Published online Sep 7, 2011. doi: 10.3748/wjg.v17.i33.3824
Revised: March 25, 2011
Accepted: April 1, 2011
Published online: September 7, 2011
AIM: To find out if by combining 2 ultrasound based elastographic methods: acoustic radiation force impulse (ARFI) elastography and transient elastography (TE), we can improve the prediction of fibrosis in patients with chronic hepatitis C.
METHODS: Our study included 197 patients with chronic hepatitis C. In each patient, we performed, in the same session, liver stiffness (LS) measurements by means of TE and ARFI, respectively, and liver biopsy (LB), assessed according to the Metavir score. 10 LS measurements were performed both by TE and ARFI; median values were calculated and expressed in kilopascals (kPa) and meters/second (m/s), respectively. Only TE and ARFI measurements with IQR < 30% and SR ≥ 60% were considered reliable.
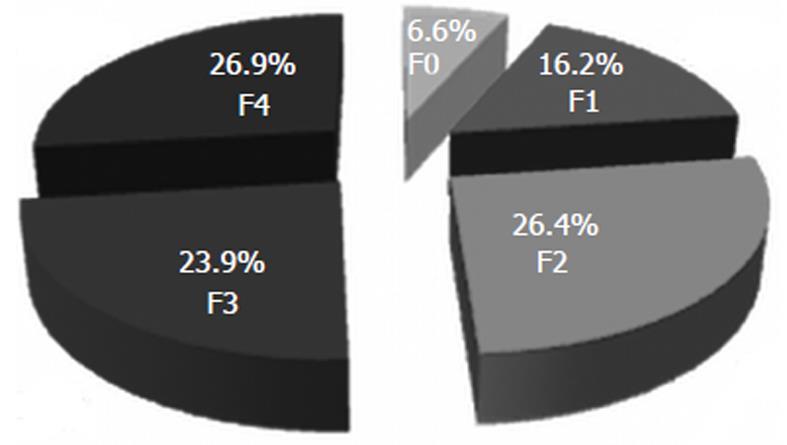
RESULTS: On LB 13 (6.6%) patients had F0, 32 (16.2%) had F1, 52 (26.4%) had F2, 47 (23.9%) had F3, and 53 (26.9%) had F4. A direct, strong correlation was found between TE measurements and fibrosis (r = 0.741), between ARFI and fibrosis (r = 0.730) and also between TE and ARFI (r = 0.675). For predicting significant fibrosis (F ≥ 2), for a cut-off of 6.7 kPa, TE had 77.5% sensitivity (Se) and 86.5% specificity (Sp) [area under the receiver operating characteristic curve (AUROC) 0.87] and for a cut-off of 1.2 m/s, ARFI had 76.9% Se and 86.7% Sp (AUROC 0.84). For predicting cirrhosis (F = 4), for a cut-off of 12.2 kPa, TE had 96.2% Se and 89.6% Sp (AUROC 0.97) and for a cut-off of 1.8 m/s, ARFI had 90.4% Se and 85.6% Sp (AUROC 0.91). When both elastographic methods were taken into consideration, for predicting significant fibrosis (F ≥ 2), (TE ≥ 6.7 kPa and ARFI ≥ 1.2 m/s) we obtained 60.5% Se, 93.3% Sp, 96.8% positive predictive value (PPV), 41.4% negative predictive value (NPV) and 68% accuracy, while for predicting cirrhosis (TE ≥ 12.2 kPa and ARFI ≥ 1.8 m/s) we obtained 84.9% Se, 94.4% Sp, 84.9% PPV, 94.4% NPV and 91.8% accuracy.
CONCLUSION: TE used in combination with ARFI is highly specific for predicting significant fibrosis; therefore when the two methods are concordant, liver biopsy can be avoided.
- Citation: Sporea I, Şirli R, Popescu A, Bota S, Badea R, Lupşor M, Focşa M, Dănilă M. Is it better to use two elastographic methods for liver fibrosis assessment? World J Gastroenterol 2011; 17(33): 3824-3829
- URL: https://www.wjgnet.com/1007-9327/full/v17/i33/3824.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i33.3824
Liver fibrosis evaluation in patients with hepatitis C virus (HCV) infection is essential for prognosis assessment and also for a decision regarding therapy. In many centers, liver biopsy (LB) is the “normal” means of fibrosis assessment. In the last few years, non-invasive methods for the evaluation of liver fibrosis have become more and more popular, especially in France and, subsequently, throughout the world.
