. Clinically, patients with abdominal nonhematogenous disseminated TL typically present with an isolated mass or a mass adhering to the surrounding organs. When abdominal neoplasm is suspected, computed tomography (CT) is used to examine the abdomen. With the widespread use of CT, physicians should be familiar with the features of CT images seen in these patients and be able to make differential diagnoses using CT findings. In this article, we present 12 patients with abdominal nonhematogenous disseminated TL and discuss the features observed on their CT scans.
We collected and retrospectively reviewed the CT scans of 12 patients who were diagnosed with abdominal nonhematogenous disseminated TL and who were admitted and treated at our institution between April 1995 and August 2009. The patients included 9 women and 3 men, and their ages ranged from 24 to 56 years (median age 43 years). The mean duration of their symptoms was 46 d (range 29-65 d).
Clinical signs and symptoms among these patients included fever (ardent fever, n = 2) and weight loss, epigastric pain (n = 8), and night sweats (n = 1). An elevated blood sedimentation rate and positive tuberculin test were present in only two patients. The initial diagnostic sonography identified an intra-abdominal mass in all of the patients; three patients presented with pancreatic masses and one patient with a hepatic mass. The chest radiographic examinations found evidence of healed TB in 3 of the 12 patients. None of our patients had any evidence or history of opportunistic infection, drug abuse, or previously treated lung TB.
Neoplasms of the pancreas, liver, or periaortic area were pre-operatively diagnosed in seven patients and were removed surgically. The final histopathological examination of the resected masses showed caseous or liquefactive substances in the center of the enlarged lymph nodes surrounded by inflammatory lymphatic tissues and no evidence of malignant cells. TL was suspected in the other 5 patients based on CT findings and clinical presentations. A diagnostic laparoscopy to biopsy the mass was performed to rule out malignancy. The subsequent anti-TB therapy confirmed the diagnosis of TL.
We used the 9800 Quick CT Scanner (General Electric Medical Systems, Milwaukee, WI; n = 5), the Picker PQ 6000 CT Scanner (Picker International, Cleveland, Ohio; n = 6), and the Brilliance 64 CT Scanner (Philips Medical Systems, Best, Netherlands; n = 1). The scans were performed with conventional techniques. Diatrizoate solution (500-750 mL of 1.5%) or water was given orally to patients one hour prior to examination. Intravenous contrast medium (Omnipaque 300 mgI/mL, GE Healthcare) was administered at 1.0-1.2 mL/kg body weight with a flow rate of 3 mL/s. For enhanced scans, images were recorded for 25 s (arterial phase) and repeated for 60 s (portal venous phase) following the administration of the intravenous contrast using the single-slice spiral CT. The images of the delay phase (300 s after contrast administration) were recorded using the multi-slice spiral CT. Contiguous axial images of 7.5 mm or 10 mm sections were obtained from the epigastrium or from the dome of the diaphragm to the pubic symphysis.
