Published online Sep 14, 2011. doi: 10.3748/wjg.v17.i34.3870
Revised: March 7, 2011
Accepted: March 14, 2011
Published online: September 14, 2011
Adult stem cells represent the self-renewing progenitors of numerous body tissues, and they are currently classified according to their origin and differentiation ability. In recent years, the research on stem cells has expanded enormously and holds therapeutic promises for many patients suffering from currently disabling diseases. This paper focuses on the possible use of stem cells in the two main clinical settings in gastroenterology, i.e., hepatic and intestinal diseases, which have a strong impact on public health worldwide. Despite encouraging results obtained in both regenerative medicine and immune-mediated conditions, further studies are needed to fully understand the biology of stem cells and carefully assess their putative oncogenic properties. Moreover, the research on stem cells arouses fervent ethical, social and political debate. The Italian Society of Gastroenterology sponsored a workshop on stem cells held in Verona during the XVI Congress of the Federation of Italian Societies of Digestive Diseases (March 6-9, 2010). Here, we report on the issues discussed, including liver and intestinal diseases that may benefit from stem cell therapy, the biology of hepatic and intestinal tissue repair, and stem cell usage in clinical trials.
- Citation: Burra P, Bizzaro D, Ciccocioppo R, Marra F, Piscaglia AC, Porretti L, Gasbarrini A, Russo FP. Therapeutic application of stem cells in gastroenterology: An up-date. World J Gastroenterol 2011; 17(34): 3870-3880
- URL: https://www.wjgnet.com/1007-9327/full/v17/i34/3870.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i34.3870
In recent years stem cells (SCs) have become increasingly important in all fields of modern medicine. Different types of SCs are eligible for cell therapy, such as mesenchymal stem cells (MSCs), hematopoietic stem cells (HSCs) and adult liver stem/progenitor cells (LPCs). Although the clinical usefulness of the related lines of research remains to be ascertained in human trials, there is fascinating potential for growth in the use of SCs as a therapeutic option. It is now well known that adult SCs are the self-renewing progenitors of numerous body tissues, classified according to their origin and ability to differentiate, and functionally responsible for the development and regeneration of tissues and organs, including the gastrointestinal tract and liver[1]. Embryonic stem cells would probably be the best cells to use in clinical research, but their use raises many ethical controversies, therefore the researchers’ attention has shifted instead to adult stem cells as a potential therapeutic tool.
SCs have recently gained great importance in gastroenterology and hepatology regarding both the pathogenesis and the treatment of liver diseases and inflammatory or immune-mediated bowel diseases, two clinical fields with a great impact for people worldwide.
But the other side of the presence of SCs in the gastrointestinal tract and liver needs to be carefully considered since the self-renewing properties characteristic of SCs may sometimes give them a key role in the processes of carcinogenesis[2].
Discussion on the therapeutic applications of SCs in gastroenterology (and other fields) was first prompted by a previously-published paper[3]. The Italian Society of Gastroenterology (SIGE) consequently sponsored a workshop on SCs in Verona during the Congress of the Federation of Italian Societies of Digestive Diseases (FISMAD) (March 6-9, 2010). The present paper reports on the issues analyzed, ranging from which liver and intestinal diseases may benefit from stem cell therapy to the biology of liver and intestinal tissue repair, and stem cell usage in clinical trials.
The liver and gastrointestinal epitheliums are known to have great regenerative potential in response to injuries and normal cell turnover[4,5].
The intestine consists of rapidly-proliferating tissue, in which cell turnover occurs every 2-7 d under normal circumstances, and even more rapidly following tissue damage[3]. This rapid regenerative potential is possible thanks to a consistent proliferation of progenitor cells that occurs in crypts. Indeed, unlike most other mammalian tissues, the stem cells of the intestine are strictly compartmentalized in crypts. Two hypotheses exist regarding the exact identity and localization of SCs. The principal hypothesis, developed in the late 1950s and experimentally supported by Potten in the 1977[6], is the so-called “+4 position model”. According to this theory, SCs are found directly above the Paneth cells, in position 4 starting from the bottom of the crypt. From there, the SCs move up and differentiate into Goblet cells, enterocytes and enteroendocrine cells, or they move down to become Paneth cells[7]. The second, and more recent, hypothesis is the “SCs zone model”, which is based on the identification of the crypt base columnar (CBC) cells hidden between the Paneth cells[8]. In 2007, Berker and colleagues demonstrated that CBC cells express a peculiar marker, the Lgr5, a Wnt target gene that encodes an orphan G protein-coupled receptor characterized by a large leucine-rich extracellular domain and seven transmembrane domains[9]. Using lineage tracing experiments, they demonstrated that the Lgr5+ cells are multipotent, giving rise to all the different intestinal epithelial cells types, and are very long-lived. Similar observations were made also in the colon, suggesting that the CBC Lrg5+ cells are authentic intestinal SCs[10]. Consequently, Lgr5 can be identified as a definitive marker of crypt SCs[11,12].
Other than the resident intestinal SCs, recent studies have suggested that mesenchymal cells derived from bone marrow (BM) have a crucial role in intestinal repair and fibrosis[13-15]. Studies conducted on both experimental animals and humans given sex-mismatched BM-derived SCs (BM-SCs) transplants showed that a population of myofibroblasts (MFs) derived from the male donor populated the mucosa in the female host intestine, and the sub-epithelial compartment in particular[16]. Given the importance of these MFs in orchestrating epithelial cell turnover and function, it may be that they have a positive therapeutic effect on gastrointestinal functions. Further studies demonstrated that donor-derived cells were able to repopulate the host’s intestinal epithelium with cells expressing all four lineage markers (goblet cells, Paneth cells, enteroendocrine and enterocytes[4]). The donor BM-SCs were also able to differentiate into all the cell types needed for neo-angiogenesis (i.e. pericytes, endothelial cells and vascular smooth muscle cells), thus contributing to tissue repair. However, the mechanisms by which SCs take effect in gut injury are still under debate. Some researchers believe that BM-SCs differentiate into intestinal SCs or progenitor epithelial cells, while others have demonstrated that a fusion with resident cells takes place. Protection against injury can be achieved even in the absence of SC differentiation, giving the impression that SCs aid native tissues via cell-cell interaction or by releasing protective substances as they transit through injured tissues[4].
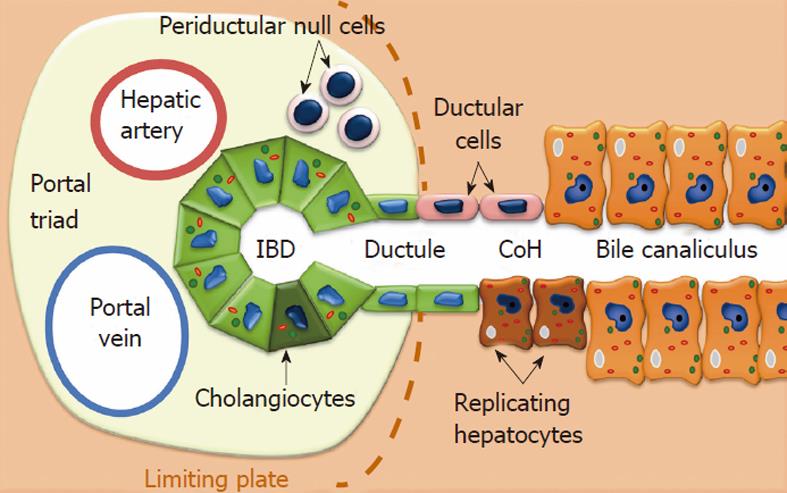
In the liver, regeneration mainly involves mature hepatocytes, highly differentiated cells with a long lifespan that can re-enter the cell cycle and restore the liver mass in response to parenchymal loss[5]. If the hepatocytes’ replication is impaired, or experimentally inhibited, then regeneration can be accomplished by the activation, expansion, and differentiation of LPCs putatively located within the canals of Hering (CoH). LPCs are responsible for the so-called “ductular reaction” in humans, which corresponds to the oval cell (OC) response seen in specific rodent models of liver injury[17]. Numerous studies have shown that both OCs and LPCs are highly clonogenic and bipotent cells (capable of differentiating into hepatocytes and cholangiocytes), and that they co-express biliary and hepatocytic markers, as well as HSC-associated antigens, such as CD34 and c-kit[18]. LPCs are a heterogeneous population with a variable stemness potential and notable phenotypic discrepancies, depending on the experimental conditions[19]. Using a functional assay, Kuwahara et al[20] found 4 possible hepatic cell populations with a stemness potential: (1) replicating hepatocytes at the parenchymal/stromal interface; (2) ductular cells of the CoH; (3) cholangiocytes of the intralobular bile ducts; and (4) periductal null cells (devoid of hepatocytic and biliary markers). The asymmetrically-dividing cells that populate these sites may represent some form of lineage hierarchy within the LPC population: periductal null cells might give rise to the cytokeratin-positive cells of the CoH, which in turn could generate the intraductal cells and periductal hepatocyte-like cells[21] (Figure 1).