Non-invasive methods for liver fibrosis assessment are: biological (serological) tests[1-5], ultrasound based (elastographic) methods, such as transient elastography (TE)[6-9], real time elastography[10-12] and acoustic radiation force impulse (ARFI) elastography[13-15] and magnetic resonance imaging (MRI) elastography[16,17]. Each method has certain advantages: only a few milliliters of blood are required for the serological tests, a special “ultrasound” examination is required for the elastographic methods and finally, a MRI examination reveals information about many abdominal organs and at the same time evaluates the liver stiffness (LS). All these methods have some disadvantages, the major one being that they are not 100% sensitive or 100% specific compared to the LB which is still considered the “gold standard”.
Some authors have proposed to combine different noninvasive methods for liver fibrosis evaluation, hoping to increase the accuracy or maybe to decrease the number of LBs needed to solve unclear cases[18]. Some years ago, Castera[18] proposed to use only ALT for the evaluation of liver activity in patients with chronic hepatitis C, and for fibrosis to combine a FibroTest with a FibroScan. If these noninvasive tests are concordant, then LB can be avoided. In this study, when the FibroScan and FibroTest results agreed, significant fibrosis (F ≥ 2) was confirmed by LB in 84% of the cases, severe fibrosis (F ≥ 3) in 95% of cases, and cirrhosis (F = 4) in 94% of the cases.
The purpose of this study is to find out if, by combining 2 ultrasound based elastographic methods: ARFI elastography and TE, we can improve the prediction of fibrosis severity in patients with chronic HCV hepatitis.
We performed a bicentric study in two university hospitals (Timisoara and Cluj-Napoca) that included 197 patients with chronic HCV hepatitis (anti HCV antibodies positive, with or without cytolysis for at least 6 mo, PCR HCV RNA positive). In all these patients, in the same session, LS was evaluated by means of TE (FibroScan®) and ARFI elastography, and LB was performed in order to assess the fibrosis stage. Patients with other causes of chronic hepatitis (HBV infection, chronic alcohol abuse, cholestatic chronic hepatitis, nonalcoholic steatohepatitis, autoimmune chronic hepatitis, haemochromatosis, Wilson’s disease) were excluded from our study. Informed consent was obtained from each patient included in the study and the study protocol was approved by the local ethical committee.
TE was performed in all patients with a FibroScan® device (EchoSens® - Paris, France) by experienced physicians (more than 500 TE), blinded to the results of LB and ARFI measurements. In each patient, 10 valid measurements were performed, after which a median value of LS was obtained, measured in kilopascals (kPa). Only patients in which LS measurements by means of TE had a success rate of at least 60%, with an interquartile range (IQR) < 30%, were included in our study. The success rate was calculated as the ratio of the number of successful acquisitions over the total number of acquisitions. IQR is the difference between the 75th percentile and the 25th percentile, essentially the range of the middle 50% of the data.
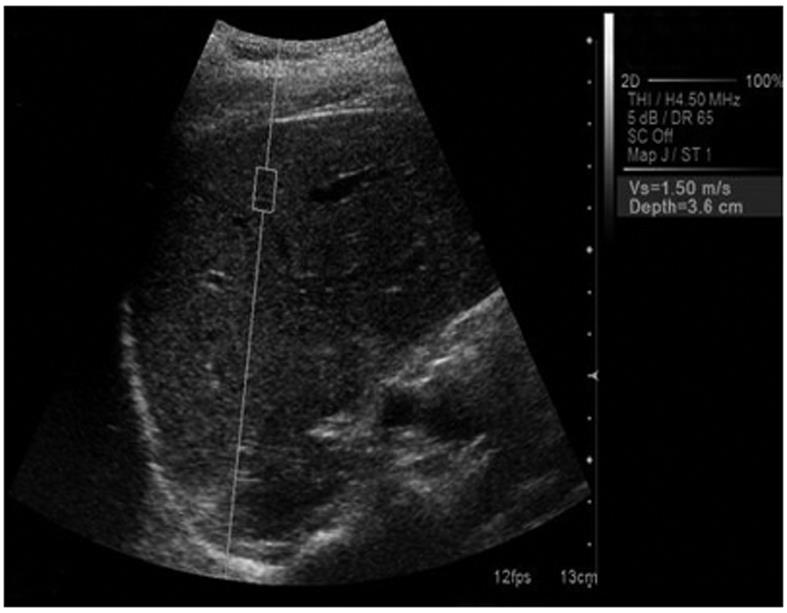
ARFI elastography was performed in all the patients with a Siemens Acuson S2000TM ultrasound system. The ultrasound probe automatically produces an acoustic “push” pulse that generates shear-waves which propagate into the liver. Their speed, measured in meters/second (m/s), is displayed on the screen. The propagation speed increases with fibrosis. The operator can select the depth at which the liver elasticity is evaluated by placing a “measuring box” (10 mm long and 5 mm wide) in the desired place (Figure 1). The patients were examined in left lateral decubitus, with the right arm in maximum abduction. Scanning was performed between the ribs in the right liver lobe in order to avoid cardiac motion (approximately in the place where we usually perform LB), 1 cm under the capsule, with minimal scanning pressure applied by the operator, while the patients were asked to stop breathing for a moment, in order to minimize breathing motion.
