Published online Sep 14, 2011. doi: 10.3748/wjg.v17.i34.3933
Revised: April 20, 2011
Accepted: April 27, 2011
Published online: September 14, 2011
AIM: To investigate the expression of Erythropoietin (Epo) and its receptor (EpoR) in gastric adenocarcinoma (GAC) and the correlation with angiogenesis and clinicopathological features.
METHODS: The expressions of Epo, EpoR and vascular endothelial growth factor (VEGF), as well as microvessel density were evaluated in 172 GAC biopsies by immunohistochemical staining. The correlations between these parameters and patient’s clinicopathological features were analyzed statistically.
RESULTS: The proportion of Epo and EpoR alterations in GAC was higher than that in adjacent normal mucosa (P = 0.035 and 0.030). Epo high-expression was associated with EpoR high-expression, Lauren type, extensive lymph node metastasis and advanced stage of GAC (P = 0.018, 0.018, 0.004 and 0), while EpoR expression was linked with older age, World Health Organization type, extensive lymph node metastasis and advanced stage (P = 0.001, 0.013, 0.008 and 0.001). VEGF high expression was significantly correlated with EpoR low-expression, Lauren type, extensive lymph node metastasis and advanced stage (P = 0.001, 0.001, 0.001 and 0.007). The expression of Epo or EpoR was associated with microvessel density (P = 0.004 and 0.046). On multivariate analysis, only lymph node metastasis, abnormal Epo expression and tumor nodes metastases stage were independently associated with survival. In addition, a strong association with the immunohistochemical expression of EpoR and the angiogenic protein, VEGF, was noted.
CONCLUSION: Increased expression of Epo and EpoR may play a significant role in the carcinogenesis, angiogenesis and progression of GAC. Epo may be an independent prognostic factor.
- Citation: Wang L, Li HG, Xia ZS, Wen JM, Lv J. Prognostic significance of erythropoietin and erythropoietin receptor in gastric adenocarcinoma. World J Gastroenterol 2011; 17(34): 3933-3940
- URL: https://www.wjgnet.com/1007-9327/full/v17/i34/3933.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i34.3933
Gastric adenocarcinoma (GAC) is one of the commonest fatal malignancies in the world. The incidence varies considerably between geographical areas, with a higher incidence in China and other Asian countries than in Western Europe and the United States[1]. Patients with tumors limited to the mucosa and submucosa have an excellent prognosis, with a 5-year survival rate of over 90% after surgery[2]. In contrast, the prognosis for patients with advanced cancers is less predictable and generally poorer. At present, therapeutic decisions are based on clinical-pathological parameters, including age, tumor nodes metastases (TNM) stage and histological grade. Although useful, these factors often fail to differentiate more aggressive tumor types from less aggressive types[3]. As a result, there is an urgent need to find special markers, which are closely related to bionomic characteristics, outcome of gastric adenocarcinoma and performance of antigen-specific therapeutic targeting strategy.
Erythropoietin (Epo) is a low-molecular-weight glycoprotein hormonal stimulator of erythropoiesis produced in the fetal liver and subsequently in the adult kidney[4]. Epo exerts its action through its specific receptor (EpoR), a member of the cytokine receptor superfamily, which is mainly expressed on erythroid colony-forming units[5]. The presence of an autocrine-paracrine Epo-EpoR system in tumors and the possible effects of Epo on the tumor microenvironment and angiogenesis are consistent with a complex biology for Epo-EpoR signaling in cancer.
Epo is a pleiotropic cytokine that exerts diverse biological effects in many non-hematopoietic tissues. There is increasing evidence suggesting a wider biological role for Epo/EpoR unrelated to erythropoiesis. Angiogenesis, the process by which new blood vessels arise from pre-existing vessels, has been shown to be one of the extra-hematopoietic functions of Epo[6]. The precise role of Epo in angiogenesis has not been clarified, although many critical functions of Epo have been reported. Endothelial cells from some sources express EpoR[7]. Moreover, Epo induces endothelial cell proliferation and migration[7-9] and has been shown to stimulate angiogenesis in rat aortic rings in vitro[10]. The expression of EpoR in tumor vascular endothelium suggests that Epo may affect the tumor microenvironment, perhaps by stimulating tumor angiogenesis[6]. In addition, Epo was considered to inhibit endothelial cell apoptosis induced by high glucose[11]. A clinical trial demonstrated that EpoR level correlated with angiogenesis and progression of patients with gastric carcinoma, and that Epo might have a trophic effect on the vasculature of the gastrointestinal tract[12].
The angiogenic potential of Epo was found to be similar to that of vascular endothelial growth factor (VEGF) when stimulating human adult myocardial endothelial cells[13]. VEGFs, as the main regulators in angiogenesis, have chemotoxic effects and promote the division of cells. Many studies have identified that the expression levels of VEGFs in malignant tumor were strongly correlated with their malignant grading, microvessel density and prognosis[14].
In the present study, we investigated the expression of Epo and EpoR in human gastric adenocarcinomas and assessed their possible association with various histopathological features, microvascular density and expression of the VEGF gene. Moreover, the association between Epo/EpoR expression and prognosis was evaluated.