In some cases the LPC are supported in the regeneration process by stem cells mobilized from BM. Various studies have in fact reported that stem cells from BM can contribute to liver regeneration through their release into the circulation, migration to the liver and differentiation into hepatocytes. However, the extents to which this occurs and the mechanisms involved remain highly controversial issues[1,22].
Estimates of liver repopulation by HSCs vary widely, ranging from 0.01% to 40%[23-34]. Petersen et al[24] originally reported that BM-SCs transplanted into sublethally irradiated rats repopulate the BM and then migrate to the liver and “transdifferentiate” into hepatocytes by entering the liver OC progenitor pathway. BM-SCs are believed to have the ability to expand clonally and to have a bipotential capacity that enables them to differentiate into both hepatocytes and bile duct epithelial cells[35,36]. This mechanism was generally accepted until studies by Wang et al[37] using lacZ marking showed that BM cells did not enter the OC pool in wild-type mice treated with DDC, nor did they contribute to liver repopulation by OCs in mouse recipients. Dabeva et al[38] also showed that BM cells transplanted into rats contributed less than 1% to the OCs expanded after inducing damage with 3 different methods: (1) 2-acetylaminofluorene/partial hepatectomy (PH); (2) retrorsine/PH; or (3) D-galactosamine-induced liver injury. The debate is still open.
Fibrosis is the progressive accumulation in the tissues of fibrillary extracellular matrix (ECM), but - for its pathophysiological role to be fully understood - it should be seen as a process of chronic wound healing taking place in a tissue continually subjected to injury.
Intestinal fibrosis is a potentially severe complication of inflammatory bowel diseases (IBD), such as Crohn’s disea-se, and its pathophysiology is still unclear. The presumably key events in the development of IBD-associated fibrosis are the exposure of MFs to inflammatory mediators, and the subsequent production of ECM and tissue remodeling[29]. Intestinal fibrosis is characterized by myofibroblast accumulation secondary to their local proliferation, migration and recruitment from the BM. Different studies have shown that BM-SCs frequently engraft in the intestine and differentiate into MFs in the lamina propria. In situ hybridization was used to detect Y-chromosomes in these cells and their myofibroblastic phenotype was confirmed by their immunostaining positivity for alpha-smooth muscle actin (α-SMA) and negativity for desmin, the mouse macrophage marker F4/80, and the hematopoietic precursor marker CD34. These results were confirmed in mice as early as 1 wk after BM transplantation, and were also seen 2 and 6 wk after cell transplantation, indicating that transplanted BM cells are capable of withstanding a sustained turnover of the MF cells in the lamina propria[39].
Intestinal MFs can also derive from alternative sources however, such as circulating fibrocytes and the process of “epithelial-mesenchymal transition (EMT)”. Fibrocytes are BM-derived circulating mesenchymal progenitors that co-express hematopoietic and mesenchymal cell markers, and produce ECM components[30]. In inflammatory processes, fibrocytes are released from the BM and migrate to the sites affected where they differentiate into epithelial, endothelial, neuronal and mesenchymal cells[30]. In several systems, significant numbers of fibroblasts may be generated by the transformation of non-mesenchymal into mesenchymal cells in a process termed EMT[40], during which epithelial cells lose their expression of E-cadherin and other components of epithelial cell junctions and acquire a mesenchymal cell phenotype[41]. This process has a role in the genesis of the fibroblasts that contribute to fibrosis in adult tissues.
In the liver, fibrosis is a multicellular, integrated process requiring a close cross-talk between hepatocytes, cholangiocytes, and non-parenchymal cells (including infiltrating inflammatory cells, Kupffer cells, hepatic stellate cells and sinusoidal endothelial cells)[42]. Nearly all forms of chronic liver disease can cause fibrosis, though its rate of progression and likelihood of leading to cirrhosis differs in the various etiologies.
All forms of fibrogenesis develop in the context of tissue damage, where hepatocytes and non-parenchymal cells produce signals that target hepatic stellate cells and other fibrogenic MFs, leading to the accumulation of ECM. The generation of reactive oxygen species and non-oxidant products of oxidative stress exacerbates the hepatocellular damage, promoting inflammation and Kupffer cell activation. Oxidative stress also directly provides pro-fibrogenic stimuli to hepatic MFs[43].
Hepatic stellate cell activation is considered the major source of MFs in liver damage, but other ECM-producing cells contribute to liver fibrosis, including fibroblasts and portal tract MFs, smooth muscle cells localized in the vessel walls, and MFs located around the centrilobular vein[42]. Recent studies have demonstrated, moreover, that epithelial cells (both hepatocytes and bile duct epithelial cells) have the ability to acquire myofibroblastic features in the process of EMT, as in the intestine[44], although the extent to which this process contributes to the development of fibrosis remains controversial.
The role of BM-SCs in the pathogenesis of liver fibrosis has recently been the object of considerable interest. It is usually impossible to track the lineage of cells in humans, although this was done in a study by Forbes et al[45] in a series of male patients with sex-mismatched liver transplants who subsequently developed graft fibrosis, and in one female patient who developed cirrhosis after receiving a BM transplant from a male. The authors used Y chromosome tracking to identify the origin of the cells participating in liver fibrosis. Substantial numbers of scar-associated MFs in fibrotic areas were found to derive from BM. Using a mouse model of liver fibrosis in which sex-mismatched BM transplants were performed, the same group found clear evidence of a BM contribution to the MFs in fibrotic scars[46], and provided evidence that the BM contributes to both the macrophage and stellate cells populations in the injured liver[47]. By subfractionating the BM-SCs compartment, it was demonstrated that, although HSCs contribute to the inflammatory cell infiltrate, the BM-derived MF-like cells originate from MSCs. Intriguingly BM-SCs are widely distributed in the scar tissue in advanced fibrosis. This suggests that, whatever the origin and topography of the injury in chronic disease, BM-derived MFs gradually begin to replace local MF recruitment. Evidence of a functional role of BM-derived MFs was provided by transplanting BM from mice bearing a reporter transgene for collagen, before inducing fibrosis, showing that the recruited MFs transcribe this gene. Also, when wild-type mice were transplanted with BM from a transgenic mouse that develops a characteristic liver scarring pattern (because it expresses a form of collagen I not susceptible to degradation by matrix metalloproteinases), CCl4 administration induced liver scarring, with characteristics similar to those seen in the BM donor mouse. These data indicate that the transfer of genetically modified BM alters the phenotype of the liver fibrosis, making it reflect the genotype of the BM donor rather than that of the recipient mouse. The same study also provided conclusive evidence that recruited cells contribute directly to fibrosis through the expression, synthesis, and secretion of collagen[47].
Despite the available evidence for the contribution of BM-SCs, the matter is still widely disputed. Indeed, a recent paper revealed an unexpectedly limited role of BM-derived cells in collagen production in two mechanistically distinct models of liver fibrosis[48]. Although some of the BM-derived cells exhibited a mesenchymal morphology resembling that of MFs, the number of BM-derived α-SMA-positive cells was much smaller than previously reported. More importantly, specific and quantitative analyses of collagen type I alpha 2 promoter activation, using a combination of enhanced green fluorescent protein and luciferase reporter genes, clearly showed that BM-derived cells produce little, if any, type I collagen during hepatic fibrogenesis.