We performed 10 measurements in every patient, and a median value was calculated, the result being measured in m/s. Only patients in which LS measurements by means of ARFI had a success rate of at least 60%, with an IQR < 30%, were included in our study. Operators were blinded to the results of LB and TE measurements.
LB was performed in all the patients using echoguided TruCut technique, with a 1.8 mm (14 G) diameter automatic needle device-Biopty Gun (Bard GMBh), or echoassisted, using Menghini type modified needles, 1.4 and 1.6 mm in diameter. Only LB fragments including at least 6 portal tracts were considered adequate for pathological interpretation and included in our study. The LBs were assessed, according to the Metavir score, by a senior pathologist (one in each center) blinded to the results of TE and ARFI measurements. Fibrosis was staged on a 0-4 scale: F0-no fibrosis; F1-portal fibrosis without septa; F2-portal fibrosis and few septa extending into lobules; F3-numerous septa extending to adjacent portal tracts or terminal hepatic venules and F4-cirrhosis.
The data we obtained from our patients were collected in a Microsoft Excel file, the statistical analysis being performed using MedCalc and GraphPad Prism programs. The predictors for the stage of fibrosis (ARFI and TE measurements) were numeric variables, so the mean and standard deviation were calculated.
Associations between assay results and fibrosis stage according to the Metavir scoring system (range: 0-4, ordinal scale), were described using the Spearman rank correlation coefficient (r).
The diagnostic performances of ARFI and TE were assessed by using receiver operating characteristics (ROC) curves. ROC curves were thus built for the detection of significant fibrosis (F ≥ 2 Metavir) and cirrhosis (F = 4 Metavir). Optimal cut-off values were chosen to maximize the sum of sensitivity (Se) and specificity (Sp). Se and Sp were calculated according to standard methods. Exact confidence intervals of 95% were calculated for each predictive test.
Our study group included 197 patients, 119 women and 78 men, mean age 50 ± 9.8 years. On LB 13 (6.6%) patients had F0, 32 (16.2%) had F1, 52 (26.4%) had F2, 47 (23.9%) had F3 and 53 (26.9%) had F4 (Figure 2).
We obtained valid TE measurements in 187/197 patients (94.9%) and valid ARFI measurements in 191/197 patients (96.9%).
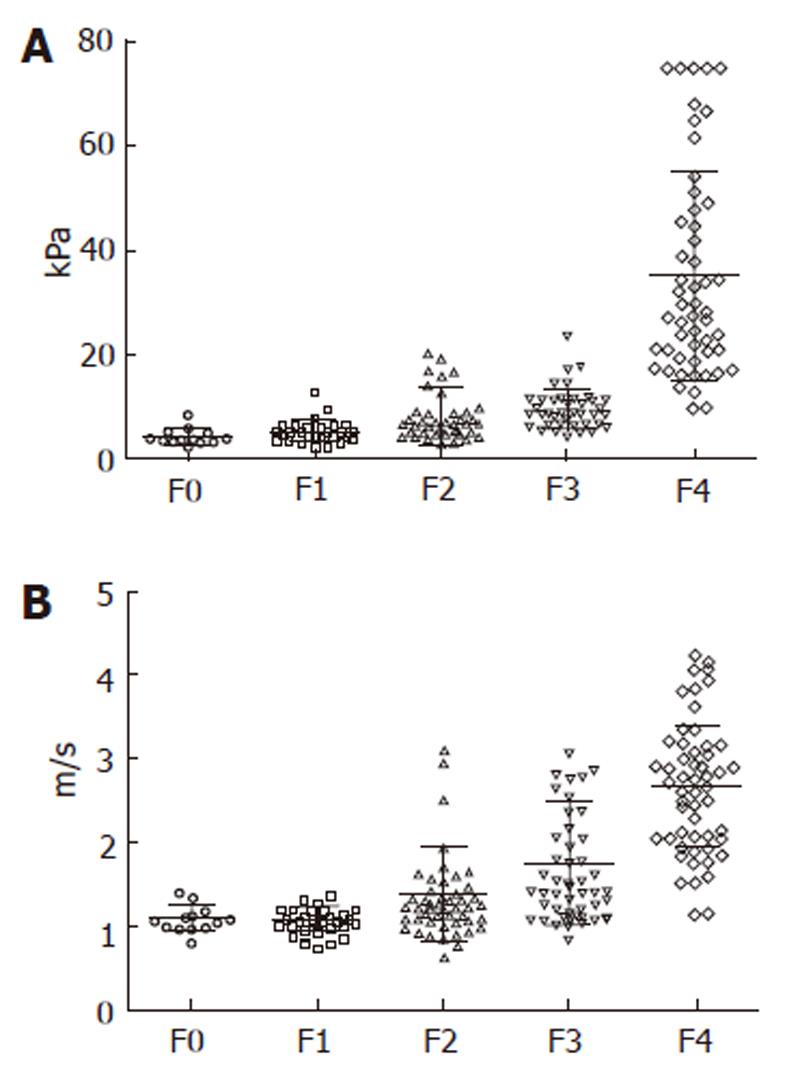
A direct, strong correlation was found between the values of liver stiffness evaluated by TE and fibrosis (r = 0.741) (Figure 3A), between the values of liver stiffness measured by ARFI and fibrosis (r = 0.730) (Figure 3B) and also between the values of liver stiffness evaluated by means both of TE and ARFI (r = 0.675).
The predictive values of TE and ARFI, alone, for F ≥ 2 and F4, respectively, are presented in Table 1.
| F≥2 | F = 4 | ||||||||||||||
| Cut-off | AUROC | Se | S | PPV | NPV | Accuracy | Cut-off | AUROC | Se | Sp | PPV | NPV | Accuracy | ||
| TE | 6.7 kPa | 0.87 | 77.5 | 86.7 | 94.8 | 54.9 | 79.6 | 12.2 kPa | 0.97 | 96.2 | 89.6 | 78.1 | 98.3 | 91.4 | |
| ARFI | 1.2 m/s | 0.84 | 76.9 | 86.7 | 95.7 | 54.1 | 79.3 | 1.8m/s | 0.91 | 90.4 | 85.6 | 50.3 | 95.8 | 83.4 | |
By combining the two elastographic methods (values both for TE and ARFI above the mentioned cut-offs) the specificity increased, statistically significant as compared to ARFI (F ≥ 2: 93.3% vs 86.7%, P = 0.04; F = 4: 94.4% vs 85.6%, P = 0.007) but not as compared to TE (F ≥ 2: 93.3% vs 86.7%, P = 0.05; F = 4: 94.4% vs 89.6%, P = 0.12), of course with lower sensitivity (Table 2), with very good positive predictive value (PPV) (96.3%) for significant fibrosis (F ≥ 2 Metavir). By combining the two elastographic methods for F4, we obtained a very high negative predictive value (NPV), along with very good PPV and accuracy (Table 2).
| F≥2 | F = 4 | ||||||||||||
| Cut-off | Se | Sp | PPV | NPV | Accuracy | Cut-off | Se | Sp | PPV | NPV | Accuracy | ||
| TE + ARFI | 6.7 kPa and 1.2 m/s | 60.5 | 93.3 | 96.8 | 41.1 | 68 | 12.2 kPa and 1.8 m/s | 84.9 | 94.4 | 84.9 | 94.4 | 91.8 | |
| TE or ARFI | 6.7 kPa or 1.2 m/s | 86.1 | 71.1 | 90.9 | 60.3 | 82.7 | 12.2 kPa or 1.8 m/s | 96.2 | 83.3 | 68 | 98.3 | 86.8 | |
The accuracy of the combined tests (TE + ARFI) was statistically significant better than ARFI alone for predicting cirrhosis (91.8% vs 83.4%, P = 0.02), but not as compared to TE alone (91.8% vs 91.4%, P = 0.96).
Discussions regarding the replacement of LB in the evaluation of liver fibrosis with non-invasive methods are currently very active. Arguments in favor of maintaining LB are: it allows a correct evaluation of fibrosis as well as of the activity of the disease; it can provide arguments for the etiology (Mallory bodies, etc) or it can evaluate the presence and severity of hepatocytes’ fatty infiltration. Arguments against this method are: it is an invasive one (there is a risk for complications, even if it is low); it is usually a stressful method for the patients; in some cases, good quality histological specimens are not obtained and, also, there are some questions regarding sampling variability.
In some countries, such as France, the non-invasive methods for fibrosis assessment have replaced the LB in a large number of cases. To become accepted worldwide, these non-invasive methods must be very accurate, in order to replace a well recognized method such as LB.
TE measures the liver stiffness of a fragment that is approximately a cylinder 1 cm in diameter and 4 cm long, 500 times bigger than the specimen obtained by LB. This examination is more or less blind, but the other method that we used for the elastographic evaluation of the liver, ARFI, is performed under clear ultrasonographic visualization of the area of interest (the operator being able to choose the area to be examined).
The criticism, especially of TE, is that it is not able to differentiate between contiguous stages of fibrosis (F0 vs F1, or F1 vs F2). On the other hand, from the point of view of the clinician, it is important to know if the patient has only mild fibrosis (probably with no need for treatment in HCV patients), or moderate or severe fibrosis. And for this purpose, TE is quite a good method (the following AUROCs were reported: 0.79 for F ≥ 2, 0.91 for F ≥ 3 and 0.97 for F = 4)[7].
The other elastographic method, ARFI technology, involves targeting an anatomic region to be interrogated for elastic properties, with the use of a region of-interest cursor, while performing real-time B-mode imaging. Tissue from the region of interest is mechanically excited by using short-duration (262 μs) acoustic pulses with a fixed transmit frequency of 2.67 MHz to generate localized tissue displacements. The displacements result in shear-wave propagation away from the region of excitation and are tracked by using US correlation-based methods[19]. The shear-wave propagation velocity is proportional to the square root of tissue elasticity. Results are expressed in meters per second (m/s). The technique is new and published data suggest that ARFI and TE have a similar predictive value for fibrosis assessment.
In a study performed by Friedrich-Rust et al[9] in which ARFI was compared to LB and blood markers in 86 patients with chronic hepatitis (HBV or HCV), the Spearman correlation coefficients between the histological fibrosis stage and ARFI, TE, FibroTest and APRI score were statistically significant: 0.71, 0.73, 0.66 and 0.45 respectively (P < 0.001).
In the study performed by Lupşor et al[14], 112 patients with chronic hepatitis were evaluated. All the patients underwent LB (fibrosis stage assessed according to the Metavir scoring system), ARFI and FibroScan. The mean ARFI values for different stages of fibrosis were: 1.079 ± 0.150 m/s (F0-F1), 1.504 ± 0.895 m/s (F2), 1.520 ± 0.575 m/s (F3) and 2.552 ± 0.782 m/s (F4). The mean values were statistically significant different only between F3 and F4. The following cut-off values were proposed for various stages of fibrosis: F ≥ 1:1.19 m/s; F ≥ 2:1.34 m/s; F ≥ 3:1.61 m/s; and F ≥ 4:2 m/s.
Since both types of elastographic evaluation are available in our Department, we tried to see if by combining them we can improve their predictive value for fibrosis assessment. Firstly, we evaluated their predictive value alone for significant fibrosis (F ≥ 2 Metavir) and cirrhosis (F = 4), after which we evaluated their predictive value in combination. If both methods were concordant (TE ≥ 6.7 kPa and ARFI ≥ 1.2 m/s), we obtained a high specificity (93.3%) for predicting significant fibrosis (F ≥ 2), also with a very good positive predictive value (96.8%), so in those cases there was no need to perform LB before initiating treatment. In our study group, 152/197 patients had significant fibrosis (F ≥ 2 Metavir) on LB. TE and ARFI were concordant for significant fibrosis in 92 of 152 patients. Therefore, we could avoid 60.5% of the LBs in our group of patients.
Also, by combining the two elastographic methods for predicting cirrhosis (F4) (TE ≥ 12.2 kPa and ARFI ≥ 1.8 m/s), the results were very good, with 94.4% Sp, 94.4% NPV and 91.8% accuracy, so the combined methods are excellent for confirming, but also for excluding the presence of cirrhosis. In our study group, 53 patients had cirrhosis (F = 4 Metavir on LB). TE and ARFI were concordant for liver cirrhosis in 45 from the 53 patients with F = 4 on LB (84.9%).
Other published data tried to combine different noninvasive methods for a better evaluation of liver stiffness. In a study published in 2005 by Castera et al[18], 183 patients with chronic HCV hepatitis were evaluated by LB, TE, FibroTest and APRI. The best performance was obtained by combining FibroScan and FibroTest, with areas under the ROC curve of 0.88 for F ≥ 2, 0.95 for F ≥ 3, and 0.95 for F = 4. When FibroScan and FibroTest results agreed, significant fibrosis (F ≥ 2) was confirmed by LB in 84% of the cases, severe fibrosis (F ≥ 3) in 95% of cases, and cirrhosis (F = 4) in 94% of the cases.
In another study published in 2010, Castera et al[20] evaluated two algorithms for liver fibrosis prediction: one combined TE and FibroTest (Castera) and the other APRI and FibroTest (SAFE biopsy). In all patients a LB was performed. Significant fibrosis (F ≥ 2 Metavir) was present in 76% of patients and cirrhosis (F4) in 25%. TE failure was observed in eight cases (2.6%). For significant fibrosis, the Castera algorithm saved 23% more liver biopsies than SAFE biopsy (71.9% vs 48.3%, respectively, P < 0.0001), but its accuracy was significantly lower (87.7% vs 97.0%, respectively; P < 0.0001). Regarding cirrhosis, the accuracy of the Castera algorithm was significantly higher than that of SAFE biopsy (95.7% vs 88.7%, respectively; P < 0.0001). The number of saved liver biopsies did not differ between the two algorithms (78.8% vs 74.8%, P = NS).
Shahenn[21] published a meta-analysis which compared the performances of TE and FibroTest for the prediction of liver fibrosis in patients with chronic HCV hepatitis. Thirteen studies were identified, 9 regarding FibroTest (1679 patients) and 4 regarding TE (546 patients). In heterogeneous analysis for significant fibrosis, the AUROC curves for FibroTest and TE were 0.81 and 0.83, respectively. At a threshold of approximately 0.60, the sensitivity and specificity of FibroTest were 47% (35%-59%) and 90% (87%-92%). For TE (threshold approximately 8 kPa), corresponding values were 64% (50%-76%) and 87% (80%-91%), respectively. However, the diagnostic accuracy of both tests was associated with the prevalence of significant fibrosis and cirrhosis in the study populations. For cirrhosis, the summary AUROCs for FibroTest and FibroScan were 0.90 and 0.95 (0.87-0.99).
In a study published in 2010 by Cross et al[22], 187 patients with chronic HCV hepatitis were evaluated by means of LB, TE and the King score. Liver fibrosis was scored using the Ishak score; significant fibrosis was defined as Ishak fibrosis stage F3-F6, and cirrhosis defined as Ishak fibrosis F5-F6. The AUROCs for TE, the King score and TE + King score for the diagnosis of Ishak F3-F6 were 0.83, 0.82 and 0.85, respectively and 0.96, 0.89 and 0.93, respectively, for the diagnosis of cirrhosis (F ≥ 5 Ishak). The negative predictive values for the diagnosis of cirrhosis, using the optimal cut-off results for TE (10.05 kPa), the King score (24.3) and the two combined (26.1), were 98%, 91% and 94%, respectively.
Our study tried to establish whether the combination of TE and ARFI could provide some advantages for the evaluation of significant fibrosis in patients with chronic hepatitis C in comparison with a single elastographic method. By combining the two elastographic methods (values both for TE and ARFI above the mentioned cut-offs), the specificity increased (of course with lower sensitivity), with very good PPV (96.3%) for significant fibrosis (F ≥ 2 Metavir). In our study group, 152/197 patients had significant fibrosis (F ≥ 2 Metavir) on LB. TE and ARFI were concordant for significant fibrosis in 92 of 152 patients. Therefore, we were able to avoid 60.5% of LB in our group of patients.
Also, by combining the two elastographic methods for predicting cirrhosis (F4) (TE ≥ 12.2 kPa and ARFI ≥ 1.8 m/s), the results were very good, with 94.4% Sp, 94.4% NPV and 91.8% accuracy, so the combined methods are not only able to confirm, but also to exclude the presence of cirrhosis.
In conclusion, LS measurements assessed by means of both TE and ARFI strongly correlate to histological fibrosis in HCV patients. TE used in combination with ARFI is highly specific (approximately 93%) for predicting significant fibrosis (F ≥ 2 Metavir), so that in patients with higher LS measurements than the proposed cut-offs for both methods, liver biopsy could be avoided. Also, in patients suspected of having severe fibrosis, if both methods are concordant, they are very good for confirming and excluding the presence of cirrhosis (94.4% Sp, 94.4% NPV).
Non-invasive methods for fibrosis assessment in chronic hepatitis, such as transient elastography (TE), are accepted more and more, tending to replace the invasive methods, especially in hepatitis C virus (HCV) chronic hepatitis. In the last few years, studies were published regarding the use of acoustic radiation force impulse (ARFI) elastography for fibrosis assessment in chronic hepatitis.
Studies were published regarding the benefits of combining non-invasive methods for fibrosis evaluation (serological tests with or without TE), but not regarding a combination of elastographic methods (TE and ARFI).
The aim of this study was to find out if by combining ARFI and TE the prediction of fibrosis in patients with chronic HCV hepatitis can be improved, and the authors concluded that TE used in combination with ARFI is highly specific for predicting significant fibrosis; therefore when the two methods are concordant liver biopsy can be avoided.
In this study that included 197 patients with chronic C hepatitis, LS measurement by means of both TE and ARFI strongly correlated to the histological fibrosis. TE used in combination with ARFI was highly specific (93.3%) for predicting significant fibrosis (F ≥ 2 Metavir); therefore in patients with higher LS measurements than the proposed cut-offs for both methods, liver biopsy could be avoided (positive predictive value 96.8%).
TE (FibroScan) is an ultrasound-based method that uses the transmission of low-frequency vibrations to create an elastic shear wave that propagates into the liver, followed by the detection wave propagation velocity, which is proportional to the tissue stiffness, with faster wave progression occurring through stiffer tissue. ARFI technology involves targeting an anatomic region to be interrogated for elastic properties, with the use of a region of-interest cursor, while performing real-time B-mode imaging. Tissue from the region of interest is mechanically excited to generate localized tissue displacements. The displacements result in shear-wave propagation away from the region of excitation and are tracked by using US correlation-based methods. The shear-wave propagation velocity is proportional to the square root of tissue elasticity.
This is a well written paper that looks at TE (a well studied technology for liver fibrosis) and acoustic radiation impulse force (a technology for which there is much less clinical data). The data is well displayed.
Peer reviewer: Ned Snyder, MD, FACP, AGAF, Professor of Medicine, Chief of Clinical Gastroenterology and Hepatology, Department of Internal Medicine, The University of Texas Medical Branch, 301 University Blvd., Galveston, Texas 77555-0764, United States
S- Editor Tian L L- Editor Ratherford A E- Editor Xiong L
| 1. | Rosenberg WM, Voelker M, Thiel R, Becka M, Burt A, Schuppan D, Hubscher S, Roskams T, Pinzani M, Arthur MJ. Serum markers detect the presence of liver fibrosis: a cohort study. Gastroenterology. 2004;127:1704-1713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 756] [Cited by in RCA: 759] [Article Influence: 36.1] [Reference Citation Analysis (0)] |
| 2. | Poynard T, Imbert-Bismut F, Ratziu V, Chevret S, Jardel C, Moussalli J, Messous D, Degos F. Biochemical markers of liver fibrosis in patients infected by hepatitis C virus: longitudinal validation in a randomized trial. J Viral Hepat. 2002;9:128-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 161] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 3. | Myers RP, Ratziu V, Imbert-Bismut F, Charlotte F, Poynard T and the MULTIVIRC Group. Biochemical markers of liver fibrosis: A comparison with histological features in patients with chronic hepatitis C. Am J Gastroenterol. 2002;97:2419-2425. [DOI] [Full Text] |
| 4. | Rossi E, Adams L, Prins A, Bulsara M, de Boer B, Garas G, MacQuillan G, Speers D, Jeffrey G. Validation of the FibroTest biochemical markers score in assessing liver fibrosis in hepatitis C patients. Clin Chem. 2003;49:450-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 171] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 5. | Myers RP, Tainturier MH, Ratziu V, Piton A, Thibault V, Imbert-Bismut F, Messous D, Charlotte F, Di Martino V, Benhamou Y. Prediction of liver histological lesions with biochemical markers in patients with chronic hepatitis B. J Hepatol. 2003;39:222-230. [PubMed] |
| 6. | Sandrin L, Fourquet B, Hasquenoph JM, Yon S, Fournier C, Mal F, Christidis C, Ziol M, Poulet B, Kazemi F. Transient elastography: a new noninvasive method for assessment of hepatic fibrosis. Ultrasound Med Biol. 2003;29:1705-1713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1967] [Cited by in RCA: 1936] [Article Influence: 88.0] [Reference Citation Analysis (0)] |
| 7. | Ziol M, Handra-Luca A, Kettaneh A, Christidis C, Mal F, Kazemi F, de Lédinghen V, Marcellin P, Dhumeaux D, Trinchet JC. Noninvasive assessment of liver fibrosis by measurement of stiffness in patients with chronic hepatitis C. Hepatology. 2005;41:48-54. [PubMed] |
| 8. | Foucher J, Chanteloup E, Vergniol J, Castéra L, Le Bail B, Adhoute X, Bertet J, Couzigou P, de Lédinghen V. Diagnosis of cirrhosis by transient elastography (FibroScan): a prospective study. Gut. 2006;55:403-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 897] [Cited by in RCA: 955] [Article Influence: 50.3] [Reference Citation Analysis (0)] |
| 9. | Friedrich-Rust M, Ong MF, Martens S, Sarrazin C, Bojunga J, Zeuzem S, Herrmann E. Performance of transient elastography for the staging of liver fibrosis: a meta-analysis. Gastroenterology. 2008;134:960-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1046] [Cited by in RCA: 1077] [Article Influence: 63.4] [Reference Citation Analysis (1)] |
| 10. | Friedrich-Rust M, Ong MF, Herrmann E, Dries V, Samaras P, Zeuzem S, Sarrazin C. Real-time elastography for noninvasive assessment of liver fibrosis in chronic viral hepatitis. AJR Am J Roentgenol. 2007;188:758-764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 238] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 11. | Tatsumi C, Kudo M, Ueshima K, Kitai S, Takahashi S, Inoue T, Minami Y, Chung H, Maekawa K, Fujimoto K. Noninvasive evaluation of hepatic fibrosis using serum fibrotic markers, transient elastography (FibroScan) and real-time tissue elastography. Intervirology. 2008;51 Suppl 1:27-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 76] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 12. | Friedrich-Rust M, Schwarz A, Ong M, Dries V, Schirmacher P, Herrmann E, Samaras P, Bojunga J, Bohle RM, Zeuzem S. Real-time tissue elastography versus FibroScan for noninvasive assessment of liver fibrosis in chronic liver disease. Ultraschall Med. 2009;30:478-484. [PubMed] |
| 13. | Friedrich-Rust M, Wunder K, Kriener S, Sotoudeh F, Richter S, Bojunga J, Herrmann E, Poynard T, Dietrich CF, Vermehren J. Liver fibrosis in viral hepatitis: noninvasive assessment with acoustic radiation force impulse imaging versus transient elastography. Radiology. 2009;252:595-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 476] [Cited by in RCA: 468] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 14. | Lupsor M, Badea R, Stefanescu H, Sparchez Z, Branda H, Serban A, Maniu A. Performance of a new elastographic method (ARFI technology) compared to unidimensional transient elastography in the noninvasive assessment of chronic hepatitis C. Preliminary results. J Gastrointestin Liver Dis. 2009;18:303-310. [PubMed] |
| 15. | Sporea I, Sirli RL, Deleanu A, Popescu A, Focsa M, Danila M, Tudora A. Acoustic radiation force impulse elastography as compared to transient elastography and liver biopsy in patients with chronic hepatopathies. Ultraschall Med. 2011;32 Suppl 1:S46-S52. [PubMed] |
| 16. | Huwart L, Peeters F, Sinkus R, Annet L, Salameh N, ter Beek LC, Horsmans Y, Van Beers BE. Liver fibrosis: non-invasive assessment with MR elastography. NMR Biomed. 2006;19:173-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 306] [Cited by in RCA: 287] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 17. | Yin M, Talwalkar JA, Glaser KJ, Manduca A, Grimm RC, Rossman PJ, Fidler JL, Ehman RL. Assessment of hepatic fibrosis with magnetic resonance elastography. Clin Gastroenterol Hepatol. 2007;5:1207-1213.e2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 771] [Cited by in RCA: 714] [Article Influence: 39.7] [Reference Citation Analysis (1)] |
| 18. | Castéra L, Vergniol J, Foucher J, Le Bail B, Chanteloup E, Haaser M, Darriet M, Couzigou P, De Lédinghen V. Prospective comparison of transient elastography, Fibrotest, APRI, and liver biopsy for the assessment of fibrosis in chronic hepatitis C. Gastroenterology. 2005;128:343-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1796] [Cited by in RCA: 1848] [Article Influence: 92.4] [Reference Citation Analysis (0)] |
| 19. | Nightingale K, Soo MS, Nightingale R, Trahey G. Acoustic radiation force impulse imaging: in vivo demonstration of clinical feasibility. Ultrasound Med Biol. 2002;28:227-235. [PubMed] |
| 20. | Castéra L, Sebastiani G, Le Bail B, de Lédinghen V, Couzigou P, Alberti A. Prospective comparison of two algorithms combining non-invasive methods for staging liver fibrosis in chronic hepatitis C. J Hepatol. 2010;52:191-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 146] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 21. | Shaheen AA, Wan AF, Myers RP. FibroTest and FibroScan for the prediction of hepatitis C-related fibrosis: a systematic review of diagnostic test accuracy. Am J Gastroenterol. 2007;102:2589-2600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 256] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 22. | Cross TJ, Calvaruso V, Maimone S, Carey I, Chang TP, Pleguezuelo M, Manousou P, Quaglia A, Grillo F, Dhillon AP. Prospective comparison of Fibroscan, King's score and liver biopsy for the assessment of cirrhosis in chronic hepatitis C infection. J Viral Hepat. 2010;17:546-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 37] [Article Influence: 2.5] [Reference Citation Analysis (0)] |