Tumor specimens were obtained from 172 patients (108 males and 64 females; mean age 55.9 years; range 24 to 82 years) who underwent surgery for gastric adenocarcinomas from November 2005 through April 2008. None had received prior chemotherapy or radiotherapy. All patients provided written informed consent.
Clinical and pathological records and slides were available for all cases. HE-stained slides of gastric adenocarcinomas were reviewed and one block with tumor and adjacent normal mucosa (ANM) tissue was selected for immunohistochemical staining. Histopathological examination indicated that 64 GAC samples were intestinal type according to Lauren-type classification, 70 were diffusal type and 38 were mixed type, respectively. According to World Health Organization (WHO) histological classification, 142 patients were diagnosed as tubular type, 12 patients were diagnosed as mucinous type, 4 patients were diagnosed as papillary type, and 14 patients were diagnosed as signet ring cell type. According to TNM classification, there were 10 cases at stage I, 18 at stage II, 80 at stage III and 64 at stage IV, respectively.
Sections (4 μm) of tissue blocks were transferred to an adhesive-coated slide. A 3-step immunoperoxidase technique using streptavidin-peroxidase (S-P) was employed for Epo, EpoR and VEGF detection. All the sections were routinely deparaffinized and rehydrated, then the sections were rinsed in phosphate-buffered saline (PBS, pH = 7.4), and were subsequently treated for antigen retrieval (10 min, microwave oven, 800 W, citrate buffer, pH = 6.0). After cooling at room temperature for 20 min, the sections were rinsed in PBS, and then immersed in 3% H2O2 for 15 min to block the endogenous enzymes. Thereafter, the sections were incubated with normal goat serum at 37 °C for 15 min to block nonspecific antibodies. The primary antibodies were a polyclonal goat antiserum for Epo (polyclonal, N-19, Santa Cruz, United States), a polyclonal rabbit antiserum for EpoR (polyclonal, H-194, Santa Cruz, United States), monoclonal rabbit antiserum for VEGF (monoclonal, ZA-0509, Zhongshan, China) and a mouse monoclonal antibody against the human endothelial cell marker CD34 (monoclonal, ZM-0046, Zhongshan, China), which were diluted at 1:50 dilution. They were used for overnight incubation at 40 °C. The sections were then rinsed in PBS and incubated with biotinylated secondary antibodies (SP kit, Zhongshan, China) and rinsed in PBS again. After interaction with streptavidin-HRP (SP kit, Zhongshan, China) and then rinsed in PBS, the sections were visualized by reaction with 3, 3’-diaminobenzidine and counterstained with hematoxylin. For both antibodies, adequate positive controls of kidney and liver were used according to the manufacturer’s recommendations, and normal goat serum and PBS substituting the primary antibody were used as negative controls.
The immunostaining results were evaluated and scored independently by two pathologists without knowledge of the clinical data of patients. Antibody staining results were scored according to the percentage of cytoplasmic positive cells as follows: (-), < 10%; (+), 11%-20%; (++), > 21%. Only epithelial labeling was scored. Epo, EpoR and VEGF high-expression was defined as > 20% tumor cells with positive staining, whereas low-expression was < 20%.
The counting of microvessels in GAC was evaluated by a previously reported method[15]. Briefly, intratumoral microvessel density (IMD) was observed in areas of most intense neovascularization or hotspots in tumor by light microscopy. After determining the area of highest neovascularization, single microvessels were manually counted on a 200 × field by two different observers without knowledge of patient outcome. Any brown-stained endothelial cell or cell cluster clearly separated from adjacent microvessels was considered as a single, countable microvessel.
All statistical analyses were performed with SPSS 13.0 software for Windows. The chi-square test was used to assess Epo, EpoR and VEGF expression with clinicopathological characteristics. Univariate analysis by Student’s t test was used to assess protein expression in relation to angiogenesis of GAC. The survival curve of patients was determined using the Kaplan-Meier method and Cox regression, and statistical evaluation was performed using the log rank test. P < 0.05 was considered statistically significant.
