Published online Sep 21, 2011. doi: 10.3748/wjg.v17.i35.4023
Revised: January 11, 2011
Accepted: January 18, 2011
Published online: September 21, 2011
AIM: To investigate the role and clinicopathological significance of aberrant expression of Notch receptors and Delta-like ligand-4 (DLL4) in extrahepatic cholangiocarcinoma and gallbladder carcinoma.
METHODS: One hundred and ten patients had surgically resected extrahepatic cholangiocarcinoma (CC) and gallbladder carcinoma specimens examined by immunohistochemistry of available paraffin blocks. Immunohistochemistry was performed using anti-Notch receptors 1-4 and anti-DLL4 antibodies. We scored the immunopositivity of Notch receptors and DLL4 expression by percentage of positive tumor cells with cytoplasmic expression and intensity of immunostaining. Coexistent nuclear localization was evaluated. Clinicopathological parameters and survival data were compared with the expression of Notch receptors 1-4 and DLL4.
RESULTS: Notch receptor proteins showed in the cytoplasm with or without nuclear expression in cancer cells, as well as showing weak cytoplasmic expression in non-neoplastic cells. By semiquantitative evaluation, positive immunostaining of Notch receptor 1 was detected in 96 cases (87.3%), Notch receptor 2 in 97 (88.2%), Notch receptor 3 in 97 (88.2%), Notch receptor 4 in 103 (93.6), and DLL4 in 84 (76.4%). In addition, coexistent nuclear localization was noted [Notch receptor 1; 18 cases (18.8%), Notch receptor 2; 40 (41.2%), Notch receptor 3; 32 (33.0%), Notch receptor 4; 99 (96.1%), DLL4; 48 (57.1%)]. Notch receptor 1 expression was correlated with advanced tumor, node, metastasis (TNM) stage (P = 0.043), Notch receptor 3 with advanced T stage (P = 0.017), tendency to express in cases with nodal metastasis (P = 0.065) and advanced TNM stage (P = 0.052). DLL4 expression tended to be related to less histological differentiation (P = 0.095). Coexistent nuclear localization of Notch receptor 3 was related to no nodal metastasis (P = 0.027) and Notch receptor 4 with less histological differentiation (P = 0.036), while DLL4 tended to be related inversely with T stage (P = 0.053). Coexistent nuclear localization of DLL4 was related to poor survival (P = 0.002).
CONCLUSION: Aberrant expression of Notch receptors 1 and 3 play a role during cancer progression, and cytoplasmic nuclear coexistence of DLL4 expression correlates with poor survival in extrahepatic CC and gallbladder carcinoma.
- Citation: Yoon HA, Noh MH, Kim BG, Han JS, Jang JS, Choi SR, Jeong JS, Chun JH. Clinicopathological significance of altered Notch signaling in extrahepatic cholangiocarcinoma and gallbladder carcinoma. World J Gastroenterol 2011; 17(35): 4023-4030
- URL: https://www.wjgnet.com/1007-9327/full/v17/i35/4023.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i35.4023
Cholangiocarcinoma (CC) is a highly malignant neoplasm that generally is diagnosed at advanced stage and is associated with a fatal outcome[1]. CC includes cancers originating by malignant transformation of biliary epithelial cells, histologically occurring in the intrahepatic and extrahepatic biliary trees with gallbladder. The extrahepatic CC accounts for 80% to 90% of CC and intrahepatic CC comprises the remainder[1]. The incidence of intrahepatic CC is increasing, in comparison with the stagnant incidence of extrahepatic CC, especially in Western countries[2], although the cause of rising incidence of intrahepatic CC has not been clarified. Intrahepatic and extrahepatic CCs differ in biologic and clinical variables, including not only histopathologic distinction and clinical characteristics, but also carcinogenesis and molecular profiling, even management. In order to define the carcinogenesis of CC, dysregulated genes and pathways involved in proliferation, evasion from apoptosis and senescence, cell cycle dysregulation, invasion, metastasis and angiogenesis, have all been investigated, suggesting a complex network of variable factors and pathways[3,4]. The development of new therapeutic modalities for molecular targeting resulting from defining of the molecular carcinogenesis of CC has been growing recently, mainly as a consequence of preclinical in vitro studies. Till now, complete surgical resection has been regarded as the only curative therapy.
Since the Notch gene was originally discovered in Drosophila, Notch signaling has been investigated in varied organisms from C. elegans to humans. Thus, Notch signaling has been noted to be an evolutionally conserved pathway which regulates physiological processes and is involved in pathological conditions[5]. There are four Notch receptors (Notch 1-4) and five ligands [Jagged 1, Jagged 2, Delta-like ligand-1, -3 and -4 (DLL1, DLL3, DLL4)] in mammals. Ligand-receptor interaction between two neighboring cells activates two sequential proteolytic cleavages of Notch receptor, mediated by the metalloprotease tumor necrosis factor -α-converting enzyme, and by γ-secretase[6]. The liberated Notch intracellular domain translocates into the nucleus, activating target genes, such as Hes, Hey, etc. Physiologically, Notch signaling regulates cellular differentiation, proliferation, apoptosis and stem cell maintenance, and participates in cell-fate specification during development of multicellular organisms[7]. Also, Notch signaling regulates biological events in adult tissue. Recently, the disruption of Notch gene was reported to be implicated in hematological malignancies[8,9]. In addition, aberrant Notch signaling has been reported in a variety of solid cancers, including breast, kidney, pancreas, prostate, cervix, endometrium, brain, lung, liver and skin[10-12]. According to the type of cancer, Notch receptors may have a role as an oncogene or a tumor suppressor gene, though the majority of studies reveal that Notch signaling promotes tumorigenesis[13]. In both physiological and pathological angiogenesis including tumor angiogenesis, the role of Notch signaling has been recognized[8].
DLL4 is an endothelial-specific ligand of the Notch signaling pathway, expressed at areas of vasculogenesis and angiogenesis[14,15]. DLL4 is induced by vascular endothelial growth factor (VEGF) and acts to the downstream of VEGF, as an autoregulatory negative feedback network for inactivation of VEGF, resulting in maturation and stabilization of microvessels[14]. Recently, in addition to endothelium, DLL4 has been shown to be expressed in epithelium, stromal cells of inflammatory cells in normal or reactive tissues, and in tumor cells of solid cancers in humans[16], raising possibilities for a role in tumorigenesis.