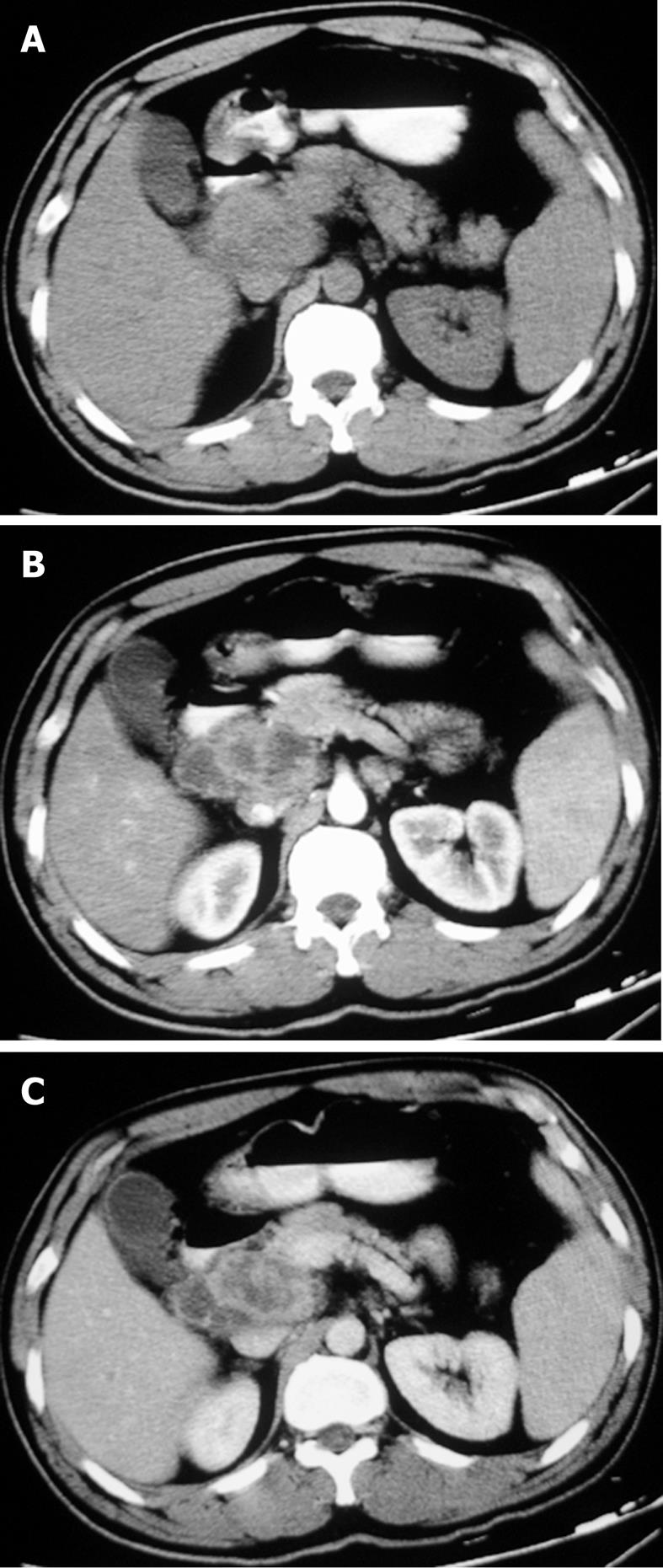
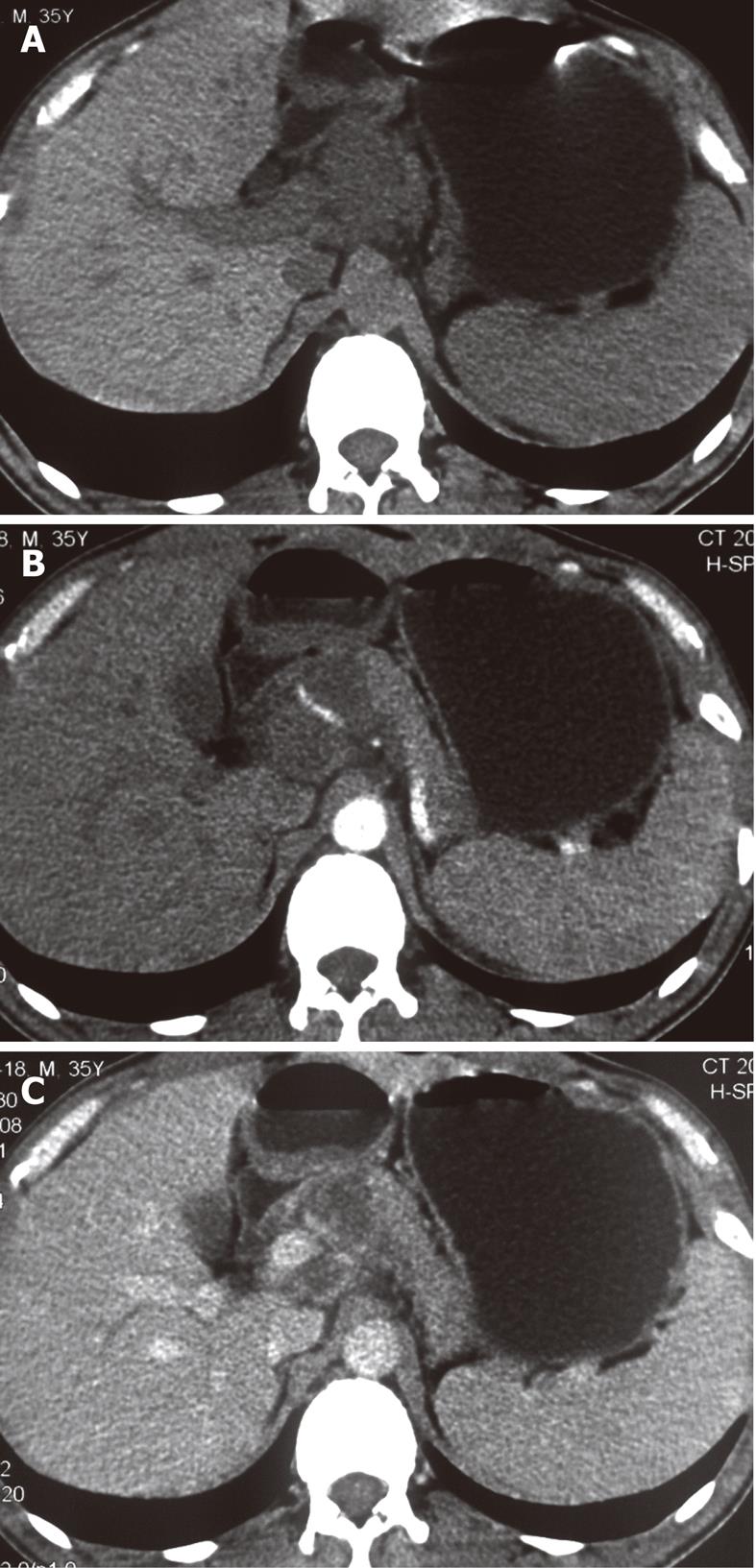
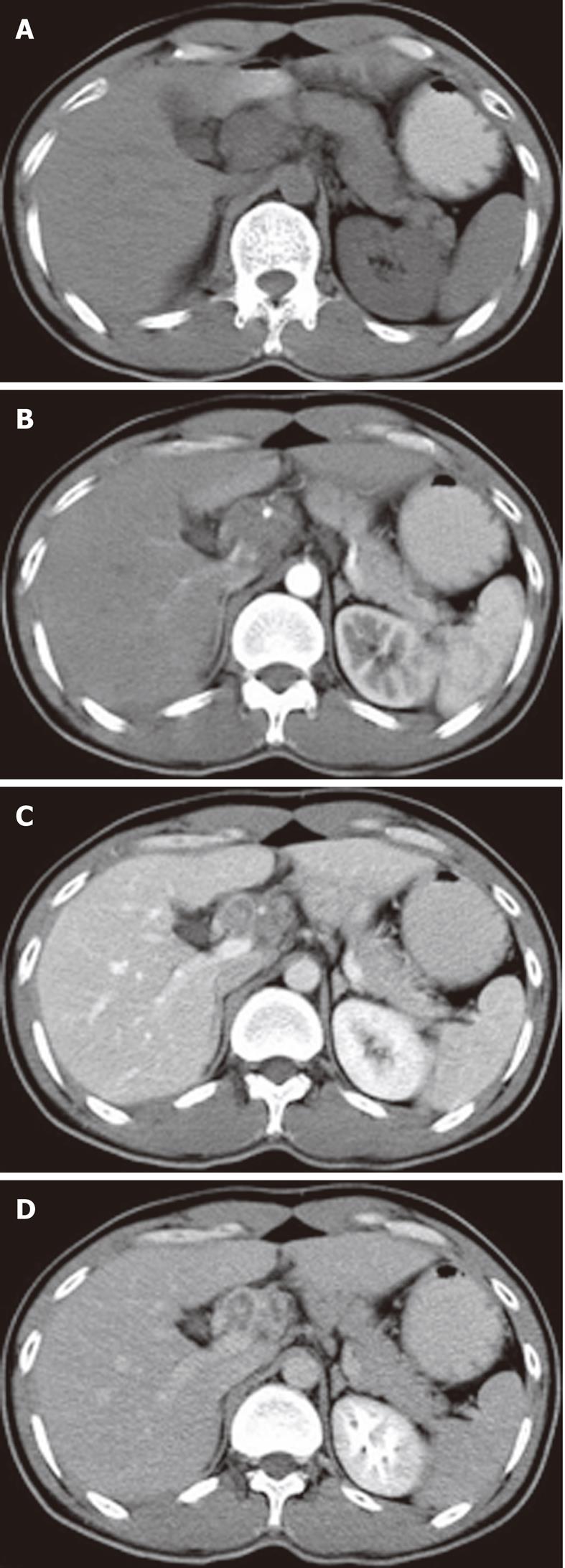
The sites of lymph node adenopathy included the periportal (n = 6), peripancreatic (n = 3), periaortic (n = 3), and small bowel mesenteric (n = 2) areas. The enlarged lymph nodes were 1.7-4.2 cm (mean 3.4 cm) in diameter. There was no calcification identified within the lesions. On the plain CT scans, the enlarged lymph nodes were of mostly heterogeneous isodensity or hypodensity, although one patient had lymph nodes of