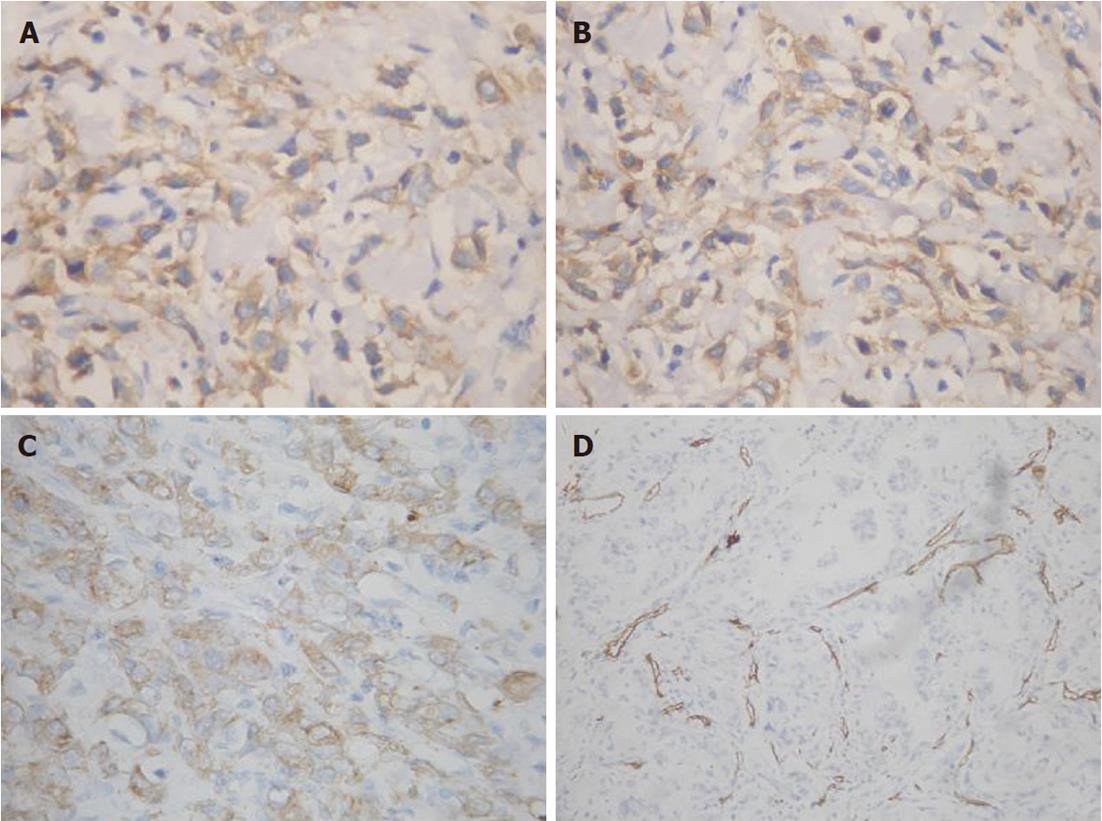
Epo and EpoR expression were strong on the cell membrane and in the cytoplasm of GAC cells (Figure 1A and B). However, the signals were weak in ANM cells. Of the sections with ANM, only 12 (6.98%) of 172 cases were stained positively with Epo antibodies and 15 (8.72%) were stained positively with EpoR antibodies. 74.4% (128/172) of carcinomas stained positively for Epo, and 60.5% (104/172) of carcinomas stained positively for EpoR. A stronger positivity was found in GAC than in ANM for both Epo and EpoR (χ2 = 4.434, P = 0.035 and χ2 = 4.719, P = 0.030, respectively).
Epo and EpoR were detected in concomitant expression in the same portion of tumor cell on serial sections. Epo expression was found to be closely related to the expression of EpoR in tumor (Table 1).
| EpoR | Epo | VEGF | ||||
| Low expression | High expression | P value | Low expression | High expression | P value | |
| 0 | 24 | 44 | 0.018 | 22 | 46 | 0.001 |
| 1 | 20 | 84 | 60 | 44 | ||
Epo high-expression (74.4%, 128/172) was significantly correlated with Lauren type, extensive lymph node metastasis and advanced stage of GAC. However, no significant correlation between Epo high-expression and older age, gender or WHO type was observed. High-expression of EpoR (60.5%, 104/172) was found to have a significant positive correlation with older age, WHO type, extensive lymph node metastasis and advanced stage. No significant correlation between EpoR high-expression and gender or Lauren type was observed (Table 2).
| Case (n = 172) | Epo | EpoR | VEGF | |||||||
| Low | High | P value | Low | High | P value | Low | High | P value | ||
| Age group | 0.557 | 0.001 | 0.115 | |||||||
| < 60 | 107 | 29 | 78 | 53 | 54 | 46 | 61 | |||
| ≥ 60 | 65 | 15 | 50 | 15 | 50 | 36 | 29 | |||
| Gender | 0.19 | 0.822 | 0.428 | |||||||
| Male | 108 | 24 | 84 | 42 | 66 | 54 | 54 | |||
| Female | 64 | 20 | 44 | 26 | 38 | 28 | 36 | |||
| Lauren type | 0.018 | 0.475 | 0.001 | |||||||
| Intestinal | 64 | 22 | 42 | 28 | 36 | 42 | 22 | |||
| Diffusal | 70 | 10 | 60 | 28 | 42 | 28 | 42 | |||
| Mixed | 38 | 12 | 26 | 12 | 26 | 12 | 26 | |||
| WHO type | 0.07 | 0.013 | 0.734 | |||||||
| Tubular | 142 | 40 | 102 | 64 | 78 | 70 | 72 | |||
| Mucinous | 12 | 2 | 10 | 2 | 10 | 4 | 8 | |||
| Papillary | 4 | 2 | 2 | 0 | 4 | 2 | 2 | |||
| Signet-ring cell | 14 | 0 | 14 | 2 | 12 | 6 | 8 | |||
| Lymph node involvement | 0.004 | 0.008 | 0.011 | |||||||
| No | 34 | 10 | 24 | 16 | 18 | 20 | 14 | |||
| 1 | 42 | 18 | 24 | 8 | 34 | 26 | 16 | |||
| > 1 | 96 | 16 | 80 | 44 | 52 | 36 | 60 | |||
| TNM stage | 0 | 0.041 | 0.007 | |||||||
| I | 10 | 4 | 6 | 4 | 6 | 2 | 8 | |||
| II | 18 | 8 | 10 | 6 | 12 | 14 | 4 | |||
| III | 80 | 28 | 52 | 24 | 56 | 32 | 48 | |||
| IV | 64 | 4 | 60 | 34 | 30 | 34 | 30 | |||
| VEGF expression | 0.298 | 0.001 | ||||||||
| Low | 82 | 18 | 64 | 22 | 60 | |||||
| High | 90 | 26 | 64 | 46 | 44 | |||||
Positive VEGF expression was found in 52.3% of (90/172) tumor tissues (Figure 1C). VEGF high-expression was significantly correlated with Lauren type, extensive lymph node metastasis and advanced stage of GAC. However, no significant correlation between VEGF high-expression and gender or WHO type was observed (Table 2). VEGF and EpoR were detected in concomitant expression in the same portion of tumor cell on serial sections. VEGF expression was found to be closely related to the expression of EpoR in tumor (Table 1).