In this study, we investigated the role of Notch receptors and DLL4 in progression of human extrahepatic CC and gallbladder carcinoma by analysis of expression with immunohistochemistry (IHC), during carcinogenesis. Furthermore, we investigated the correlations between aberrant expression of Notch receptors and DLL4, and clinicopathological parameters with survival.
One hundred and ten patients with surgically resected extrahepatic CC and gallbladder carcinoma were included in this study, from January 1999 till December 2008 at Dong-A University Hospital, Busan, South Korea. None of the patients recruited in this study received chemotherapy or radiotherapy before surgery. After review of pathological information and medical records, available cases for IHC with paraffin blocks were collected. The pathologic reviews were performed by two pathologists who are experienced in biliary cancer pathology. Outcomes were determined from the date of surgery until death or 31 December 2008, which resulted in a follow-up period of from 1 to 88 mo (mean, 28 mo). Cases lost to follow-up or who died from problems other than extrahepatic CC and gallbladder carcinoma were censored during the survival analysis. The sites of extrahepatic biliary tract consisted of common bile duct and gallbladder. All cases were adenocarcinomas. Clinicopathological parameters such as age, gender, site, tumor differentiation and tumor, node, metastasis (TNM) with staging according to American Joint Committee on Cancer classification were evaluated by reviewing medical and pathological records. The study was approved by the Institutional Review Board of Dong-A University Hospital, Busan, South Korea (10-10-6).
Core tissue biopsy specimens (diameter 2 mm) were obtained from individual paraffin-embedded extrahepatic CC and gallbladder carcinoma (donor blocks) and arranged in new recipient paraffin blocks (tissue array blocks) using a trephine apparatus (Superbiochips Laboratories, Seoul, South Korea). Non-neoplastic biliary mucosa specimens were included in each of the array blocks. Each tissue array block contained up to 60 cores.
Immunohistochemistry was performed using tissue array paraffin blocks. Utilized antibodies are summarized in Table 1. With antibodies to Notch receptors 1-4, avidin-biotin-peroxidase complex (ABC) method was applied and with anti-DLL4, Ventana Autostaininer System was used. Four to six micron thick sections from array blocks were dewaxed in xylene, rehydrated using a graded alcohol series and placed in an endogenous peroxide blocker for 15 min and washed with buffer. The slides were then placed in citrate buffer (10% citrate buffer stock in distilled water, pH 6.0) and microwaved for 10 min for antigen retrieval. Non-reactive staining was blocked using 1% horse serum in Tris buffered saline (pH 6.0) for 3 min. Primary antibodies to Notch receptors 1-4 were applied and antibody binding was detected using avidin-biotin-peroxidase complex (Universal Elite ABC kit PK-6200; Vectastain, Burlingame, CA, United States) for 10 min and diaminobenzidine tetrahydrochloride solution (Kit HK153-5K; Biogenex, San Ramon, CA, United States). For DLL4, antigen retrieval was performed using CC1 antigen retrieval buffer (Ventana Medical Systems, Tucson, AZ, United States). Next, the sections were incubated with anti-DLL4, and stained on the Ventana automated slide stainer (NEXES) using the Ventana diaminobenzidine detection kit (Ventana Medical Systems, Tucson, AZ, United States).
| Antibody | Dilution | Company | Non-neoplastic tissue | Aberrant expression in cancer |
| Notch 1 | 0.111 | Santa Cruz | Variable | Cytoplasm/nucleus |
| Notch 2 | 0.111 | Santa Cruz | Variable | Cytoplasm/nucleus |
| Notch 3 | 0.111 | Santa Cruz | Variable | Cytoplasm/nucleus |
| Notch 4 | 1:50 | Santa Cruz | Variable | Cytoplasm/nucleus |
| DLL4 | 1:50 | Sigma | Variable | Cytoplasm/nucleus |
There are few analysis criteria for the immunopositivity of Notch receptors and DLL4 expression. The scoring was based on distribution and intensity according to a previous report[12]. Briefly, the percentage of positive tumor cells with cytoplasmic expression was determined semi-quantitatively and each sample was scored on a scale of 0-4, in which 0: negative, 1: positive staining in 1%-25% of cells, 2: in 26%-50%, 3: 51%-75%, and 4: 76%-100%. The intensity of immunostaining was determined as 0: negative staining, 1: weakly positive staining, 2: moderately positive staining, and 3: strongly positive staining. The immunoreactive score of each section was calculated by the sum of these two parameters. The total sum score was transformed into a three tier system and graded as negative (sum: 0), low (sum: 2-4) and high (sum: 5-7).
The two-tailed χ2 test was performed to determine the significance of the difference between the covariates. Survival durations were calculated using the Kaplan-Meier method. The log-rank test was used to compare cumulative survival in the patient groups. The SPSS software program (version 12.0; SPSS Inc., Chicago, IL) was used in the analyses.
One hundred and ten patients comprised 47 males (42.7%) and 63 females (57.3%), with a range from 37 to 81 years. The mean patient age was 63 years. Carcinomas from the extrahepatic common bile duct were 47 cases (42.7%) and from the gallbladder, 63 cases (57.3%). Well differentiated adenocarcinomas were 53 cases (48.2%); moderately differentiated, 46 cases (41.8%); and poorly differentiated, 11 cases (10.0%). Seventeen cases were T1 (15.5%), T2: 55 cases (50.0%), T3: 32 cases (29.1%), and T4: 6 cases (5.4%). Seventy-eight patients showed no evidence of lymph node metastasis (70.9%) and 32 patients (29.1%) showed lymph node metastasis. Seven patients (6.4%) showed distant metastasis. Fifty-one cases were stage I (46.4%), stage II: 45 cases (40.9%), stage III: 6 cases (5.4%), and stage IV: 8 cases (7.3%).