uniform low density. The margins of the involved lymph nodes were poorly defined. The lymph nodes could not be distinguished from the mass of the pancreas in three patients. After the administration of the contrast material, the lesions showed peripheral or ring-like enhancement with an expanded low-density central area in all the cases (Figures 1-4).
Some (n = 5) had a conglomerated and multilocular appearance instead of single node involvement (Figures 1-3). The peripheral enhancement of the nodes was low in the arterial phase but high in the venous or delay phase in two patients (Figures 2 and 3). The involved lymph nodes were easily distinguished from the pancreas (Figures 1 and 2)
in three cases. The common hepatic artery was embedded in the enlarged lymph nodes (Figures 2 and 3) in two patients. None of the patients displayed clinical signs of ascites and/or peritoneum thickening.
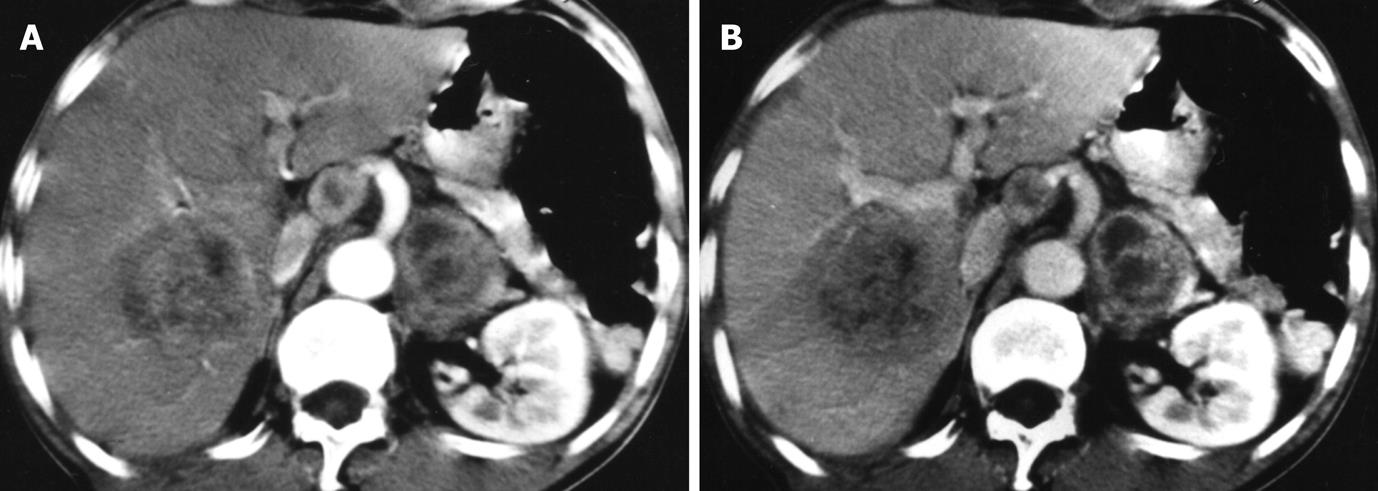
One patient had hepatic TB and retroperitoneal lymphadenopathy. The large liver lesion showed irregular rim enhancement with enlarged lymph nodes (Figure 4).