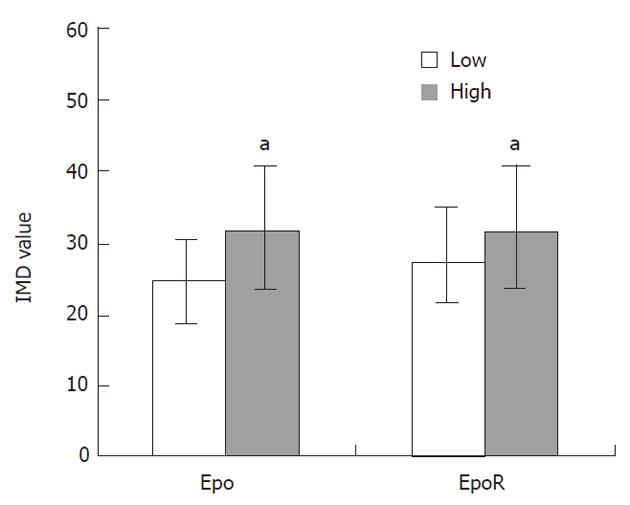
Microvessels in GAC, indicated by CD34 immunostaining, were observed to be scattered in the tumor cell nests (Figure 1D), and were scored as IMD. The correlation between IMD and protein expression in GAC is shown in Figure 2. Mean IMD value was 31.62 ± 14.01 and 31.57 ± 14.01 in the cases with high Epo or EpoR expression, respectively, which were significantly higher than those in the cases with low Epo or EpoR expression (24.82 ± 11.74 and 27.29 ± 13.06, P-values were 0.004 and 0.046, respectively). We examined the relationship between other clinicopathological characteristics and IMD in tumors; however, no significant correlations were found. Therefore, angiogenesis in GAC seemed to be independent of gender, age, WHO type, lymph node metastasis, TNM stage and VEGF expression in patients.
In this study, 150 cases had adequate follow-up data for the final analysis, whereas 22 cases were excluded from survival analysis because the patients were lost to follow-up. The 150 cases were followed up for 1 to 56 mo (mean 27.4 mo), and 98 patients (65.3%) died of tumor during this period.
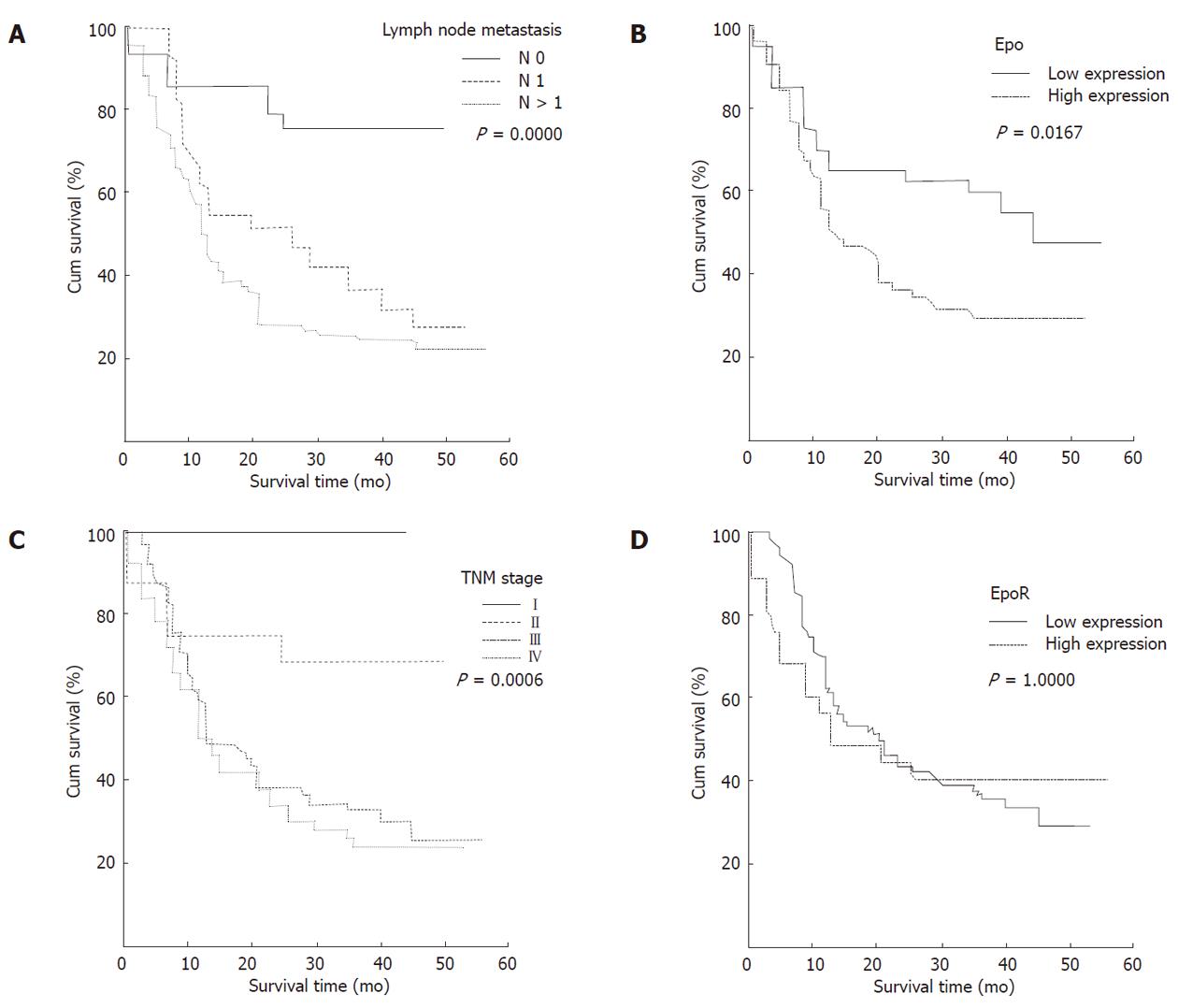
Univariate survival analysis demonstrated that patients with high Epo immunoreactivity, had a significantly worse overall survival compared to patients with low Epo immunoreactivity (log rank test: P = 0.0167, Figure 3B). Median survival time of patients with low Epo expression was 45 mo, which was much longer than patients with high positivity whose median survival time was 14 mo.
Patient survival was also associated with lymph node metastasis and advanced clinical stage (P = 0.000 and P = 0.0006, Figure 3A, 3C). The difference in survival rate was not significant between the GAC patients with different EpoR expressions (P = 1.0000). When patients were stratified according to tumor high-expression of both Epo and EpoR (84 cases), the difference in survival rate was not significant between the patients with both high-expression of Epo and EpoR compared with patients with low-expression of both Epo and EpoR or single protein high-expression (P = 0.5306). In addition, the difference in survival rate was not significant between the patients with different VEGF expression status in tumor cells (P = 0.9756).
On multivariate analysis, only lymph node metasta-sis (hazard ratio, 1.672; 95% confidence interval, 1.2199-2.2916, P = 0.0014), abnormal Epo expression of GAC tumor cells (hazard ratio, 1.6517; 95% confidence interval, 0.9979-2.7338, P = 0.0509) and TNM stage (hazard ratio, 1.4292; 95% confidence interval, 1.0403-1.9636, P = 0.0276) were independently associated with survival (Table 3).
| Variable | B | SE | Wald | df | Sig | R |
| LN metastasis | 0.514 | 0.161 | 10.212 | 1 | 0.001 | 0.096 |
| Epo | 0.502 | 0.257 | 3.810 | 1 | 0.051 | 0.045 |
| TNM | 0.357 | 0.162 | 4.856 | 1 | 0.028 | 0.056 |
Epo is used to manage anemia in cancer patients[16,17]. Patients treated with recombinant human Epo not only have increased levels of hemoglobin, but their performance status also improves significantly, and they enjoy a significantly enhanced quality of life[17]. The expression of the Epo-EpoR system in tumor vascular endothelium suggests that this system may affect the tumor microenvironment, perhaps by stimulating tumor angiogenesis[6,18,19]. In this study, we found a correlation between the expression of Epo or EpoR and CD34 in vascular endothelial cells using immunohistochemistry. Compared with that in cases with low Epo or EpoR expression, microvessel density in tumor in the cases with high Epo or EpoR expression was significantly higher. These results suggested that the Epo-EpoR system is an important factor in gastric adenocarcinoma angiogenesis. The possible effects of Epo/EpoR on tumor angiogenesis are consistent with the complex biology of Epo-EpoR signaling in cancer. Thus, the potential role of the Epo-EpoR system in angiogenesis may be considered as a subsidiary of its possible function in improving overall tissue oxygenation[11].
Epo and EpoR were detected in solid tumors of the brain[20], breast[21,22], kidney[23], female genital tract[24], squamous cell carcinoma of the uterine cervix and tongue[25,26], and were implicated in tumor growth, invasion and metastasis. In the present study, we clearly demonstrated the presence of Epo and EpoR proteins in a series of GAC by immunohistochemistry. Epo and EpoR were highly expressed in GAC as compared to ANM. These results suggest that Epo and EpoR may be involved in the pathogenesis of GAC.