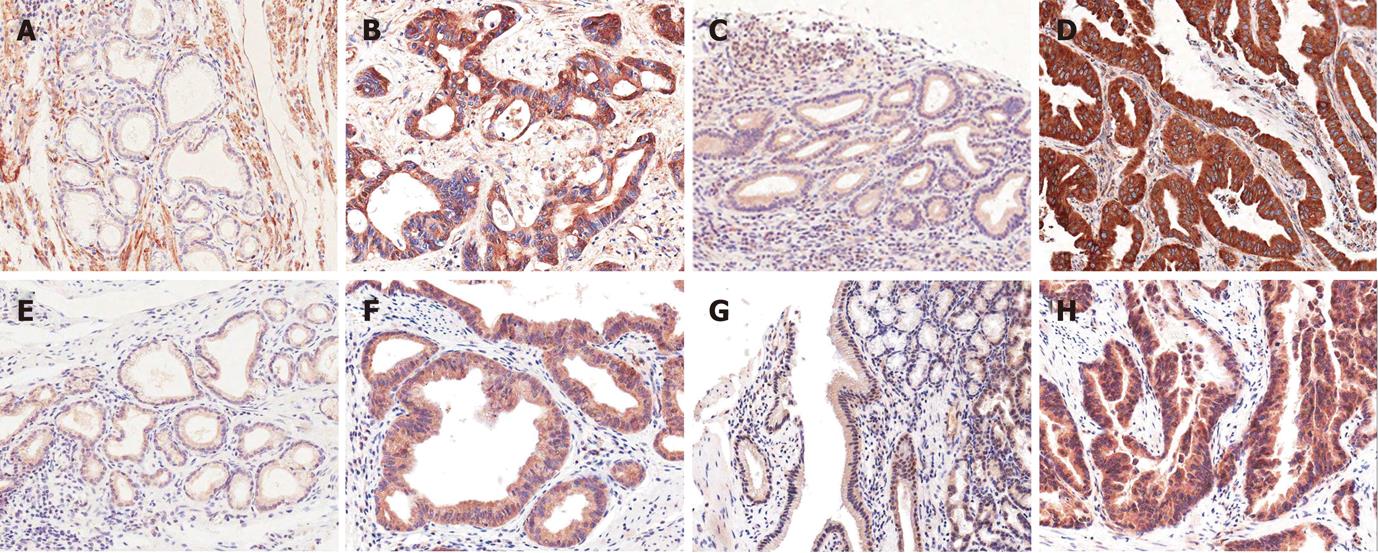
Expression of Notch receptors: Notch receptors 1, 2, 3 and 4 were expressed in non-neoplastic biliary epithelial cells, mesenchymal cells and sometimes inflammatory cells with variable intensities, as well as in micro-vessels
(Figure 1A, C, E and G). They were mainly expressed in the cytoplasm of CC cells (Figure 1B, D, F and H) and were evaluated semiquantitatively (Table 2). Notch receptor 1 showed 55 cases (50.0%) of low grade immunoreactivity and 41 cases (37.3%) of high grade immunoreactivity, Notch receptor 2: 60 cases (54.6%) of low grade and 37 cases (33.6%) of high grade, Notch receptor 3: 56 cases (50.9%) of low grade and 41 cases (37.3%) of high grade, and Notch receptor 4: 61 cases (55.4%) of low grade and 42 cases (38.2%) of high grade immunoreactivity.
| Negativity | Positivity | Total | ||
| Low grade | High grade | |||
| Notch 1 | 14 (12.7) | 55 (50.0) | 41 (37.3) | 110 (100) |
| Notch 2 | 13 (11.8) | 60 (54.6) | 37 (33.6) | 110 (100) |
| Notch 3 | 13 (11.8) | 56 (50.9) | 41 (37.3) | 110 (100) |
| Notch 4 | 7 (6.4) | 61 (55.4) | 42 (38.2) | 110 (100) |
| DLL4 | 26 (23.6) | 63 (57.3) | 21 (19.1) | 110 (100) |
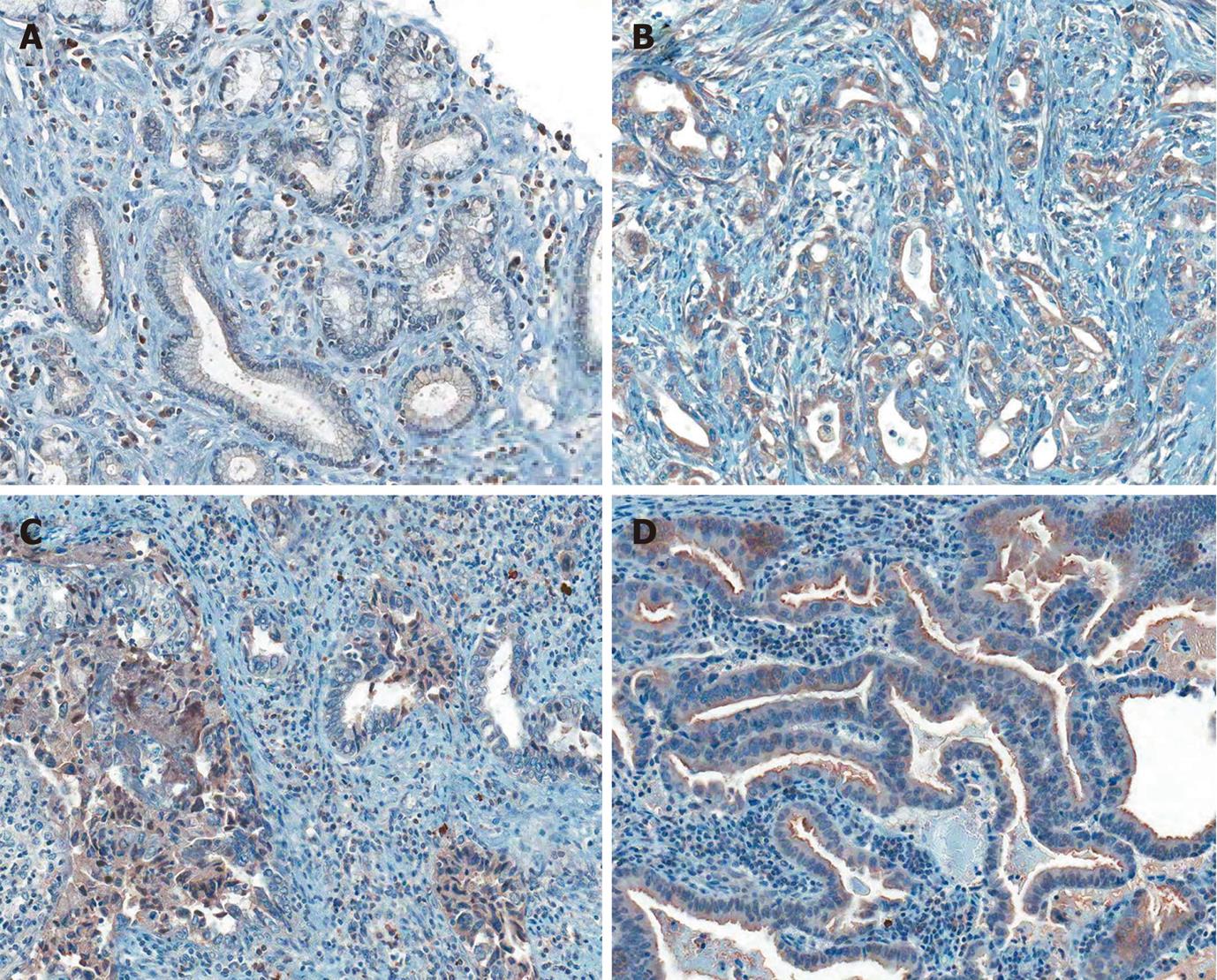
In some cases with cytoplasmic positive immunoreaction, coexistent distinct nuclear staining was observed (Figure 2). The number of coexistent cytoplasmonuclear staining in Notch receptor 1 (+) carcinomas were 18 cases (18.8%), Notch receptor 2: 40 cases (41.2%) and Notch receptor 3: 32 cases (32.7%), and most of the Notch receptor 4 cases (95.2%) showed cytoplasmonuclear staining except 5 cases (Table 3). In addition to cytoplasm and nucleus, occasionally incomplete membranous staining along with staining of luminal borders of neoplastic glands was noted.