The incidence of abdominal TL is low. The disease is thought to result from one of three major routes of transmission of TB. The most common route is through the ingestion of contaminated materials containing the tubercle bacilli. The second source is the hematogeneous spread of the bacteria from a distant site of infection (commonly the lungs). The third route is the direct spread from the serosa of the adjacent infected organs or structures[5]. Our case series identified three patients with X-ray evidence of healed lung TB; none of the patients had an active infection or had been previously treated. Therefore, hematogeneous infection was impossible. This finding suggests that the only possible route of these infections was the ingestion of TB bacteria into the gastrointestinal tract. The tubercle bacilli are absorbed into the intestinal submucosal layer and then carried to the draining lymph nodes of the jejunum, ileum and ascending colon. The lymphatic drainage pattern supports our observation that the affected areas included the periportal, peripancreatic, upper periaortic, and mesenteric regions[6]. Because the tubercle bacilli are hardly absorbed from the left side of the colon, the lower periaortic nodes are rarely involved in cases of nonhematogenous disseminated TL[7].
The diagnosis of abdominal hematogenous disseminated TL can be made clinically when a patient has active pulmonary miliary TB[7]. The signs and symptoms of abdominal nonhematogenous disseminated TL in patients include epigastric pain, fever, weight loss, fatigue, and abdominal mass. Other indicative symptoms include the presence of TB at other sites, positive skin TB tests, and night sweats, although these are uncommon in patients with nonhematogenous disseminated TL[8]. In our review, only two patients had a positive skin TB test and one patient had night sweats. Obstructive jaundice is a rare complication and may also be caused by periportal lymphadenopathy[9,10], portal vein thrombosis, or portal hypertension[11]. Although the clinical presentation of abdominal nonhematogenous disseminated TL has been well characterized, clinical diagnosis is limited because the signs and symptoms are nonspecific.
The CT scan is a useful tool in detecting lesions and making presumptive diagnoses in the abdomen. Lymphadenopathy is the most common manifestation of abdominal TB found on the CT[12,13]. Plain CT findings are nonspecific in patients with abdominal nonhematogenous disseminated TL. The enlarged lymph nodes display low or soft tissue attenuation values and cannot be used to differentiate TL from neoplasm[14,15]. On the other hand, contrast-enhanced CT scans can detect the peripheral or rim-like enhancement with a low-attenuation center that was seen in all of our patients. This radiographic feature corresponds histologically to the peripheral inflammatory reaction and neovascularity around central liquefaction or caseous necrosis. Homogeneous enhancement on the CT scan[6] may reflect an earlier pathologic stage of the disease (i.e., the non caseating epithelioid and giant cell granulomas that precede necrosis) in which the size of the enlarged lymph nodes is often less than 1 cm in diameter. Non-enhancement on the CT scan is presumably due to the diminished inflammatory reaction associated with AIDS or with other immunocompromising diseases in patients. The individuals in this study were selected based upon the following criteria: (1) enlarged lymph nodes greater than 1 cm in diameter and (2) negative HIV infection status and a non-immunocompromised state. These parameters excluded the possibility of the confounding variables discussed above.