It is well recognized that inflammatory and immune-mediated bowel diseases, such as Crohn’s disease and celiac disease, are due to a dysregulation of the immune response in genetically susceptible individuals. Both these conditions have a strong impact on public health due to their increasing prevalence and incidence in Western populations.
Medical therapy for these disorders has improved dramatically in the last decade with the introduction of targeted biological therapies, the optimization of older therapies and a better understanding of the mucosal immune system and genetics involved in the pathogenesis of IBD. Nevertheless, a considerable number of patients remain refractory to therapy, or become unresponsive to, or intolerant to therapy[49]. Unconventional strategies have consequently been investigated, identifying the use of SCs as an effective alternative approach to IBD.
The possibility that SCs might represent an effective treatment in IBD and celiac disease initially emerged from several case reports of remission being induced in patients undergoing hematopoietic stem cell transplantation (HSCT) for concomitant hematological malignancies. Theoretically, allogeneic HSCT could be beneficial by replacing the genetic predisposition to IBD, and autologous HSCT might also offer the advantage of a more intense immunosuppression than would otherwise be given, thereby clearing the body of committed lymphocyte clones. On this basis, two phase I clinical trials were carried out on a total of 16 patients who underwent autologous HSCT for refractory Crohn’s disease[50,51]. The results were promising, with remission observed in 14/16 patients, but the adverse effects were far from negligible. As for celiac disease, autologous HSCT was used in a phase I clinical trial to treat 7 patients suffering from refractory celiac disease type II, prompting a significant reduction in the number of aberrant T cells and an improvement in both clinical and serological parameters in 6/7 cases after a mean follow-up of 15.5 mo[52]. In contrast, when 15 patients with enteropathy-associated T cell lymphoma as a complication of celiac disease were treated with autologous HSCT in three phase I trials, 10 patients died of progressive disease, indicating that the HSCT afforded no benefit in prognostic terms[53-55].
MSCs can be isolated from various tissues, such as BM, adipose or muscle tissue, fetal tissues and perivascular tissue, and they can support hematopoiesis and differentiation towards adipogenic, osteogenic[56], and myogenic lineages[57]. These cells are identified by their expression of a particular panel of surface molecules, e.g., CD105, CD73, CD90, and the absence of CD14, CD34, CD45, and HLA-DR. They elicit no proliferative response from alloreactive lymphocytes because of the negligible levels of extracellular MHC class I and II determinants (though they are present intracellularly). MSCs are also endowed with important immunomodulatory functions in all the cells involved in both the innate and adaptive immune responses[58]. In vitro studies have demonstrated that MSCs can inhibit or suppress several T cell functions, such as proliferation after mitogen and antigen stimulation and inducing cell cycle arrest, probably via the release of chemokines, nitric oxide and the enzyme indoleamine 2, 3-dioxygenase. MSCs also do not preferentially target any T cell subset and their inhibition can also extend to B cells, NK cells, and dendritic cells. More precisely, dendritic cells cultured in the presence of MSCs have an impaired T cell stimulatory activity in a mixed lymphocyte reaction, with a shift from predominantly pro-inflammatory Th1 to anti-inflammatory Th2 cells, thus skewing the immune response to T-cell tolerance. Combined, these results support a role for MSCs in preventing rejection after organ transplantation and in the treatment of immune-mediated diseases.
A parallel series of in vivo studies conducted on animal models of gastrointestinal injury (gastric and colonic ulcers, and IBD), such as dextran sulfate sodium- and trinitrobenzene sulfoxide-induced colitis, showed beneficial effects of MSCs after both systemic infusion and topical injection[59-61]. These studies showed that MSCs preferentially homed onto areas of mucosal injury, promoted tissue repair and neo-angiogenesis, reduced inflammation, and restored the immune cell balance.
To date, over 60 MSC clinical trials have been registered and/or are underway according to the website (http://www.clinicaltrials.gov) in the fields of immune-mediated and cardiovascular diseases, orthopedics, and organ transplantation. As for gastrointestinal diseases, MSCs were used in a single systemic infusion in an open-label phase 2 trial testing Prochymal (ex vivo cultured human MSCs, Osiris Therapeutics) for the treatment of refractory Crohn’s disease. All 10 patients treated had a statistically significant, mean 105-point reduction in their CDAI score by day 28, and there appeared to be a positive correlation between the dose of cells infused and the clinical response, with patients on high doses achieving a better response[62]. The most impressive results, however, emerged from a multicenter, phase II trial in which patients with severe, acute GvHD refractory to conventional therapies were treated with two MSC infusions, resulting in an overall survival rate of 53% two years later[63].
Given the immunomodulatory function of MSCs and their ability to home onto sites of tissue injury, the Ciccocioppo group in Pavia demonstrated that BM-derived MSCs from Crohn’s disease patients can be isolated and expanded, and that this cell population exhibits the same biological characteristics as those from healthy controls. MSCs from Crohn’s disease patients may therefore be considered for use in cell therapy in an autologous setting.
In general, the convenient isolation procedure, the lack of significant immunogenicity (which allow for allogeneic transplantation without using immunosuppressive drugs), the absence of ethical controversies, the potential to differentiate into tissue-specific cell types with a trophic activity, and their immunosuppressive and immunomodulatory effects make MSCs particularly interesting with a view to their potential uses in regenerative therapy in many gastrointestinal diseases[64].
Liver disorders affect hundreds of millions of patients worldwide. Liver failure can be defined as the inability of the liver to perform its normal, physiological synthetic and metabolic functions due to severe hepatic injury. Classically, liver failure is divided into acute (ALF) and chronic forms (CLF). ALF is characterized by the sudden onset of hyperbilirubinemia, hepatic encephalopathy and coagulopathy with no underlying liver disease; this remains a dramatic, unpredictable condition, with high morbidity and mortality rates[65]. CLF usually occurs in the context of hepatic cirrhosis, which can be the result of many possible causes, including excessive alcohol consumption, chronic hepatitis B or C, autoimmunity, or metabolic disorders. The natural course of chronic liver disease is often complicated by acute episodes of potentially reversible decompensation, triggered by a precipitating event, such as infection or upper gastrointestinal bleeding; this situation is frequently called acute-on-chronic liver failure (AoCLF)[66].
With the help of intensive care and artificial liver support, a substantial proportion of ALF patients may recover spontaneously, but orthotopic liver transplantation (OLT) is the only curative option for end-stage liver diseases and remains the final resort of proven benefit for ALF too, raising the one-year survival rate of ALF patients from 50% to 75%[67]. Organ shortage remains a major limitation however, and alternative solutions are being examined. Hepatocyte transplantation, or the use of these cells in bioartificial livers[68,69], have attracted attention in the past three decades, but their exploitation in clinical practice still poses considerable problems, including the difficulty of obtaining hepatocytes and particularly of maintaining their viability and differentiated function when cultured in vitro. These issues have been investigated with the aim of improving results[70,71], but the use of SCs and/or growth factors seems to be a more appealing and applicable solution for promoting liver repair[3,72,73].
The possible therapeutic value of BM-SCs was first investigated by intraportally transplanting autologous CD133+ cells in patients with liver cancer undergoing portal embolization prior to extensive liver resection (LR)[74]. A significant improvement in Child-Pugh score and albumin levels was reported in 9 cirrhotic patients given a portal vein infusion of unsorted autologous BM-SCs[75]. Improved liver function after LR was also recently documented in patients with cirrhosis and hepatocellular carcinoma (HCC) after they underwent autologous BM-SCs transplantation prior to surgery[76].