| Notch1 | Notch 2 | Notch 3 | Notch 4 | DLL4 | |||||||||||
| Nuclear localization | - | + | P value | - | + | P value | - | + | P value | - | + | P value | - | + | P value |
| Total | 78 | 18 | 57 | 40 | 65 | 32 | 4 | 99 | 36 | 48 | |||||
| Differentiation | 0.545 | 0.418 | 0.556 | 0.036 | 0.175 | ||||||||||
| Well | 37 | 11 | 29 | 18 | 32 | 17 | 2 | 47 | 17 | 20 | |||||
| Moderate | 34 | 6 | 22 | 20 | 27 | 14 | 0 | 43 | 14 | 26 | |||||
| Poorly | 7 | 1 | 6 | 2 | 6 | 1 | 2 | 9 | 5 | 2 | |||||
| T stage | 0.846 | 0.594 | 0.269 | 0.365 | 0.053 | ||||||||||
| T1 | 12 | 4 | 11 | 5 | 8 | 9 | 0 | 16 | 5 | 9 | |||||
| T2 | 43 | 8 | 25 | 23 | 33 | 13 | 1 | 51 | 15 | 26 | |||||
| T3 | 18 | 5 | 18 | 10 | 20 | 8 | 3 | 26 | 11 | 13 | |||||
| T4 | 5 | 1 | 3 | 2 | 4 | 2 | 0 | 6 | 5 | 0 | |||||
| N stage | 0.100 | 0.408 | 0.027 | 0.34 | 0.558 | ||||||||||
| N0 | 52 | 16 | 44 | 27 | 41 | 28 | 2 | 70 | 27 | 32 | |||||
| N1 | 26 | 2 | 13 | 13 | 24 | 4 | 2 | 29 | 9 | 16 | |||||
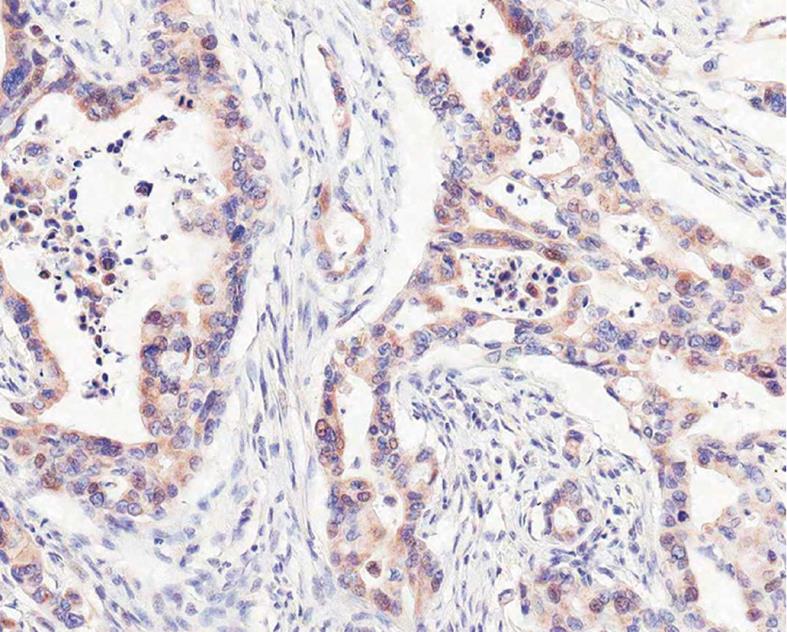
Expression of DLL4: DLL4 was expressed in endothelial cells and non-neoplastic biliary epithelial cells, mesenchymal cells and sometimes inflammatory cells with variable intensities (Figure 3A). The cancer cells expressed DLL4 mainly in cytoplasm (Figure 3B), showing low grade: 63 cases (57.3%) and high grade: 21 cases (19.1%) (Table 2). As with Notch receptor protein expression, 48 cases (57.1%) out of DLL 4 (+) 84 cases showed cytoplasmonuclear coexistent immunostaining (Figure 3C, Table 4). Occasionally, luminal borders of neoplastic glands showed distinct membranous immunostaining (Figure 3D).