Other imaging modalities used for the detection of abdominal nonhematogenous disseminated TL have been described in other studies. Sonography shows TL as a hypoechoic[16] and homogeneous mass. These features are nonspecific and cannot be used to differentiate TL from a neoplasm[17]. Limited literature addresses the application of magnetic resonance imaging (MRI) in the diagnosis of abdominal TL. Kim et al[18] described MRI findings of abdominal TL in a series of 11 patients who were all diagnosed correctly as having TL. The lesions may show a variety of signal intensities depending on the stage of evolution and are frequently hypointense on the T1-weighted images and hyperintense on the T2-weighted images. The enhancement pattern is similar to that of the CT scans, which show predominant peripheral rim-like enhancement[18,19]. Furthermore, the MRI seems to be useful in differentiating the enlarged lymph nodes around the pancreas from a cystic neoplasm. Limited evidence suggests that the MRI scan has a valuable role in the diagnosis of abdominal TL. However, MRI scanners are not always available in developing countries. 18F-fluorodeoxyglucose positron emission tomography (FDG-PET) CT imaging is increasingly used as a tool for detecting malignancies. The accumulation of FDG in tuberculous lymph nodes prevents its use as a diagnostic tool for TL[20].
Intra-abdominal lymphoma can be confused with abdominal nonhematogenous disseminated TL both clinically and on CT scans. Lymphoma often involves the abdominal lymph nodes in the entire periaortic region, including above and below the third lumbar areas[6,21]. The enlarged lymph nodes in lymphoma can grow to be as large as 4.0 cm in diameter and appear homogeneous on CT scans in most patients[6,21,22]. Yu et al[21] considered embedded vessels to be a unique finding of lymphoma. Nonhematogenous disseminated TL involves the mesentery lymph nodes, the lesser omentum, and the upper periaortic regions in most cases and is rarely found in the lower periaortic region. In general, the size of the lymph nodes is less than 4 cm in diameter. The CT scan shows the peripheral enhancement in TL. On rare occasions, the peripheral enhancement may, however, be seen in untreated lymphoma and lymphoma following radiotherapy. Two of our cases even had embedded vessels that could be seen on a CT scan, and our initial misinterpretation led to a diagnosis of neoplasm. Therefore, the distribution characteristics of the anatomic regions, morphological patterns including size, and enhancement features on the CT scans are all helpful in distinguishing TL from lymphoma, although the results may not be conclusive in some cases.
Another important goal in the development of CT imaging as a screening tool for abdominal nonhematogenous disseminated TL is to differentiate this disease from metastatic lymphadenopathy. Most of the metastases are easily diagnosed because of the presence of a primary tumor, and they generally show homogeneous enhancement on the contrast-enhanced CT scans. Some malignant adenopathies, especially testicular tumors, can result in abdominal metastatic lymphadenopathy with peripheral rim enhancement[12]. Diagnosis aims to locate the primary tumors first. The anatomic distribution characteristics can sometimes provide clues to the differential diagnosis. For testicular tumors, the metastases may be located in the renal perihilar, paralumbar, and aortic bifurcation regions[12]. Moreover, other infectious diseases, such as Whipple’s disease, can also appear on contrast-enhanced CT scans and may look similar to TL. Although these diseases are rare, they should be considered in the differential diagnosis.
Three patients received an initial diagnosis of pancreatic tumor following sonography and CT scans. The retrospective review of the CT scans found the clear boundary of lymphadenopathy presented on contrast-enhanced images and could be easily distinguished from the pancreas. This misdiagnosis was likely the result of unfamiliarity with identifying the features of non-malignant lymphadenopathies using CT imaging.
CT or ultrasound-guided needle aspiration cytology has been used to confirm diagnoses[23,24]. These methods are quicker and less invasive than surgery; moreover, these methods can offer high diagnostic accuracy. In a study by Suri et al[25], ultrasound-guided needle aspiration cytology yielded a positive diagnosis in 78.6% of 14 cases of abdominal TL and a false negative result in only one patient. However, this technique is heavily operator dependent, particularly when not enough tissues are harvested and when the nodes are situated in close proximity to major blood vessels or important viscera, posing a risk to the patient. In cases where this technique is inconvenient or its result is ambiguous, diagnostic laparoscopy could be scheduled to collect sufficient tissues for histological and microbiological examinations, except in those patients with significant risk of perforation[26,27].
In conclusion, the characteristics of anatomic distribution, morphological pattern, and enhancement features on contrast-enhanced CT scans will help to differentiate between abdominal nonhematogenous disseminated TL and lymph node neoplasms. Ultrasound-guided needle aspiration cytology performed by an experienced operator should be the first method of diagnosis, although diagnostic laparoscopy is a more reliable method for selected patients.