Nakamatsu et al[27] demonstrated that Epo was not detectable in normal or cirrhotic liver tissues without tumors using radioimmunoassay, while immunoreactive EpoR was detectable in the endothelium of intervening vessels of all hepatic tumors using immunohistochemistry. The reason for selective expression of EpoR in the tumor vessels is unclear. The immature nature of tumor vessels compared with mature hepatic vessels may be related to the selective expression of EpoR. In the present study, Epo expression was significantly associated with Lauren type, while EpoR expression was significantly associated with WHO type. These results may suggest that the signaling pathway of Epo is different from that of EpoR in GAC. The most interesting finding of this study is the association between EpoR expression and age. EpoR expression was higher in older patients than in younger patients. The reason for such selective expression in GAC is not clear.
Phenotypic traits of malignant tumor may be caused by microenvironmental selection pressure during carcinogenesis. Hypoxia can drive a tumor towards more malignant phenotypes[28], such as invasion and migration of tumor cells, which may be the basis for lymph node metastasis and distant metastasis. Our previous study demonstrated that increased levels of Epo and EpoR promote invasiveness and lymph node metastases of human tongue squamous cell carcinoma[26]. In the current study, the observed relationship between high Epo or EpoR expression and lymph node metastasis as well as advanced tumor stage indicated that Epo or EpoR might be possible mediators that contribute to the extensive lymph node metastasis and accelerated progression of GAC.
In the Epo-EpoR system, both pathways were important in executing their multifunctions. The co-expression of Epo and EpoR in tumor cells suggests the involvement of an autocrine Epo-EpoR signaling loop. However, in the survival analysis, although high Epo expression in tumor cells was closely associated with lower survival rate of patients with GAC, high EpoR expression was not. Moreover, on multivariate analysis of prognostic factors in GAC, only high Epo expression emerged as an independent factor which influenced the prognosis of GAC patients. These results indicate that the tumor-cell-derived Epo-EpoR system may contribute, in part, to metastasis of lymph node and progression of GAC through an autocrine pathway. Epo is an independent prognostic factor in predicting the prognosis of GAC.
Epo is a hypoxia-inducible stimulator of erythropoiesis. Acting via its receptor (EpoR), Epo up-regulates bcl-2 and inhibits apoptosis of erythroid cells, and then, rescues neurons from hypoxic damage[29]. Although we did not detect expression and regulation of bcl-2 in GAC tumor cells, it is reasonable to believe that only abnormal Epo expression, including absent and endocytosed expression resulting in dysfunction of Epo complexes, plays important roles in metastasis and progression of GAC. Yasuda et al[30] blocked Epo signaling in xenografts of stomach choriocarcinoma in nude mice by ip injections of EpoR antagonist, and found inhibition of angiogenesis and tumor cells, and destruction of tumor masses. The mechanism of Epo and EpoR protein overexpression in GAC merits further investigation to establish these proteins as therapeutic targets.
The expression, regulation and biological significance of the Epo/EpoR system and VEGF in malignant tumor are complicated. It is well known that VEGF induced by hypoxia might mediate hypoxia-initiated angiogenesis[31], while hypoxia could rapidly activate hypoxia inducible factor-3α (HIF-3α) gene expression[32]. Under hypoxic stimulation, HIF-1 has been shown to activate the transcription of Epo[30]. Epo and EpoR gene expressions are under the direct control of hypoxia through stabilization of the HIF1α transcription factor that binds to the hypoxia-responsive element of the Epo gene[33]. Nakano et al[34] demonstrated the important roles of the vascular EpoR system, including induction of postischemic angiogenesis, secretion of VEGF from ischemic muscle and BM-derived cells, and enhancement of VEGFR-2 in ischemic tissue. These results suggested that EpoR might be important for VEGF secretion and angiogenesis.
In the current study, VEGF expression was significantly correlated with Lauren type, lymph node metastasis, TNM stage and EpoR reactivity. To the best of our knowledge, this is the first study to demonstrate a tight relationship between EpoR and VEGF expression in GAC. High EpoR reactivity was linked with low VEGF expression, possibly as a result of an intracellular signal overflow. Thus, we infer a rivalry action existing between EpoR and VEGF when combining with the receptor on tumor surface.
Gastric adenocarcinoma is one of the commonest fatal malignancies worldwide especially in China and other Asian countries. The prognosis for patients with advanced cancers is generally poor. There is an urgent need to find special markers closely related to tumor outcome and therapy.
Erythropoietin (Epo) and its specific receptor (EpoR) are members of the cytokine receptor superfamily. The possible effects of Epo on tumor microenvironment and angiogenesis are consistent with the complex biology of Epo-EpoR signaling in cancer. However, the precise role of Epo-EpoR in gastric adenocarcinoma is still not clear. In this study, the authors demonstrate that the Epo-EpoR system is an important factor in gastric adenocarcinoma angiogenesis.
Epo and the EpoR have been implicated in some solid tumors. However, there are very few data in the medical literature regarding the role of Epo and the EpoR in the carcinogenesis or progression of gastric adenocarcinoma. This study demonstrates that Epo and EpoR are over-expressed in gastric adenocarcinoma. Furthermore, the authors suggest that Epo or EpoR might be possible mediators that contribute to the extensive lymph node metastasis and accelerated progression of gastric adenocarcinoma.
By understanding the important roles of the Epo-EpoR system in metastasis and progression of gastric adenocarcinoma, this study may represent a future strategy for therapeutic intervention in the treatment of patients with gastric adenocarcinoma.
Epo is a pleiotropic cytokine that exerts diverse biological effects in many non-hematopoietic tissues such as stimulating tumor angiogenesis. Epo exerts its action through EpoR.
The authors examined the expression of Epo and EpoR in gastric adenocarcinoma and the correlation with angiogenesis and clinicopathological features. It revealed that Epo and EpoR system provides advantage to the carcinogenesis, angiogenesis, and malignant progression of gastric adenocarcinoma. The results are interesting and indicate that Epo might be an independent prognostic factor in gastric adenocarcinoma.
Peer reviewer: Guida Portela-Gomes, MD, PhD, Associate Professor in Experimental Pathology and Assistant Professor in Gastroenterology, Department of Gastroenterology, Faculty of Medicine, University of Lisbon, Rua Domingos Sequeira-128, Estoril 2765-525, Portugal
S- Editor Sun H L- Editor Webster JR E- Editor Li JY
| 1. | Lambert R, Guilloux A, Oshima A, Pompe-Kirn V, Bray F, Parkin M, Ajiki W, Tsukuma H. Incidence and mortality from stomach cancer in Japan, Slovenia and the USA. Int J Cancer. 2002;97:811-818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 86] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 2. | Siewert JR, Böttcher K, Stein HJ, Roder JD. Relevant prognostic factors in gastric cancer: ten-year results of the German Gastric Cancer Study. Ann Surg. 1998;228:449-461. [PubMed] |
| 3. | Laimer K, Fong D, Gastl G, Obrist P, Kloss F, Tuli T, Gassner R, Rasse M, Norer B, Spizzo G. EpCAM expression in squamous cell carcinoma of the oral cavity: frequency and relationship to clinicopathologic features. Oral Oncol. 2008;44:72-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 4. | Schuster SJ, Koury ST, Bohrer M, Salceda S, Caro J. Cellular sites of extrarenal and renal erythropoietin production in anaemic rats. Br J Haematol. 1992;81:153-159. [PubMed] |
| 5. | Lacombe C, Mayeux P. Biology of erythropoietin. Haematologica. 1998;83:724-732. [PubMed] |
| 6. | Ribatti D, Vacca A, Roccaro AM, Crivellato E, Presta M. Erythropoietin as an angiogenic factor. Eur J Clin Invest. 2003;33:891-896. [PubMed] |
| 7. | Anagnostou A, Liu Z, Steiner M, Chin K, Lee ES, Kessimian N, Noguchi CT. Erythropoietin receptor mRNA expression in human endothelial cells. Proc Natl Acad Sci USA. 1994;91:3974-3978. [PubMed] |
| 8. | Anagnostou A, Lee ES, Kessimian N, Levinson R, Steiner M. Erythropoietin has a mitogenic and positive chemotactic effect on endothelial cells. Proc Natl Acad Sci USA. 1990;87:5978-5982. [PubMed] |
| 9. | Ashley RA, Dubuque SH, Dvorak B, Woodward SS, Williams SK, Kling PJ. Erythropoietin stimulates vasculogenesis in neonatal rat mesenteric microvascular endothelial cells. Pediatr Res. 2002;51:472-478. [PubMed] |
| 10. | Carlini RG, Reyes AA, Rothstein M. Recombinant human erythropoietin stimulates angiogenesis in vitro. Kidney Int. 1995;47:740-745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 171] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 11. | Sekiguchi N, Inoguchi T, Kobayashi K, Nawata H. Effect of erythropoietin on endothelial cell apoptosis induced by high glucose. Diabetes Res Clin Pract. 2004;66 Suppl 1:S103-S107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 12. | Ribatti D, Marzullo A, Nico B, Crivellato E, Ria R, Vacca A. Erythropoietin as an angiogenic factor in gastric carcinoma. Histopathology. 2003;42:246-250. [PubMed] |
| 13. | Jaquet K, Krause K, Tawakol-Khodai M, Geidel S, Kuck KH. Erythropoietin and VEGF exhibit equal angiogenic potential. Microvasc Res. 2002;64:326-333. [PubMed] |
| 14. | Choi WW, Lewis MM, Lawson D, Yin-Goen Q, Birdsong GG, Cotsonis GA, Cohen C, Young AN. Angiogenic and lymphangiogenic microvessel density in breast carcinoma: correlation with clinicopathologic parameters and VEGF-family gene expression. Mod Pathol. 2005;18:143-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 164] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 15. | Rubio L, Burgos JS, Morera C, Vera-Sempere FJ. Morphometric study of tumor angiogenesis as a new prognostic factor in nasopharyngeal carcinoma patients. Pathol Oncol Res. 2000;6:210-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 16. | Ludwig H, Pecherstorfer M, Leitgeb C, Fritz E. Recombinant human erythropoietin for the treatment of chronic anemia in multiple myeloma and squamous cell carcinoma. Stem Cells. 1993;11:348-355. [PubMed] |
| 17. | Ludwig H, Sundal E, Pecherstorfer M, Leitgeb C, Bauernhofer T, Beinhauer A, Samonigg H, Kappeler AW, Fritz E. Recombinant human erythropoietin for the correction of cancer associated anemia with and without concomitant cytotoxic chemotherapy. Cancer. 1995;76:2319-2329. [PubMed] |
| 18. | Ribatti D, Poliani PL, Longo V, Mangieri D, Nico B, Vacca A. Erythropoietin/erythropoietin receptor system is involved in angiogenesis in human neuroblastoma. Histopathology. 2007;50:636-641. [PubMed] |
| 19. | Ribatti D, Marzullo A, Gentile A, Longo V, Nico B, Vacca A, Dammacco F. Erythropoietin/erythropoietin-receptor system is involved in angiogenesis in human hepatocellular carcinoma. Histopathology. 2007;50:591-596. [PubMed] |
| 20. | Batra S, Perelman N, Luck LR, Shimada H, Malik P. Pediatric tumor cells express erythropoietin and a functional erythropoietin receptor that promotes angiogenesis and tumor cell survival. Lab Invest. 2003;83:1477-1487. [PubMed] |
| 21. | Acs G, Zhang PJ, Rebbeck TR, Acs P, Verma A. Immunohistochemical expression of erythropoietin and erythropoietin receptor in breast carcinoma. Cancer. 2002;95:969-981. [PubMed] |
| 22. | Arcasoy MO, Amin K, Karayal AF, Chou SC, Raleigh JA, Varia MA, Haroon ZA. Functional significance of erythropoietin receptor expression in breast cancer. Lab Invest. 2002;82:911-918. [PubMed] |
| 23. | Westenfelder C, Baranowski RL. Erythropoietin stimulates proliferation of human renal carcinoma cells. Kidney Int. 2000;58:647-657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 164] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 24. | Yasuda Y, Fujita Y, Masuda S, Musha T, Ueda K, Tanaka H, Fujita H, Matsuo T, Nagao M, Sasaki R. Erythropoietin is involved in growth and angiogenesis in malignant tumours of female reproductive organs. Carcinogenesis. 2002;23:1797-1805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 91] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 25. | Acs G, Zhang PJ, McGrath CM, Acs P, McBroom J, Mohyeldin A, Liu S, Lu H, Verma A. Hypoxia-inducible erythropoietin signaling in squamous dysplasia and squamous cell carcinoma of the uterine cervix and its potential role in cervical carcinogenesis and tumor progression. Am J Pathol. 2003;162:1789-1806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 26. | Li HG, Li JS, Chen WL, Wang L, Wu DH, Lin ZY. Prognostic significance of erythropoietin and erythropoietin receptor in tongue squamous cell carcinoma. Br J Oral Maxillofac Surg. 2009;47:470-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 27. | Nakamatsu K, Nishimura Y, Suzuki M, Kanamori S, Maenishi O, Yasuda Y. Erythropoietin/erythropoietin-receptor system as an angiogenic factor in chemically induced murine hepatic tumors. Int J Clin Oncol. 2004;9:184-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 28. | Lee WY, Huang SC, Hsu KF, Tzeng CC, Shen WL. Roles for hypoxia-regulated genes during cervical carcinogenesis: somatic evolution during the hypoxia-glycolysis-acidosis sequence. Gynecol Oncol. 2008;108:377-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 43] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 29. | Sättler MB, Merkler D, Maier K, Stadelmann C, Ehrenreich H, Bähr M, Diem R. Neuroprotective effects and intracellular signaling pathways of erythropoietin in a rat model of multiple sclerosis. Cell Death Differ. 2004;11 Suppl 2:S181-S192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 128] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 30. | Yasuda Y, Fujita Y, Matsuo T, Koinuma S, Hara S, Tazaki A, Onozaki M, Hashimoto M, Musha T, Ogawa K. Erythropoietin regulates tumour growth of human malignancies. Carcinogenesis. 2003;24:1021-1029. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 200] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 31. | Shweiki D, Itin A, Soffer D, Keshet E. Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature. 1992;359:843-845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3206] [Cited by in RCA: 3224] [Article Influence: 97.7] [Reference Citation Analysis (0)] |
| 32. | Heidbreder M, Fröhlich F, Jöhren O, Dendorfer A, Qadri F, Dominiak P. Hypoxia rapidly activates HIF-3alpha mRNA expression. FASEB J. 2003;17:1541-1543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 130] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 33. | Manalo DJ, Rowan A, Lavoie T, Natarajan L, Kelly BD, Ye SQ, Garcia JG, Semenza GL. Transcriptional regulation of vascular endothelial cell responses to hypoxia by HIF-1. Blood. 2005;105:659-669. [PubMed] |
| 34. | Nakano M, Satoh K, Fukumoto Y, Ito Y, Kagaya Y, Ishii N, Sugamura K, Shimokawa H. Important role of erythropoietin receptor to promote VEGF expression and angiogenesis in peripheral ischemia in mice. Circ Res. 2007;100:662-669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 136] [Article Influence: 7.6] [Reference Citation Analysis (0)] |