Other clinical approaches rely on the administration of G-CSF in combination with leukocyte apheresis and reinfusion of mobilized HSC. The feasibility, safety and BM-SCs mobilization patterns following G-CSF treatment has been assessed in patients with cirrhosis[77]. Yannaki et al[78] reported on the successful use of boost infusions of mobilized CD34+ cells after a standard G-CSF regimen in 2 patients. A significant biochemical and histopathological improvement was achieved by infusing G-CSF-mobilized CD34+ HSCs in a patient with drug-induced ALF[79]. In a phase I clinical trial on 5 patients with AoCLF, administering G-CSF and then reinfusing the CD34+ cells improved liver function in more than 50% of cases during a 60-d follow-up[80]. The same patients were then monitored for up to 18 mo, during which time the procedure was judged to be safe and the beneficial effects lasted around 12 mo[81]. In 9 patients with alcohol-related cirrhosis, the reinfusion of CD34+ HSCs (collected after G-CSF mobilization and expanded in vitro) was well tolerated and beneficial to liver function[82]. In another trial, 40 patients with HBV-related cirrhosis were randomized to receive G-CSF alone or in combination with the reinfusion of peripheral blood monocytes in the hepatic artery. During a 6-mo follow-up, a significant biochemical and clinical improvement was seen in both groups[83]. Finally, G-CSF has been used alone to treat end-stage liver diseases in a few small clinical trials: overall, the procedure proved safe and was well tolerated, though these studies were too small to draw any conclusions regarding patient survival or treatment efficacy[77,84-86]. It is worth noting, however, that G-CSF administration was associated with the induction of endogenous liver SCs proliferation within 7 d in the largest randomized trial published to date, conducted by Sphar et al[86].
Most of the above-mentioned clinical trials have their limitations, having been conducted on small groups of patients, with no controls, and using outcome parameters that are easily biased, as mentioned elsewhere[87,88]. It seems likely that the main role for SC-based therapies in hepatology will be as a bridge to transplantation, or as a way to maintain patients who are not eligible for OLT, using repeated infusions of SCs and/or growth factors to stabilize their liver function. Some conceptual and technical issues nonetheless continue to restrict the diffusion of such treatments in clinical practice[87,89] and, until these open questions have been properly answered, SC-based therapies for liver diseases should be limited to well-designed and adequately powered clinical trials.
The cellular origin of most solid tumors is largely unknown, but different tumor subtypes seem to reflect different origins at the time of cancer initiation. Cells within the tumor population also often exhibit different phenotypic and functional characteristics, which lend heterogeneity to the tumor mass[90].
At least two models have been proposed to account for the heterogeneity and inherent differences in tumor-generating capacity of SCs, including the cancer stem cells (CSCs) and the clonal evolution models[91,92]. In the CSCs model, based on a cell hierarchy within the tumor, only a minority of tumor cells can generate a tumor, based on their self-renewal properties and enormous proliferative potential. The clonal evolution model, on the other hand, postulates that genetic and epigenetic changes can occur stochastically over time in individual cancer cells, giving them a selective advantage in proliferating and forming a tumor mass. These two models are not mutually exclusive in cancers following a stem cell model, because CSCs would be expected to evolve via the clonal evolution of transformed SCs[2]. The hypothesis that stem cells could be targets of malignant transformation has led to an awareness of the similarities between CSCs and normal SCs, including their surface marker phenotype and molecular machinery relating to self-renewal and differentiation. In the last ten years, evidence has been accumulating to indicate that CSCs are not only involved in the perpetuation of hematopoietic tumors, but also play a part in various solid cancers, including those of the breast, brain, prostate, colon, and liver.
Colorectal cancer is the second leading cause of cancer-related death in the western world. A number of studies have produced evidence of the existence of colon CSCs and demonstrated that the tumorigenic cell population of colorectal cancer can be isolated from its expression of specific cell surface biomarkers. The existence of colon CSCs was first reported by the Dick and De Maria research groups[93,94], each of which identified a small population of cancer cells capable of initiating tumor growth in immunodeficient mice and staining positive for the marker CD133. This marker (also known as prominin-1) was initially identified as a marker of Drosophila neuroblasts[95]. Although it is expressed on different types of stem cell and may play a part in cell polarity, it has no known key role in stem cell function. CD133 is reportedly a potential marker of CSCs in various tumors[96], but its role as a marker of colorectal cancer was recently questioned by Shmelkov et al[97], who showed that both CD133-positive and CD133-negative metastatic colon cancer cells can initiate a tumor. For the time being it is not clear whether all reported markers really do mark CSCs or merely lead to their enrichment, and whether these markers are directly linked to stem cell functions. So the search for the perfect CSC marker, which could be useful as a therapeutic target, goes on.
The cellular origin of HCC has long been debated, but whether it originates from mature hepatocytes or stem/progenitor cells, or both, remain to be seen. It has been suggested that intrahepatic stem cells can give rise to human HCC and cholangiocarcinoma (CC)[98], since oval cell activation has been demonstrated in rodent models of HCC and CC[99,100]. A role for intrahepatic stem cells in carcinogenesis is also supported by a histological subtype of liver malignancy that displays features of both HCC and CC (HC-CC), combined with the presence of numerous liver progenitor cells[101,102].
Putative CSCs have recently been isolated from both cancer cell lines and primary HCC tissue using different cell surface markers specific for normal stem cells (Table 1). CD133 seems to play a crucial part in HCC too. Cells positive for CD133 have been reported to exhibit a greater tumorigenicity than the corresponding CD133-negative cells in HCC cell lines[103,104]. Another frequently-used CSCs marker for isolating liver CSCs is EpCAM[105,106]. As mentioned earlier, the classification of HCC patients based on EpCAM expression has a prognostic significance. In addition, the prospectively isolated EpCAM cells exhibited an adult SC-like gene expression profile. Given that the heterogeneity of human liver cancer may be related to its CSC origin, hepatic CSCs cannot be identified by the expression of a single marker. A clear liver CSCs profile has yet to be established, and Colombo et al[107] recently implemented a long-term culture system for isolating and characterizing human liver CSCs. Different HCC cell lines and clones from single HCC specimens were obtained, probably generated from different clonogenic cells, some of which had SC-like features. These HCC cell lines revealed a different morphology, antigen profile, proliferative potential, and in vitro antitumor drug resistance. The progenies of clone-initiating cells tend to reproduce the original population over time, suggesting the existence of hierarchically different cells and thus supporting the CSCs model. On the other hand, other HCC cell lines, which showed less aggressive features in vivo and were unable to generate different clones in vitro, seem to support the clonal evolution model. Taken together, these preliminary data appear to indicate that liver cancer heterogeneity can be explained by a model incorporating both the cancer stem cell hypothesis and clonal evolution mechanisms.
Stem cells may have the potential for replacing cells lost as a result of many devastating diseases, such as acute and chronic liver diseases and inflammatory or immune-mediated bowel diseases. There is little doubt that this potential benefit underpins the huge interest in stem cell research. Nonetheless, despite encouraging results obtained in regenerative medicine and the promise of future therapies, more studies are needed to thoroughly understand the biology of stem cells, overcome barriers related to immune response, and assess their oncogenic properties. Moreover, some conceptual and technical issues still restrict the diffusion of such treatments in clinical practice. There is still no consensus on the best methods to use for stem cell purification, the ideal route of delivery, amount of cells to infuse, and timing of infusions. Moreover, the risk of malignant transformation and/or pro-fibrogenic effects of SC-based therapies cannot be ruled out, and this imposes the need for a careful evaluation and longer follow-up periods to ascertain the safety and efficacy of SCs in therapeutic applications. Finally, while stem cell experimentation is appealing to researchers and medical specialists, it is also arousing fervent ethical, social and political debate.
Adopting scientific methods based on randomized and controlled trials should produce the necessary results on the real therapeutic role of stem cells. Indeed, the latest findings and ongoing technical improvements entitle researchers to have high hopes for the near future. The scientific researchers and physicians of today continue to hope that the mythological idea of regeneration in the story of Prometheus can be made real by developing therapies to restore lost, damaged or aging cells and tissues in the human body[3]. Much has been done, but there is still a lot to do. The future is open and promising.