The authors thank Jian-Sheng Wang for assisting with the manuscript.
Tuberculous lymphadenopathy (TL) is the most common manifestation of abdominal tuberculosis. The diagnosis of abdominal hematogenous disseminated TL can be made clinically in patients with active pulmonary miliary tuberculosis. Patients with abdominal nonhematogenous disseminated TL typically present with an isolated mass or a mass adhering to the surrounding organs, and these masses may easily be confused with neoplasm. With the widespread use of computed tomography (CT), physicians should be familiar with the features of CT images for these patients and be able to make differential diagnoses based upon such findings.
CT is a useful method in the diagnosis and differential diagnosis of abdominal lesions. This study reports on CT findings from 12 patients with abdominal nonhematogenous disseminated TL.
Abdominal nonhematogenous disseminated TL still presents a diagnostic dilemma. The authors reviewed 12 patients with this condition and also reviewed the related literature to develop a diagnostic algorithm.
The recognition of relatively specific CT findings of abdominal nonhematogenous disseminated TL may help avoid misdiagnosis and unnecessary invasive procedures, allowing for the prompt consideration of antituberculosis therapy.
The authors describe typical CT features of abdominal nonhematogenous disseminated tuberculous lymphadenopathy in 12 patients. The paper is well organised and informative.
Peer reviewers: Dr. Andreas G Schreyer, Professor, Department of Radiology, University Hospital Regensburg, Franz-Josef-Strauss-Allee 11, Regensburg 93053, Germany; Vineet Ahuja, Associate Professor, Department of Gastroenterology and Human Nutrition, All India Institute of Medical Sciences, New Delhi 110029, India
S- Editor Tian L L- Editor Ma JY E- Editor Xiong L
| 1. | World Health Organization. Global tuberculosis control 2010. Geneva: WHO Press; 2010; 5-7. |
| 2. | Harisinghani MG, McLoud TC, Shepard JA, Ko JP, Shroff MM, Mueller PR. Tuberculosis from head to toe. Radiographics. 2000;20:449-70; quiz 528-9, 532. [PubMed] |
| 3. | Burrill J, Williams CJ, Bain G, Conder G, Hine AL, Misra RR. Tuberculosis: a radiologic review. Radiographics. 2007;27:1255-1273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 303] [Cited by in RCA: 278] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 4. | Akhan O, Pringot J. Imaging of abdominal tuberculosis. Eur Radiol. 2002;12:312-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 122] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 5. | Pereira JM, Madureira AJ, Vieira A, Ramos I. Abdominal tuberculosis: imaging features. Eur J Radiol. 2005;55:173-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 106] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 6. | Yang ZG, Min PQ, Sone S, He ZY, Liao ZY, Zhou XP, Yang GQ, Silverman PM. Tuberculosis versus lymphomas in the abdominal lymph nodes: evaluation with contrast-enhanced CT. AJR Am J Roentgenol. 1999;172:619-623. [PubMed] |
| 7. | Li Y, Yang ZG, Guo YK, Min PQ, Yu JQ, Ma ES, Hu J. Distribution and characteristics of hematogenous disseminated tuberculosis within the abdomen on contrast-enhanced CT. Abdom Imaging. 2007;32:484-488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 8. | Demir K, Okten A, Kaymakoglu S, Dincer D, Besisik F, Cevikbas U, Ozdil S, Bostas G, Mungan Z, Cakaloglu Y. Tuberculous peritonitis--reports of 26 cases, detailing diagnostic and therapeutic problems. Eur J Gastroenterol Hepatol. 2001;13:581-585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 81] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 9. | Colovic R, Grubor N, Jesic R, Micev M, Jovanovic T, Colovic N, Atkinson HD. Tuberculous lymphadenitis as a cause of obstructive jaundice: a case report and literature review. World J Gastroenterol. 2008;14:3098-3100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 27] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 10. | Obama K, Kanai M, Taki Y, Nakamoto Y, Takabayashi A. Tuberculous lymphadenitis as a cause of obstructive jaundice: report of a case. Surg Today. 2003;33:229-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 11. | Chiu C, Peng Y, Chang W, Hsieh T, Chao Y, Chu H. Massive Esophageal Variceal Bleeding as the Initial Presentation of Peripancreatic Tuberculoma with Portal Hypertension. Tzu Chi Medical Journal. 2009;21:172-177. |