| Notch 1 | Notch 2 | Notch 3 | Notch 4 | DLL4 | |||||||||||||||||
| Total (n = 110) | - | + | ++ | P value | - | + | ++ | P value | - | + | ++ | P value | - | + | ++ | P value | - | + | ++ | P value | |
| Gender | 0.207 | 0.005 | 0.2 | 0.144 | 0.517 | ||||||||||||||||
| Male | 47 | 9 | 21 | 17 | 11 | 22 | 14 | 8 | 20 | 19 | 5 | 22 | 20 | 13 | 24 | 10 | |||||
| Female | 63 | 5 | 34 | 24 | 2 | 38 | 23 | 5 | 36 | 22 | 2 | 39 | 22 | 13 | 39 | 11 | |||||
| Age | 0.251 | 0.34 | 0.107 | 0.761 | 0.873 | ||||||||||||||||
| < 60 | 34 | 3 | 21 | 10 | 5 | 15 | 14 | 1 | 21 | 12 | 1 | 20 | 13 | 7 | 20 | 7 | |||||
| ≥ 60 | 76 | 11 | 34 | 31 | 8 | 45 | 23 | 12 | 35 | 29 | 6 | 41 | 29 | 19 | 43 | 14 | |||||
| Differentiation | 0.391 | 0.266 | 0.101 | 0.414 | 0.095 | ||||||||||||||||
| Well | 53 | 5 | 30 | 18 | 6 | 29 | 18 | 4 | 28 | 21 | 4 | 29 | 20 | 16 | 31 | 6 | |||||
| Moderate | 46 | 6 | 20 | 20 | 4 | 24 | 18 | 5 | 23 | 18 | 3 | 23 | 20 | 6 | 27 | 13 | |||||
| Poorly | 11 | 3 | 5 | 3 | 3 | 7 | 1 | 4 | 5 | 2 | 0 | 9 | 2 | 4 | 5 | 2 | |||||
| T stage | 0.103 | 0.285 | 0.017 | 0.294 | 0.614 | ||||||||||||||||
| T1 | 17 | 1 | 8 | 8 | 1 | 11 | 5 | 0 | 13 | 4 | 1 | 12 | 4 | 3 | 12 | 2 | |||||
| T2 | 55 | 4 | 32 | 19 | 7 | 31 | 17 | 9 | 26 | 20 | 3 | 25 | 27 | 14 | 31 | 10 | |||||
| T3 | 32 | 9 | 11 | 12 | 4 | 13 | 15 | 4 | 11 | 17 | 3 | 19 | 10 | 8 | 15 | 9 | |||||
| T4 | 6 | 0 | 4 | 2 | 1 | 5 | 0 | 0 | 6 | 0 | 0 | 5 | 1 | 1 | 5 | 0 | |||||
| N stage | 0.999 | 0.223 | 0.065 | 0.795 | 0.301 | ||||||||||||||||
| N0 | 78 | 10 | 39 | 29 | 7 | 46 | 25 | 9 | 45 | 24 | 6 | 42 | 30 | 19 | 47 | 12 | |||||
| N1 | 32 | 4 | 16 | 12 | 6 | 14 | 12 | 4 | 11 | 17 | 1 | 19 | 12 | 7 | 16 | 9 | |||||
| M stage | 0.467 | 0.443 | 0.999 | 0.999 | 0.221 | ||||||||||||||||
| M0 | 103 | 13 | 53 | 37 | 13 | 57 | 33 | 13 | 52 | 38 | 7 | 57 | 39 | 25 | 60 | 18 | |||||
| M1 | 7 | 1 | 2 | 4 | 0 | 3 | 4 | 0 | 4 | 3 | 0 | 4 | 3 | 1 | 3 | 3 | |||||
| TNM stage | 0.043 | 0.144 | 0.052 | 0.898 | 0.36 | ||||||||||||||||
| I | 51 | 2 | 29 | 20 | 4 | 31 | 16 | 6 | 30 | 15 | 3 | 27 | 21 | 11 | 33 | 7 | |||||
| II | 45 | 11 | 20 | 14 | 8 | 21 | 16 | 7 | 16 | 22 | 4 | 24 | 17 | 12 | 22 | 11 | |||||
| III | 6 | 0 | 4 | 2 | 1 | 5 | 0 | 0 | 6 | 0 | 0 | 5 | 1 | 1 | 5 | 0 | |||||
| IV | 8 | 1 | 2 | 5 | 0 | 3 | 5 | 0 | 4 | 4 | 0 | 5 | 3 | 2 | 3 | 3 | |||||
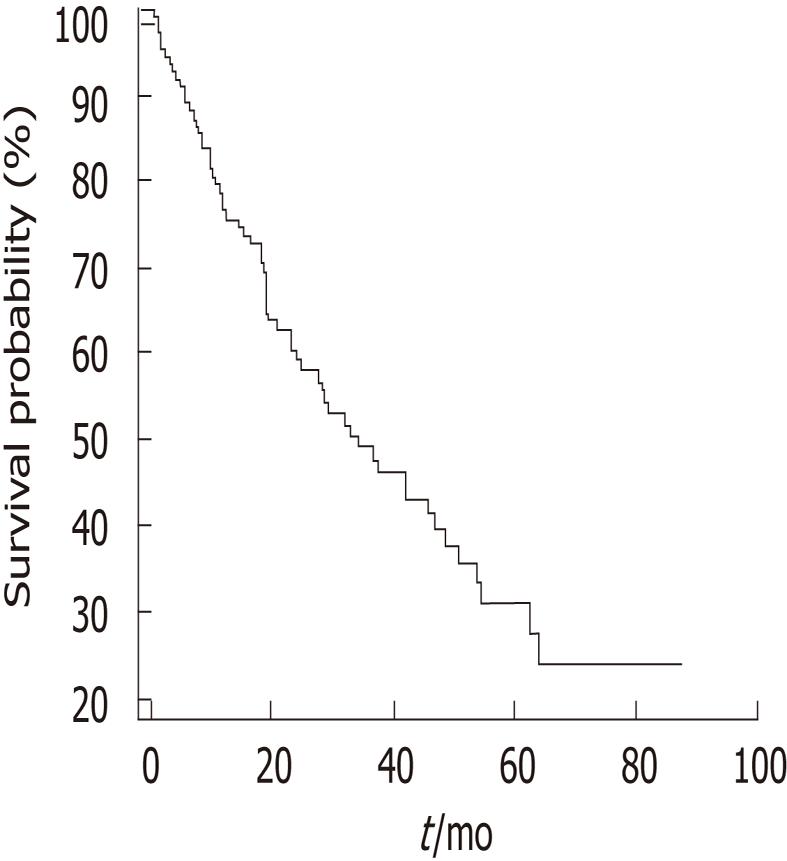
Table 4 summarizes the correlations between expression of Notch receptors and DLL4, and clinicopathological parameters, including statistical analyses. Notch receptor 1 was expressed at advanced TNM stage, representing a statistically significant correlation (P = 0.043). Notch receptor 2 expression was positively correlated with female gender (P = 0.005). Notch receptor 3 was expressed at advanced T stage (P = 0.017) and tended to express in cases with lymph node metastasis (P = 0.065) and at advanced TNM stage (P = 0.052). The expression of Notch receptor 4 was not correlated with clinicopathological parameters. High DLL4 expression tended to be related to less histological differentiation (P = 0.095). The median survival of 110 extrahepatic CC and gallbladder carcinoma patients was 34.1 mo (Figure 4). There was no significant correlation between the expression of Notch receptors 1-4 and DLL4, and survival (Notch receptor 1; P = 0.487, Notch receptor 2; P = 0.922, Notch receptor 3; P = 0.391, Notch receptor 4; P = 0.474, DLL4; P = 0.441).
Cytoplasmonuclear coexistent localization of Notch receptor 3 was correlated with no lymph node metastasis (P = 0.027), Notch receptor 4 correlated with less histological differentiation (P = 0.036), and DLL 4 tended to be inversely related to advanced T stage (P = 0.053) (Table 4). The other clinicopathological parameters were not correlated with cytoplasmonuclear coexistent localization of Notch receptors 1-4 and DLL4 expression.
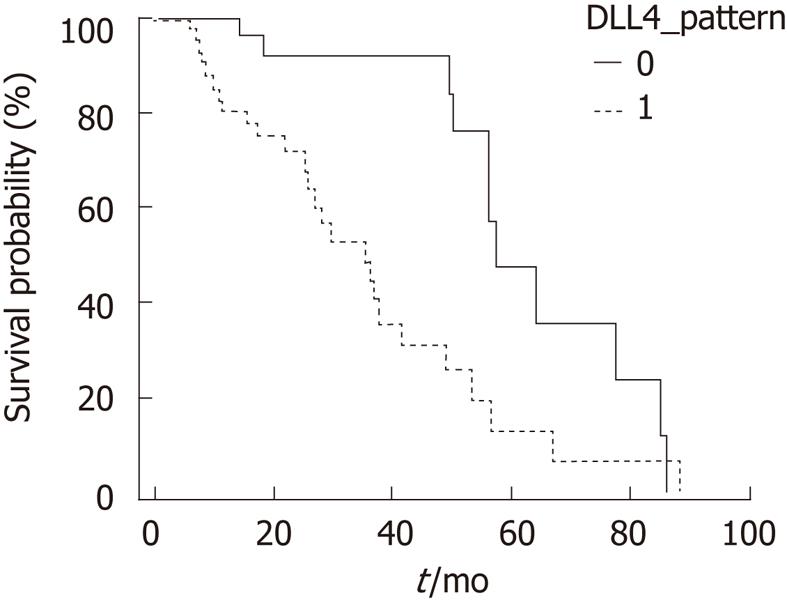
There was no significant correlation between the cytoplasmonuclear coexistent localization of Notch receptor 1-4 expression and survival (Notch receptor 1: P = 0.280, Notch receptor 2: P = 0.204, Notch receptor 3: P = 0.768, Notch receptor 4: P = 0.425). Cytoplasmonuclear coexistent localization of DLL4 expression was related to poor survival in a statistically significant manner (P = 0.002, Figure 5).
The Notch signaling pathway has been shown to be expressed in a variety of adult tissues in mammals, and furthermore is involved in tumorigenesis with neoangiogenesis of many malignant tumors. In addition, it participates in the development of multicellular organisms by its self renewal potential and induction of differentiation[10]. Few studies in the field of Notch signaling involving biliary epithelial cells have been reported with regard to developmental biology and neoplastic transformation. Notch receptor 2 signaling has been reported to be related to the regulation of biliary epithelial cell differentiation, induction of tubulogenesis during early intrahepatic bile duct development in mice, and mutations of Jagged 1 and Notch receptor 2 resulting in Alagille syndrome, a rare hereditary multi-organ disorder involving impaired intrahepatic bile ducts[5,17]. Ishimura et al[18] reported that Notch receptor 1, but not other Notch receptors, was up-regulated in cholangiocytes when induced by inflammatory mediator nitric oxide synthase, and associated with malignant transformation. However, controversies about the role of Notch receptors in tumorigenesis as an oncogene or a tumor suppressor have arisen; Notch receptors are reported generally as an oncogene in most human cancers[10]. As a well-studied example, Notch receptor 1 functioned as an oncogene in T-cell leukemia through t(7; 9) chromosomal translocation of Notch receptor 1 and T-cell receptor Jß locus[8,9]. Activation of Notch signaling is reported in human non-hematopoietic solid tumors originating from mammary duct, colon, kidney, pancreas, and liver, etc[16]. Others have shown Notch signaling as tumor suppressor in skin cancer and small cell lung cancer, and two faces of Notch as oncogene or tumor suppressor in cervical cancer[10].
In this study, aberrantly high expression of Notch receptors 1-4 in extrahepatic CC and gallbladder carcinoma was observed by immunohistochemical study. In human breast cancer, the high expression of Notch receptor 1, Notch receptor 3 and Jagged 1 was reported to be correlated with poor predicted mortality, and the high-level co-expression of Notch receptor 1 and Jagged 1 was associated with poor overall survival[19]. We found that the high expression of Notch receptor 1 related to advanced TNM stage (P = 0.043), and the high expression of Notch receptor 3 related to advanced T stage (P = 0.017) and tended to be related to nodal metastasis (P = 0.065) and advanced TNM stage (P = 0.052) in extrahepatic CC and gallbladder carcinoma. These results suggest that the up-regulation of Notch receptors 1 and 3 has a possible role in tumor progression of extrahepatic CC and gallbladder carcinoma, reflecting its role as an oncogene. However, there were inherent limitations in the number of studied cases for this study. One further limitation we must point out is that the studied cases were mostly surgically removed cancers, thus this study did not include late advanced or early cancers. In addition, there was no relation between high expression of Notch receptors and the overall survival.
Regarding the biologic roles of Notch signaling, liberated Notch intracellular domain after two sequential proteolytic cleavages translocates into the nucleus, and then activates transcription of target genes[3]. In addition, the nuclear translocation of Notch receptor proteins is required for target gene activation, especially in malignant transformation[20]. For the evaluation of gene expression in tissue, in situ IHC is valuable in morphologic identification of individual cell and intracellular localization of specific proteins. The reason why IHC should be applied in cases of human cancer tissue composed of neoplastic and non-neoplastic cells is because generally microvasculature cells, stromal cells and inflammatory cells express Notch signaling proteins, thus extracted samples contain non-neoplastic cells as well as cancer cells. We found that Notch receptors 1-4 were expressed in the cytoplasm of cancer cells, some of which were associated with nuclear co-localization. In hepatocellular carcinoma, the nuclear localization of Notch receptor 1 and Notch receptor 4 expression was reported to be involved in carcinoma development, representing target gene activation through translocated Notch receptor protein components[12]. However, although coexistent cytoplasmonuclear localization of Notch receptors 1-4-expressing CC cells was found with variable ratios, only the coexistent cytoplasmonuclear localization of Notch receptor 3 correlated with no nodal metastasis (P = 0.027) and Notch receptor 4 with less histologic differentiation (P = 0.036). Not many studies in human cancers have dealt with the cytoplasmic and/or nuclear expression of Notch receptor proteins, especially not in biliary epithelial carcinomas. Study of the mechanism of action regarding cytoplasmic high-expression of Notch receptors 1 and 3 in tumor progression during cholangiocarcinogenesis is needed through collection of more cases and application of biological tools.
As one of the five known Notch receptor ligands, the up-regulation of DLL4 expression was found to be involved in vasculogenesis and vessel maturation in human cancers[21,22]. Recently, the expression of DLL4 was reported in human cancer cells as well as in normal epithelial cells, stromal cells, and endothelial cells of neo-angiogenesis[16], thus interest was focused on possible roles in the development and progression of tumors, due to the nuclear or membranous localization. This study demonstrated that DLL4 is highly expressed in cancer cells, mainly in cytoplasm similar to Notch receptors, and that the up-regulation of DLL4 tended to be related to less histologic differentiation (P = 0.095) of extrahepatic CC and gallbladder carcinoma. Furthermore, the coexistent cytoplasmonuclear localization of DLL4 expression was observed and indicated poor survival (P = 0.002). Similar to Notch receptors, there are few reports concerning clinicopathological impact of DLL4 expression with intracellular localization. The up-regulation and cytoplasmonuclear localization of DLL4 expression is suggested to be involved in progression of cholangiocarcinogenesis, and has a probable role as a poor prognosticator. Overall, more cases should be studied for the identification of biological roles, according to intracellular compartmentalization in the expression of Notch receptors and DLL4.
In conclusion, these results imply that the up-regulation of Notch receptors 1 and 3 correlates with cancer progression, and that the coexistent cytoplasmonuclear localization of DLL4 expression correlates with poor survival in extrahepatic CC and gallbladder carcinoma. Further investigation on a large scale should be performed in order to understand the contribution of the involvement of Notch signaling in extrahepatic CC and gallbladder carcinoma.
Cholangiocarcinoma (CC) is the second most frequent primary liver cancer and the incidence is increasing. It has a poorer prognosis than hepatocellular carcinoma. Recently, in mammals, it was reported that the Notch signaling pathway is engaged in not only embryonic development, differentiation and specification of cell fate, but also in tumorigenesis with consequent possibilities of anti-cancer therapy targets. Basically, in human extrahepatic CC, including gallbladder cancer, the expression patterns of the Notch receptors and the representative ligand, Delta-like ligand-4, in cancer cells should be clarified.
There are four Notch receptors (Notch 1-4) and five ligands [Jagged 1, Jagged 2, Delta-like ligand-1, -3 and -4 (DLL1, DLL3, DLL4)] in mammals. Recently, they have been reported to be involved in tumorigenesis as oncogenes or as tumor suppressors, and proposed as prognostic factors or anti-cancer targets in aggressive or advanced cancers. In particular, antibodies to Notch receptor 4 and DLL4 have the possibility to be coupled with chemotherapeutic drugs as targeted therapy.
Abnormal Notch signaling has been reported in many human solid tumors, especially in breast cancer. With regard to cholangiocarcinoma, only its high expression has been reported with little study of the clinical impact. As with other solid tumors, the expression of Notch receptors in CC shows a possible role in cancer progression. The study of DLL4 expression in cancer cells has hardly been looked at. The coexistent cytoplasmonuclear localization of DLL4 expression has a novel value as a poor survival indicator.
The up-regulation and nuclear translocation of DLL4 has a probable role in the evaluation of survival as a poor prognosticator. These results of altered Notch signaling in CC can provide fundamentals for further investigation on an expanded scale of human extrahepatic CC and gallbladder carcinoma, mechanism of action through cross-talk of Notch receptors and their ligands, and other signaling networks.
Four Notch receptors (Notch 1-4) and five ligands (Jagged 1, Jagged 2, DLL1, DLL3, DLL4) are found in mammals. Ligand-receptor interaction between two neighboring cells is involved in developmental, physiologic and pathologic processes.
The paper is reasonably important and includes data on a large cohort of patient's with extrahepatic cholangiocarcinoma and gallbladder cancer.
Peer reviewer: Dr. Neil L Julie, MD, Gastroenterologist,15225 Shady Grove Road Suite 103, Rockville, MD 20850, United States
S- Editor Tian L L- Editor Logan S E- Editor Xiong L
| 1. | de Groen PC, Gores GJ, LaRusso NF, Gunderson LL, Nagorney DM. Biliary tract cancers. N Engl J Med. 1999;341:1368-1378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 708] [Cited by in RCA: 682] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 2. | Welzel TM, McGlynn KA, Hsing AW, O'Brien TR, Pfeiffer RM. Impact of classification of hilar cholangiocarcinomas (Klatskin tumors) on the incidence of intra- and extrahepatic cholangiocarcinoma in the United States. J Natl Cancer Inst. 2006;98:873-875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 267] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 3. | Blechacz B, Gores GJ. Cholangiocarcinoma: advances in pathogenesis, diagnosis, and treatment. Hepatology. 2008;48:308-321. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 553] [Cited by in RCA: 536] [Article Influence: 31.5] [Reference Citation Analysis (0)] |
| 4. | Berthiaume EP, Wands J. The molecular pathogenesis of cholangiocarcinoma. Semin Liver Dis. 2004;24:127-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 68] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 5. | Shi W, Harris AL. Notch signaling in breast cancer and tumor angiogenesis: cross-talk and therapeutic potentials. J Mammary Gland Biol Neoplasia. 2006;11:41-52. [PubMed] |
| 6. | Blaumueller CM, Qi H, Zagouras P, Artavanis-Tsakonas S. Intracellular cleavage of Notch leads to a heterodimeric receptor on the plasma membrane. Cell. 1997;90:281-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 454] [Cited by in RCA: 458] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 7. | Artavanis-Tsakonas S, Rand MD, Lake RJ. Notch signaling: cell fate control and signal integration in development. Science. 1999;284:770-776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4480] [Cited by in RCA: 4555] [Article Influence: 175.2] [Reference Citation Analysis (0)] |
| 8. | Ellisen LW, Bird J, West DC, Soreng AL, Reynolds TC, Smith SD, Sklar J. TAN-1, the human homolog of the Drosophila notch gene, is broken by chromosomal translocations in T lymphoblastic neoplasms. Cell. 1991;66:649-661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1289] [Cited by in RCA: 1290] [Article Influence: 37.9] [Reference Citation Analysis (0)] |
| 9. | Weng AP, Ferrando AA, Lee W, Morris JP, Silverman LB, Sanchez-Irizarry C, Blacklow SC, Look AT, Aster JC. Activating mutations of NOTCH1 in human T cell acute lymphoblastic leukemia. Science. 2004;306:269-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2075] [Cited by in RCA: 2131] [Article Influence: 101.5] [Reference Citation Analysis (0)] |
| 10. | Radtke F, Raj K. The role of Notch in tumorigenesis: oncogene or tumour suppressor? Nat Rev Cancer. 2003;3:756-767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 600] [Cited by in RCA: 616] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 11. | Leong KG, Karsan A. Recent insights into the role of Notch signaling in tumorigenesis. Blood. 2006;107:2223-2233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 374] [Cited by in RCA: 391] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 12. | Gao J, Song Z, Chen Y, Xia L, Wang J, Fan R, Du R, Zhang F, Hong L, Song J. Deregulated expression of Notch receptors in human hepatocellular carcinoma. Dig Liver Dis. 2008;40:114-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 62] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 13. | Nickoloff BJ, Osborne BA, Miele L. Notch signaling as a therapeutic target in cancer: a new approach to the development of cell fate modifying agents. Oncogene. 2003;22:6598-6608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 224] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 14. | Hellström M, Phng LK, Hofmann JJ, Wallgard E, Coultas L, Lindblom P, Alva J, Nilsson AK, Karlsson L, Gaiano N. Dll4 signalling through Notch1 regulates formation of tip cells during angiogenesis. Nature. 2007;445:776-780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1232] [Cited by in RCA: 1304] [Article Influence: 72.4] [Reference Citation Analysis (0)] |
| 15. | Ridgway J, Zhang G, Wu Y, Stawicki S, Liang WC, Chanthery Y, Kowalski J, Watts RJ, Callahan C, Kasman I. Inhibition of Dll4 signalling inhibits tumour growth by deregulating angiogenesis. Nature. 2006;444:1083-1087. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 743] [Cited by in RCA: 767] [Article Influence: 42.6] [Reference Citation Analysis (0)] |
| 16. | Martinez JC, Müller MM, Turley H, Steers G, Choteau L, Li JL, Sainson R, Harris AL, Pezzella F, Gatter KC. Nuclear and membrane expression of the angiogenesis regulator delta-like ligand 4 (DLL4) in normal and malignant human tissues. Histopathology. 2009;54:598-606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 17. | Tchorz JS, Kinter J, Müller M, Tornillo L, Heim MH, Bettler B. Notch2 signaling promotes biliary epithelial cell fate specification and tubulogenesis during bile duct development in mice. Hepatology. 2009;50:871-879. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 94] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 18. | Ishimura N, Bronk SF, Gores GJ. Inducible nitric oxide synthase up-regulates Notch-1 in mouse cholangiocytes: implications for carcinogenesis. Gastroenterology. 2005;128:1354-1368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 87] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 19. | Reedijk M, Odorcic S, Chang L, Zhang H, Miller N, McCready DR, Lockwood G, Egan SE. High-level coexpression of JAG1 and NOTCH1 is observed in human breast cancer and is associated with poor overall survival. Cancer Res. 2005;65:8530-8537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 551] [Cited by in RCA: 592] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 20. | Jeffries S, Capobianco AJ. Neoplastic transformation by Notch requires nuclear localization. Mol Cell Biol. 2000;20:3928-3941. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 103] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 21. | Gale NW, Dominguez MG, Noguera I, Pan L, Hughes V, Valenzuela DM, Murphy AJ, Adams NC, Lin HC, Holash J. Haploinsufficiency of delta-like 4 ligand results in embryonic lethality due to major defects in arterial and vascular development. Proc Natl Acad Sci U S A. 2004;101:15949-15954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 461] [Cited by in RCA: 469] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 22. | Mailhos C, Modlich U, Lewis J, Harris A, Bicknell R, Ish-Horowicz D. Delta4, an endothelial specific notch ligand expressed at sites of physiological and tumor angiogenesis. Differentiation. 2001;69:135-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 216] [Article Influence: 9.0] [Reference Citation Analysis (0)] |