The authors are grateful to all the members of the “Stem Cells in Gastroenterology” Study Section of the SIGE who contributed to a unique and stimulating debate. We also gratefully acknowledge the support of the SIGE and its highly professional staff. “Stem Cells in Gastroenterology” Study Section of the SIGE: Pietro Andreone (Bologna), Diletta Arcidiacono (Padua), Michele Barone (Bari), Vincenzo Boccaccio (Pavia), Massimiliano Cadamuro (Padua), Andrea Cappon (Padua), Tatiana Chioato (Padua), Carolina Ciacci (Naples), Federico Colombo (Milano), Gino Roberto Corazza (Pavia), Alfredo Di Leo (Bari), Luca Fabris (Padua), Isabel Freitas (Pavia), Silvia Gaia (Turin), Stefania Lorenzini (Bologna), Maurizio Parola (Turin), Daniele Prati (Lecco), Maria Luisa Russo (Pavia), Cristina Ubezio (Pavia), Lorenzo Valfrè di Bonzo (Turin), Giovanni Zanellati (Pavia).
Peer reviewer: Toshihiro Mitaka, Professor, Pathophysiology, Department of Cancer Research Institute, South-1, West-17, Chuo-ku, Sapporo 060-85567, Japan
S- Editor Sun H L- Editor Rutherford A E- Editor Li JY
| 1. | Wagers AJ, Weissman IL. Plasticity of adult stem cells. Cell. 2004;116:639-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 856] [Cited by in RCA: 770] [Article Influence: 36.7] [Reference Citation Analysis (0)] |
| 2. | Shackleton M, Quintana E, Fearon ER, Morrison SJ. Heterogeneity in cancer: cancer stem cells versus clonal evolution. Cell. 2009;138:822-829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 833] [Cited by in RCA: 830] [Article Influence: 51.9] [Reference Citation Analysis (0)] |
| 3. | Burra P, Tomat S, Villa E, Gasbarrini A, Costa AN, Conconi MT, Forbes SJ, Farinati F, Cozzi E, Alison MR. Experimental hepatology applied to stem cells. Dig Liver Dis. 2008;40:54-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 4. | Okamoto R, Matsumoto T, Watanabe M. Regeneration of the intestinal epithelia: regulation of bone marrow-derived epithelial cell differentiation towards secretory lineage cells. Hum Cell. 2006;19:71-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 5. | Michalopoulos GK. Liver regeneration. J Cell Physiol. 2007;213:286-300. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1246] [Cited by in RCA: 1158] [Article Influence: 64.3] [Reference Citation Analysis (0)] |
| 6. | Potten CS. Extreme sensitivity of some intestinal crypt cells to X and gamma irradiation. Nature. 1977;269:518-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 340] [Cited by in RCA: 331] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 7. | Scoville DH, Sato T, He XC, Li L. Current view: intestinal stem cells and signaling. Gastroenterology. 2008;134:849-864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 315] [Cited by in RCA: 313] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 8. | Cheng H, Leblond CP. Origin, differentiation and renewal of the four main epithelial cell types in the mouse small intestine. I. Columnar cell. Am J Anat. 1974;141:461-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 520] [Cited by in RCA: 513] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 9. | Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003-1007. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3854] [Cited by in RCA: 4333] [Article Influence: 240.7] [Reference Citation Analysis (0)] |
| 10. | Clevers H. Searching for adult stem cells in the intestine. EMBO Mol Med. 2009;1:255-259. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 11. | Sato T, van Es JH, Snippert HJ, Stange DE, Vries RG, van den Born M, Barker N, Shroyer NF, van de Wetering M, Clevers H. Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature. 2011;469:415-418. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2119] [Cited by in RCA: 1931] [Article Influence: 137.9] [Reference Citation Analysis (0)] |
| 12. | Snippert HJ, van der Flier LG, Sato T, van Es JH, van den Born M, Kroon-Veenboer C, Barker N, Klein AM, van Rheenen J, Simons BD. Intestinal crypt homeostasis results from neutral competition between symmetrically dividing Lgr5 stem cells. Cell. 2010;143:134-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1401] [Cited by in RCA: 1500] [Article Influence: 100.0] [Reference Citation Analysis (0)] |
| 13. | Brittan M, Wright NA. Gastrointestinal stem cells. J Pathol. 2002;197:492-509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 168] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 14. | Brittan M, Wright NA. The gastrointestinal stem cell. Cell Prolif. 2004;37:35-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 47] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 15. | Pucilowska JB, McNaughton KK, Mohapatra NK, Hoyt EC, Zimmermann EM, Sartor RB, Lund PK. IGF-I and procollagen alpha1(I) are coexpressed in a subset of mesenchymal cells in active Crohn's disease. Am J Physiol Gastrointest Liver Physiol. 2000;279:G1307-G1322. [PubMed] |
| 16. | Andoh A, Bamba S, Fujiyama Y, Brittan M, Wright NA. Colonic subepithelial myofibroblasts in mucosal inflammation and repair: contribution of bone marrow-derived stem cells to the gut regenerative response. J Gastroenterol. 2005;40:1089-1099. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 64] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 17. | Zhou H, Rogler LE, Teperman L, Morgan G, Rogler CE. Identification of hepatocytic and bile ductular cell lineages and candidate stem cells in bipolar ductular reactions in cirrhotic human liver. Hepatology. 2007;45:716-724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 109] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 18. | Theise ND. Gastrointestinal stem cells. III. Emergent themes of liver stem cell biology: niche, quiescence, self-renewal, and plasticity. Am J Physiol Gastrointest Liver Physiol. 2006;290:G189-G193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 46] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 19. | Jelnes P, Santoni-Rugiu E, Rasmussen M, Friis SL, Nielsen JH, Tygstrup N, Bisgaard HC. Remarkable heterogeneity displayed by oval cells in rat and mouse models of stem cell-mediated liver regeneration. Hepatology. 2007;45:1462-1470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 84] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 20. | Kuwahara R, Kofman AV, Landis CS, Swenson ES, Barendswaard E, Theise ND. The hepatic stem cell niche: identification by label-retaining cell assay. Hepatology. 2008;47:1994-2002. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 210] [Cited by in RCA: 198] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 21. | Petersen B, Shupe T. Location is everything: the liver stem cell niche. Hepatology. 2008;47:1810-1812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 22. | Fausto N. Liver regeneration and repair: hepatocytes, progenitor cells, and stem cells. Hepatology. 2004;39:1477-1487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 537] [Cited by in RCA: 517] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 23. | Lorenzini S, Andreone P. Stem cell therapy for human liver cirrhosis: a cautious analysis of the results. Stem Cells. 2007;25:2383-2384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 32] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 24. | Petersen BE, Bowen WC, Patrene KD, Mars WM, Sullivan AK, Murase N, Boggs SS, Greenberger JS, Goff JP. Bone marrow as a potential source of hepatic oval cells. Science. 1999;284:1168-1170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1795] [Cited by in RCA: 1669] [Article Influence: 64.2] [Reference Citation Analysis (0)] |
| 25. | Theise ND, Badve S, Saxena R, Henegariu O, Sell S, Crawford JM, Krause DS. Derivation of hepatocytes from bone marrow cells in mice after radiation-induced myeloablation. Hepatology. 2000;31:235-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 752] [Cited by in RCA: 696] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 26. | Theise ND, Nimmakayalu M, Gardner R, Illei PB, Morgan G, Teperman L, Henegariu O, Krause DS. Liver from bone marrow in humans. Hepatology. 2000;32:11-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 900] [Cited by in RCA: 851] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 27. | Lagasse E, Connors H, Al-Dhalimy M, Reitsma M, Dohse M, Osborne L, Wang X, Finegold M, Weissman IL, Grompe M. Purified hematopoietic stem cells can differentiate into hepatocytes in vivo. Nat Med. 2000;6:1229-1234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1764] [Cited by in RCA: 1631] [Article Influence: 65.2] [Reference Citation Analysis (0)] |
| 28. | Wang X, Montini E, Al-Dhalimy M, Lagasse E, Finegold M, Grompe M. Kinetics of liver repopulation after bone marrow transplantation. Am J Pathol. 2002;161:565-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 190] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 29. | Körbling M, Katz RL, Khanna A, Ruifrok AC, Rondon G, Albitar M, Champlin RE, Estrov Z. Hepatocytes and epithelial cells of donor origin in recipients of peripheral-blood stem cells. N Engl J Med. 2002;346:738-746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 588] [Cited by in RCA: 545] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 30. | Wagers AJ, Sherwood RI, Christensen JL, Weissman IL. Little evidence for developmental plasticity of adult hematopoietic stem cells. Science. 2002;297:2256-2259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1136] [Cited by in RCA: 1027] [Article Influence: 44.7] [Reference Citation Analysis (0)] |
| 31. | Mallet VO, Mitchell C, Mezey E, Fabre M, Guidotti JE, Renia L, Coulombel L, Kahn A, Gilgenkrantz H. Bone marrow transplantation in mice leads to a minor population of hepatocytes that can be selectively amplified in vivo. Hepatology. 2002;35:799-804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 87] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 32. | Kanazawa Y, Verma IM. Little evidence of bone marrow-derived hepatocytes in the replacement of injured liver. Proc Natl Acad Sci USA. 2003;100 Suppl 1:11850-11853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 107] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 33. | Fujii H, Hirose T, Oe S, Yasuchika K, Azuma H, Fujikawa T, Nagao M, Yamaoka Y. Contribution of bone marrow cells to liver regeneration after partial hepatectomy in mice. J Hepatol. 2002;36:653-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 113] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 34. | Dahlke MH, Popp FC, Bahlmann FH, Aselmann H, Jäger MD, Neipp M, Piso P, Klempnauer J, Schlitt HJ. Liver regeneration in a retrorsine/CCl4-induced acute liver failure model: do bone marrow-derived cells contribute? J Hepatol. 2003;39:365-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 45] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 35. | FARBER E. Similarities in the sequence of early histological changes induced in the liver of the rat by ethionine, 2-acetylamino-fluorene, and 3'-methyl-4-dimethylaminoazobenzene. Cancer Res. 1956;16:142-148. [PubMed] |
| 36. | Evarts RP, Nagy P, Marsden E, Thorgeirsson SS. A precursor-product relationship exists between oval cells and hepatocytes in rat liver. Carcinogenesis. 1987;8:1737-1740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 318] [Cited by in RCA: 284] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 37. | Wang X, Foster M, Al-Dhalimy M, Lagasse E, Finegold M, Grompe M. The origin and liver repopulating capacity of murine oval cells. Proc Natl Acad Sci USA. 2003;100 Suppl 1:11881-11888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 332] [Cited by in RCA: 304] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 38. | Dabeva MD, Shafritz DA. Hepatic stem cells and liver repopulation. Semin Liver Dis. 2003;23:349-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 34] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 39. | Brittan M, Hunt T, Jeffery R, Poulsom R, Forbes SJ, Hodivala-Dilke K, Goldman J, Alison MR, Wright NA. Bone marrow derivation of pericryptal myofibroblasts in the mouse and human small intestine and colon. Gut. 2002;50:752-757. [PubMed] |
| 40. | Kalluri R, Neilson EG. Epithelial-mesenchymal transition and its implications for fibrosis. J Clin Invest. 2003;112:1776-1784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 1050] [Article Influence: 50.0] [Reference Citation Analysis (0)] |
| 41. | Acloque H, Adams MS, Fishwick K, Bronner-Fraser M, Nieto MA. Epithelial-mesenchymal transitions: the importance of changing cell state in development and disease. J Clin Invest. 2009;119:1438-1449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 947] [Cited by in RCA: 1069] [Article Influence: 66.8] [Reference Citation Analysis (0)] |
| 42. | Friedman SL. Mechanisms of hepatic fibrogenesis. Gastroenterology. 2008;134:1655-1669. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2139] [Cited by in RCA: 2163] [Article Influence: 127.2] [Reference Citation Analysis (0)] |
| 43. | Parola M, Robino G. Oxidative stress-related molecules and liver fibrosis. J Hepatol. 2001;35:297-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 541] [Cited by in RCA: 562] [Article Influence: 23.4] [Reference Citation Analysis (1)] |
| 44. | Omenetti A, Porrello A, Jung Y, Yang L, Popov Y, Choi SS, Witek RP, Alpini G, Venter J, Vandongen HM. Hedgehog signaling regulates epithelial-mesenchymal transition during biliary fibrosis in rodents and humans. J Clin Invest. 2008;118:3331-3342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 191] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 45. | Forbes SJ, Russo FP, Rey V, Burra P, Rugge M, Wright NA, Alison MR. A significant proportion of myofibroblasts are of bone marrow origin in human liver fibrosis. Gastroenterology. 2004;126:955-963. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 335] [Cited by in RCA: 310] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 46. | Metz CN. Fibrocytes: a unique cell population implicated in wound healing. Cell Mol Life Sci. 2003;60:1342-1350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 168] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 47. | Russo FP, Alison MR, Bigger BW, Amofah E, Florou A, Amin F, Bou-Gharios G, Jeffery R, Iredale JP, Forbes SJ. The bone marrow functionally contributes to liver fibrosis. Gastroenterology. 2006;130:1807-1821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 388] [Cited by in RCA: 357] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 48. | Higashiyama R, Moro T, Nakao S, Mikami K, Fukumitsu H, Ueda Y, Ikeda K, Adachi E, Bou-Gharios G, Okazaki I. Negligible contribution of bone marrow-derived cells to collagen production during hepatic fibrogenesis in mice. Gastroenterology. 2009;137:1459-1466.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 93] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 49. | Schmidt KJ, Büning J, Jankowiak C, Lehnert H, Fellermann K. Crohn's targeted therapy: myth or real goal? Curr Drug Discov Technol. 2009;6:290-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 50. | Oyama Y, Craig RM, Traynor AE, Quigley K, Statkute L, Halverson A, Brush M, Verda L, Kowalska B, Krosnjar N. Autologous hematopoietic stem cell transplantation in patients with refractory Crohn's disease. Gastroenterology. 2005;128:552-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 195] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 51. | Cassinotti A, Annaloro C, Ardizzone S, Onida F, Della Volpe A, Clerici M, Usardi P, Greco S, Maconi G, Porro GB. Autologous haematopoietic stem cell transplantation without CD34+ cell selection in refractory Crohn's disease. Gut. 2008;57:211-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 119] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 52. | Al-toma A, Visser OJ, van Roessel HM, von Blomberg BM, Verbeek WH, Scholten PE, Ossenkoppele GJ, Huijgens PC, Mulder CJ. Autologous hematopoietic stem cell transplantation in refractory celiac disease with aberrant T cells. Blood. 2007;109:2243-2249. [PubMed] |
| 53. | Jantunen E, Juvonen E, Wiklund T, Putkonen M, Nousiainen T. High-dose therapy supported by autologous stem cell transplantation in patients with enteropathy-associated T-cell lymphoma. Leuk Lymphoma. 2003;44:2163-2164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 54. | Al-Toma A, Verbeek WH, Visser OJ, Kuijpers KC, Oudejans JJ, Kluin-Nelemans HC, Mulder CJ, Huijgens PC. Disappointing outcome of autologous stem cell transplantation for enteropathy-associated T-cell lymphoma. Dig Liver Dis. 2007;39:634-641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 58] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 55. | Bishton MJ, Haynes AP. Combination chemotherapy followed by autologous stem cell transplant for enteropathy-associated T cell lymphoma. Br J Haematol. 2007;136:111-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 56] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 56. | Kern S, Eichler H, Stoeve J, Klüter H, Bieback K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells. 2006;24:1294-1301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2248] [Cited by in RCA: 2347] [Article Influence: 123.5] [Reference Citation Analysis (0)] |
| 57. | Conconi MT, Burra P, Di Liddo R, Calore C, Turetta M, Bellini S, Bo P, Nussdorfer GG, Parnigotto PP. CD105(+) cells from Wharton's jelly show in vitro and in vivo myogenic differentiative potential. Int J Mol Med. 2006;18:1089-1096. [PubMed] |
| 58. | Nauta AJ, Fibbe WE. Immunomodulatory properties of mesenchymal stromal cells. Blood. 2007;110:3499-3506. [PubMed] |
| 59. | González MA, Gonzalez-Rey E, Rico L, Büscher D, Delgado M. Adipose-derived mesenchymal stem cells alleviate experimental colitis by inhibiting inflammatory and autoimmune responses. Gastroenterology. 2009;136:978-989. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 462] [Cited by in RCA: 484] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 60. | Lockhart BP, Tsiang H. Actin-independent maturation of rabies virus in neuronal cultures. J Gen Virol. 1991;72:2257-2261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 105] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 61. | Hayashi Y, Tsuji S, Tsujii M, Nishida T, Ishii S, Iijima H, Nakamura T, Eguchi H, Miyoshi E, Hayashi N. Topical implantation of mesenchymal stem cells has beneficial effects on healing of experimental colitis in rats. J Pharmacol Exp Ther. 2008;326:523-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 105] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 62. | Taupin P. OTI-010 Osiris Therapeutics/JCR Pharmaceuticals. Curr Opin Investig Drugs. 2006;7:473-481. [PubMed] |
| 63. | Le Blanc K, Frassoni F, Ball L, Locatelli F, Roelofs H, Lewis I, Lanino E, Sundberg B, Bernardo ME, Remberger M. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet. 2008;371:1579-1586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2047] [Cited by in RCA: 2028] [Article Influence: 119.3] [Reference Citation Analysis (0)] |
| 64. | Satija NK, Singh VK, Verma YK, Gupta P, Sharma S, Afrin F, Sharma M, Sharma P, Tripathi RP, Gurudutta GU. Mesenchymal stem cell-based therapy: a new paradigm in regenerative medicine. J Cell Mol Med. 2009;13:4385-4402. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 172] [Cited by in RCA: 183] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 65. | Polson J, Lee WM. AASLD position paper: the management of acute liver failure. Hepatology. 2005;41:1179-1197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 644] [Cited by in RCA: 642] [Article Influence: 32.1] [Reference Citation Analysis (0)] |
| 66. | Jalan R, Williams R. Acute-on-chronic liver failure: pathophysiological basis of therapeutic options. Blood Purif. 2002;20:252-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 222] [Article Influence: 10.1] [Reference Citation Analysis (1)] |
| 67. | Bismuth H, Samuel D, Castaing D, Williams R, Pereira SP. Liver transplantation in Europe for patients with acute liver failure. Semin Liver Dis. 1996;16:415-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 88] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 68. | Gupta S, Gorla GR, Irani AN. Hepatocyte transplantation: emerging insights into mechanisms of liver repopulation and their relevance to potential therapies. J Hepatol. 1999;30:162-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 61] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 69. | Grant MH, Morgan C, Henderson C, Malsch G, Seifert B, Albrecht W, Groth T. The viability and function of primary rat hepatocytes cultured on polymeric membranes developed for hybrid artificial liver devices. J Biomed Mater Res A. 2005;73:367-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 70. | Tomat S, Burra P, Gringeri E, Cillo U, Calabrese F, Giacometti C, Carraro P, Macchi C, Nussdorfer GG, Parnigotto PP. Metabolic activity of rat hepatocytes cultured on homologous acellular matrix and transplanted into Gunn rats. Int J Mol Med. 2006;18:837-842. [PubMed] |
| 71. | Burra P, Tomat S, Conconi MT, Macchi C, Russo FP, Parnigotto PP, Naccarato R, Nussdorfer GG. Acellular liver matrix improves the survival and functions of isolated rat hepatocytes cultured in vitro. Int J Mol Med. 2004;14:511-515. [PubMed] |
| 72. | Mimeault M, Hauke R, Batra SK. Stem cells: a revolution in therapeutics-recent advances in stem cell biology and their therapeutic applications in regenerative medicine and cancer therapies. Clin Pharmacol Ther. 2007;82:252-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 293] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 73. | Piscaglia AC, Di Campli C, Gasbarrini G, Gasbarrini A. Stem cells: new tools in gastroenterology and hepatology. Dig Liver Dis. 2003;35:507-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 74. | am Esch JS, Knoefel WT, Klein M, Ghodsizad A, Fuerst G, Poll LW, Piechaczek C, Burchardt ER, Feifel N, Stoldt V. Portal application of autologous CD133+ bone marrow cells to the liver: a novel concept to support hepatic regeneration. Stem Cells. 2005;23:463-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 187] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 75. | Terai S, Ishikawa T, Omori K, Aoyama K, Marumoto Y, Urata Y, Yokoyama Y, Uchida K, Yamasaki T, Fujii Y. Improved liver function in patients with liver cirrhosis after autologous bone marrow cell infusion therapy. Stem Cells. 2006;24:2292-2298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 363] [Cited by in RCA: 349] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 76. | Ismail A, Fouad O, Abdelnasser A, Chowdhury A, Selim A. Stem cell therapy improves the outcome of liver resection in cirrhotics. J Gastrointest Cancer. 2010;41:17-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 36] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 77. | Gaia S, Smedile A, Omedè P, Olivero A, Sanavio F, Balzola F, Ottobrelli A, Abate ML, Marzano A, Rizzetto M. Feasibility and safety of G-CSF administration to induce bone marrow-derived cells mobilization in patients with end stage liver disease. J Hepatol. 2006;45:13-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 125] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 78. | Yannaki E, Anagnostopoulos A, Kapetanos D, Xagorari A, Iordanidis F, Batsis I, Kaloyannidis P, Athanasiou E, Dourvas G, Kitis G. Lasting amelioration in the clinical course of decompensated alcoholic cirrhosis with boost infusions of mobilized peripheral blood stem cells. Exp Hematol. 2006;34:1583-1587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 67] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 79. | Gasbarrini A, Rapaccini GL, Rutella S, Zocco MA, Tittoto P, Leone G, Pola P, Gasbarrini G, Di Campli C. Rescue therapy by portal infusion of autologous stem cells in a case of drug-induced hepatitis. Dig Liver Dis. 2007;39:878-882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 38] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 80. | Gordon MY, Levicar N, Pai M, Bachellier P, Dimarakis I, Al-Allaf F, M'Hamdi H, Thalji T, Welsh JP, Marley SB. Characterization and clinical application of human CD34+ stem/progenitor cell populations mobilized into the blood by granulocyte colony-stimulating factor. Stem Cells. 2006;24:1822-1830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 197] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 81. | Levicar N, Pai M, Habib NA, Tait P, Jiao LR, Marley SB, Davis J, Dazzi F, Smadja C, Jensen SL. Long-term clinical results of autologous infusion of mobilized adult bone marrow derived CD34+ cells in patients with chronic liver disease. Cell Prolif. 2008;41 Suppl 1:115-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 64] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 82. | Pai M, Zacharoulis D, Milicevic MN, Helmy S, Jiao LR, Levicar N, Tait P, Scott M, Marley SB, Jestice K. Autologous infusion of expanded mobilized adult bone marrow-derived CD34+ cells into patients with alcoholic liver cirrhosis. Am J Gastroenterol. 2008;103:1952-1958. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 153] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 83. | Han Y, Yan L, Han G, Zhou X, Hong L, Yin Z, Zhang X, Wang S, Wang J, Sun A. Controlled trials in hepatitis B virus-related decompensate liver cirrhosis: peripheral blood monocyte transplant versus granulocyte-colony-stimulating factor mobilization therapy. Cytotherapy. 2008;10:390-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 45] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 84. | Di Campli C, Zocco MA, Saulnier N, Grieco A, Rapaccini G, Addolorato G, Rumi C, Santoliquido A, Leone G, Gasbarrini G. Safety and efficacy profile of G-CSF therapy in patients with acute on chronic liver failure. Dig Liver Dis. 2007;39:1071-1076. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 51] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 85. | Lorenzini S, Isidori A, Catani L, Gramenzi A, Talarico S, Bonifazi F, Giudice V, Conte R, Baccarani M, Bernardi M. Stem cell mobilization and collection in patients with liver cirrhosis. Aliment Pharmacol Ther. 2008;27:932-939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 41] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 86. | Spahr L, Lambert JF, Rubbia-Brandt L, Chalandon Y, Frossard JL, Giostra E, Hadengue A. Granulocyte-colony stimulating factor induces proliferation of hepatic progenitors in alcoholic steatohepatitis: a randomized trial. Hepatology. 2008;48:221-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 177] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 87. | Piscaglia AC, Novi M, Campanale M, Gasbarrini A. Stem cell-based therapy in gastroenterology and hepatology. Minim Invasive Ther Allied Technol. 2008;17:100-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 26] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 88. | Gilchrist ES, Plevris JN. Bone marrow-derived stem cells in liver repair: 10 years down the line. Liver Transpl. 2010;16:118-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 69] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 89. | Kallis YN, Alison MR, Forbes SJ. Bone marrow stem cells and liver disease. Gut. 2007;56:716-724. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 112] [Cited by in RCA: 108] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 90. | Heppner GH, Miller BE. Tumor heterogeneity: biological implications and therapeutic consequences. Cancer Metastasis Rev. 1983;2:5-23. [PubMed] |
| 91. | Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997;3:730-737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4851] [Cited by in RCA: 4861] [Article Influence: 173.6] [Reference Citation Analysis (1)] |
| 92. | Nowell PC. The clonal evolution of tumor cell populations. Science. 1976;194:23-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4428] [Cited by in RCA: 4183] [Article Influence: 85.4] [Reference Citation Analysis (1)] |
| 93. | O'Brien CA, Pollett A, Gallinger S, Dick JE. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature. 2007;445:106-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2977] [Cited by in RCA: 3051] [Article Influence: 160.6] [Reference Citation Analysis (0)] |
| 94. | Ricci-Vitiani L, Lombardi DG, Pilozzi E, Biffoni M, Todaro M, Peschle C, De Maria R. Identification and expansion of human colon-cancer-initiating cells. Nature. 2007;445:111-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2952] [Cited by in RCA: 3035] [Article Influence: 159.7] [Reference Citation Analysis (0)] |
| 95. | Weigmann A, Corbeil D, Hellwig A, Huttner WB. Prominin, a novel microvilli-specific polytopic membrane protein of the apical surface of epithelial cells, is targeted to plasmalemmal protrusions of non-epithelial cells. Proc Natl Acad Sci USA. 1997;94:12425-12430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 457] [Cited by in RCA: 479] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 96. | Mizrak D, Brittan M, Alison MR. CD133: molecule of the moment. J Pathol. 2008;214:3-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 396] [Cited by in RCA: 404] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 97. | Shmelkov SV, Butler JM, Hooper AT, Hormigo A, Kushner J, Milde T, St Clair R, Baljevic M, White I, Jin DK. CD133 expression is not restricted to stem cells, and both CD133+ and CD133- metastatic colon cancer cells initiate tumors. J Clin Invest. 2008;118:2111-2120. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 430] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 98. | Alison MR. Liver stem cells: implications for hepatocarcinogenesis. Stem Cell Rev. 2005;1:253-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 96] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 99. | Dumble ML, Croager EJ, Yeoh GC, Quail EA. Generation and characterization of p53 null transformed hepatic progenitor cells: oval cells give rise to hepatocellular carcinoma. Carcinogenesis. 2002;23:435-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 172] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 100. | Steinberg P, Steinbrecher R, Radaeva S, Schirmacher P, Dienes HP, Oesch F, Bannasch P. Oval cell lines OC/CDE 6 and OC/CDE 22 give rise to cholangio-cellular and undifferentiated carcinomas after transformation. Lab Invest. 1994;71:700-709. [PubMed] |
| 101. | Theise ND, Yao JL, Harada K, Hytiroglou P, Portmann B, Thung SN, Tsui W, Ohta H, Nakanuma Y. Hepatic 'stem cell' malignancies in adults: four cases. Histopathology. 2003;43:263-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 154] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 102. | Lee JS, Heo J, Libbrecht L, Chu IS, Kaposi-Novak P, Calvisi DF, Mikaelyan A, Roberts LR, Demetris AJ, Sun Z. A novel prognostic subtype of human hepatocellular carcinoma derived from hepatic progenitor cells. Nat Med. 2006;12:410-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 729] [Cited by in RCA: 748] [Article Influence: 39.4] [Reference Citation Analysis (0)] |
| 103. | Yin S, Li J, Hu C, Chen X, Yao M, Yan M, Jiang G, Ge C, Xie H, Wan D. CD133 positive hepatocellular carcinoma cells possess high capacity for tumorigenicity. Int J Cancer. 2007;120:1444-1450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 419] [Cited by in RCA: 434] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 104. | Ma S, Chan KW, Hu L, Lee TK, Wo JY, Ng IO, Zheng BJ, Guan XY. Identification and characterization of tumorigenic liver cancer stem/progenitor cells. Gastroenterology. 2007;132:2542-2556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 899] [Cited by in RCA: 922] [Article Influence: 51.2] [Reference Citation Analysis (0)] |
| 105. | Yamashita T, Ji J, Budhu A, Forgues M, Yang W, Wang HY, Jia H, Ye Q, Qin LX, Wauthier E. EpCAM-positive hepatocellular carcinoma cells are tumor-initiating cells with stem/progenitor cell features. Gastroenterology. 2009;136:1012-1024. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 936] [Cited by in RCA: 958] [Article Influence: 59.9] [Reference Citation Analysis (0)] |
| 106. | Munz M, Baeuerle PA, Gires O. The emerging role of EpCAM in cancer and stem cell signaling. Cancer Res. 2009;69:5627-5629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 375] [Cited by in RCA: 415] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 107. | Colombo F, Baldan F, Mazzucchelli S, Martin-Padura I, Marighetti P, Cattaneo A, Foglieni B, Spreafico M, Guerneri S, Baccarin M. Evidence of distinct tumour-propagating cell populations with different properties in primary human hepatocellular carcinoma. PLoS One. 2011;6:e21369. |
| 108. | Terris B, Cavard C, Perret C. EpCAM, a new marker for cancer stem cells in hepatocellular carcinoma. J Hepatol. 2010;52:280-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 170] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 109. | Zhu Z, Hao X, Yan M, Yao M, Ge C, Gu J, Li J. Cancer stem/progenitor cells are highly enriched in CD133+CD44+ population in hepatocellular carcinoma. Int J Cancer. 2010;126:2067-2078. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 225] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 110. | Ma S, Chan KW, Lee TK, Tang KH, Wo JY, Zheng BJ, Guan XY. Aldehyde dehydrogenase discriminates the CD133 liver cancer stem cell populations. Mol Cancer Res. 2008;6:1146-1153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 333] [Cited by in RCA: 367] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 111. | Xu XL, Xing BC, Han HB, Zhao W, Hu MH, Xu ZL, Li JY, Xie Y, Gu J, Wang Y. The properties of tumor-initiating cells from a hepatocellular carcinoma patient's primary and recurrent tumor. Carcinogenesis. 2010;31:167-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 75] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 112. | Yang ZF, Ho DW, Ng MN, Lau CK, Yu WC, Ngai P, Chu PW, Lam CT, Poon RT, Fan ST. Significance of CD90+ cancer stem cells in human liver cancer. Cancer Cell. 2008;13:153-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 883] [Cited by in RCA: 927] [Article Influence: 54.5] [Reference Citation Analysis (0)] |
| 113. | Chiba T, Kita K, Zheng YW, Yokosuka O, Saisho H, Iwama A, Nakauchi H, Taniguchi H. Side population purified from hepatocellular carcinoma cells harbors cancer stem cell-like properties. Hepatology. 2006;44:240-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 503] [Cited by in RCA: 494] [Article Influence: 26.0] [Reference Citation Analysis (0)] |