| 12. | Suri S, Gupta S, Suri R. Computed tomography in abdominal tuberculosis. Br J Radiol. 1999;72:92-98. [PubMed] |
| 13. | Gulati MS, Sarma D, Paul SB. CT appearances in abdominal tuberculosis. A pictorial essay. Clin Imaging. 1999;23:51-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 31] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 14. | Kim YS, Moon JS, Lee JW, Kim I, Ryu SH, Paik IW. Solitary intra-abdominal tuberculous lymphadenopathy mimicking duodenal GIST. Korean J Intern Med. 2005;20:72-75. [PubMed] |
| 15. | Barbalinardo RJ, Hamilton GB, Eliot GR, Lazaro EJ, Haycock C. Tuberculous retroperitoneal lymphadenopathy mimicking metastatic pancreatic carcinoma. J Natl Med Assoc. 1986;78:385-387. [PubMed] |
| 16. | Malik A, Saxena NC. Ultrasound in abdominal tuberculosis. Abdom Imaging. 2003;28:574-579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 49] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 17. | Mathieu D, Ladeb MF, Guigui B, Rousseau M, Vasile N. Periportal tuberculous adenitis: CT features. Radiology. 1986;161:713-715. [PubMed] |
| 18. | Kim SY, Kim MJ, Chung JJ, Lee JT, Yoo HS. Abdominal tuberculous lymphadenopathy: MR imaging findings. Abdom Imaging. 2000;25:627-632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 19. | De Backer AI, Mortelé KJ, Deeren D, Vanschoubroeck IJ, De Keulenaer BL. Abdominal tuberculous lymphadenopathy: MRI features. Eur Radiol. 2005;15:2104-2109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 22] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 20. | Li YJ, Zhang Y, Gao S, Bai RJ. Systemic disseminated tuberculosis mimicking malignancy on F-18 FDG PET-CT. Clin Nucl Med. 2008;33:49-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 21. | Yu RS, Zhang WM, Liu YQ. CT diagnosis of 52 patients with lymphoma in abdominal lymph nodes. World J Gastroenterol. 2006;12:7869-7873. [PubMed] |
| 22. | Dong P, Wang B, Sun QY, Cui H. Tuberculosis versus non-Hodgkin's lymphomas involving small bowel mesentery: evaluation with contrast-enhanced computed tomography. World J Gastroenterol. 2008;14:3914-3918. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 11] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 23. | Gupta S, Rajak CL, Sood BP, Gulati M, Rajwanshi A, Suri S. Sonographically guided fine needle aspiration biopsy of abdominal lymph nodes: experience in 102 patients. J Ultrasound Med. 1999;18:135-139. [PubMed] |
| 24. | Xia F, Poon RT, Wang SG, Bie P, Huang XQ, Dong JH. Tuberculosis of pancreas and peripancreatic lymph nodes in immunocompetent patients: experience from China. World J Gastroenterol. 2003;9:1361-1364. [PubMed] |
| 25. | Suri R, Gupta S, Gupta SK, Singh K, Suri S. Ultrasound guided fine needle aspiration cytology in abdominal tuberculosis. Br J Radiol. 1998;71:723-727. [PubMed] |
| 26. | Tan KK, Chen K, Sim R. The spectrum of abdominal tuberculosis in a developed country: a single institution's experience over 7 years. J Gastrointest Surg. 2009;13:142-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 50] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 27. | Bhandarkar DS, Shah RS, Katara AN, Shankar M, Chandiramani VA, Udwadia TE. Laparoscopic biopsy in patients with abdominal lymphadenopathy. J Minim Access Surg. 2007;3:14-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |