Published online Aug 28, 2011. doi: 10.3748/wjg.v17.i32.3739
Revised: March 29, 2011
Accepted: April 5, 2011
Published online: August 28, 2011
AIM: To investigate the effects of sleeve gastrectomy on adipose tissue infiltration and lectin-like oxidized low density lipoprotein receptor-1 (LOX-1) expression in rat aortas.
METHODS: Twenty-four rats were randomized into three groups: normal chow (control), high fat diet (HD) and high fat diet with sleeve gastrectomy (SG). After surgery, the HD and SG groups were fed a high fat diet. Animals were sacrificed and plasma high density lipoprotein (HDL) and low density lipoprotein (LDL) levels were determined. LOX-1 protein and LOX-1 mRNA expression was also measured. Aortas were stained with Nile red to visualize adipose tissue.
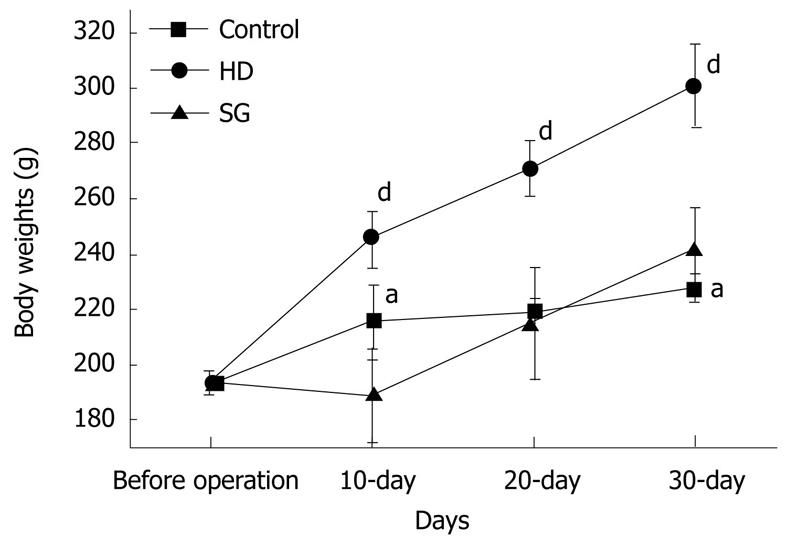
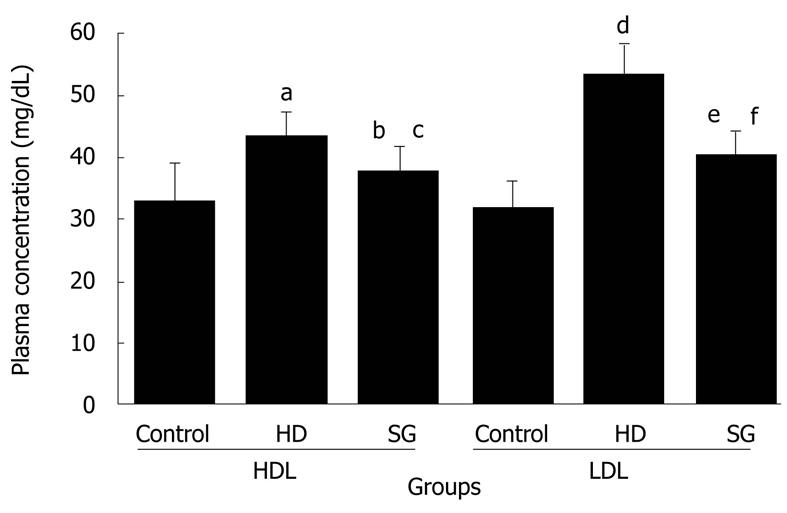
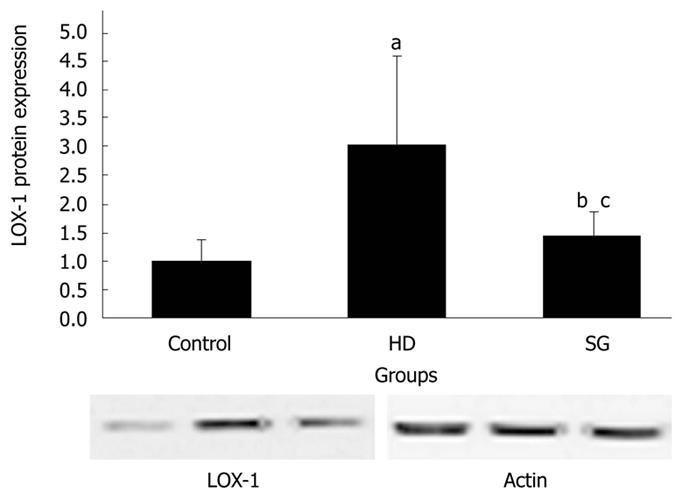
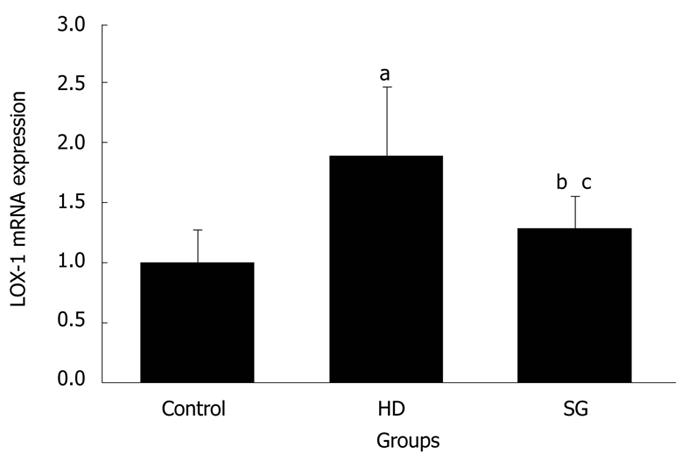
RESULT: Body weights were higher in the HD group compared to the other groups. HDL levels in control, HD, and SG groups were 32.9 ± 6.2 mg/dL, 43.4 ± 4.0 mg/dL and 37.5 ± 4.3 mg/dL, respectively. LDL levels in control, HD, and SG groups were 31.8 ± 4.5 mg/dL, 53.3 ± 5.1 mg/dL and 40.5 ± 3.7 mg/dL, respectively. LOX-1 protein and LOX-1 mRNA expression was greater in the HD group versus the other groups. Staining for adipose tissue in aortas was greater in the HD group in comparison to the other groups. Thus, a high fat diet elevates LOX-1 protein and mRNA expression in aorta.
CONCLUSION: Sleeve gastrectomy decreases plasma LDL levels, and downregulates LOX-1 protein and mRNA expression.
- Citation: Bai J, Wang Y, Liu Y, Geng DH, Liu JG. Sleeve gastrectomy prevents lipoprotein receptor-1 expression in aortas of obese rats. World J Gastroenterol 2011; 17(32): 3739-3744
- URL: https://www.wjgnet.com/1007-9327/full/v17/i32/3739.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i32.3739
Morbid obesity is a serious health problem worldwide. The incidence of diet-induced obesity in the United States has risen to 32%[1]. Approximately 127 million individuals are overweight, of which 60 million are obese and 8-10 million have morbid obesity with serious medical comorbidities, such as increased disability, morbidity and early mortality[1-3].
Atherosclerosis is an important comorbidity of obesity that accounts for over 500 000 deaths annually in the United States. Diseases associated with atherosclerosis, such as myocardial infarction and stroke, account for the majority of deaths in industrialized countries. Atherosclerosis is a complex, multifactorial disease with both genetic and environmental determinants.
In clinical trials on atherosclerosis and hypertension, researchers found a direct association between the amount of weight loss and blood pressure reduction following a 36 mo weight loss intervention[4,5]. Prospective cohort studies have also found that the prevalence of atherosclerosis and hypertension decreases with weight loss[6-8]. Additionally, several researchers have found a positive relationship between weight gain and atherosclerosis[6,9-12].
Lectin-like oxidized low density lipoprotein receptor-1 (LOX-1) is a type of oxidized low density lipoprotein (OX-LDL) receptor[13]. After binding with OX-LDL, LOX-1 can induce vascular smooth muscle cell migration to the tunica intima via extracellular signal-regulated kinase (ERK) activation. It can also promote vascular smooth muscle cell proliferation and increase lipid intake, and thereby pathological vascular changes that significantly affect the formation and progress of atherosclerotic disease[14].
Thus, we hypothesized that sleeve gastrectomy would result in weight loss, and thereby prevent LOX-1 protein and LOX-1 mRNA expression, as well as adipose tissue infiltration in the aorta.
Twenty-four male, 8-week-old, Wistar rats weighing 180 g-200 g (Beijing Laboratory Animal Research Center, China) were acclimatized for 7 d, and then randomized into three groups: normal chow (control), high fat diet (HD) and high fat diet with sleeve gastrectomy (SG). The normal diet consisted of 10% kcal of fat (D12450B diet, Research Diets Inc, New Brunswick, NJ), whereas the high fat diet consisted of 60% kcal of fat (D12492 diet, Research Diets Inc, New Brunswick, NJ). Throughout the study, rats were kept in individual metabolic cages with a natural light/dark cycle, at a temperature of 18 °C ± 2 °C and humidity of 50% ± 2%.
Rats were anesthetized with an intraperitoneal injection of 300 mg/kg chloral hydrate and placed in the supine position on a surgical board with their extremities immobilized. An epigastric incision of approximately 1.5 cm-2 cm in length was made. The incision was kept open with a blade retractor, and the gastric omentum dissociated to reveal the gastric cardium. The gastric cavity was then closed with vascular clamps and cut off with a cauterizer, which also induced hemostasis. A gastric tube was made from the distal antrum (1.5 mm-2 mm from the pylorus) to the Hiss angle using an 8-0 unabsorbable suture. The fundus was completely removed (i.e., 70%-80% of total stomach). After the gastric tube was constructed, the peritoneal cavity was cleaned with saline and closed with a 6-0 silk suture. In the control group, a sham operation was performed as described above with the exception of the stomach incisions. All animals were given 5 mL of sterile, warmed saline subcutaneously to avoid dehydration, and allowed to recover from anesthesia and surgery. Rats were then returned to their home cages, and provided with food and water ad libitum 24 h after the surgery.
Following the surgery, rats in the HD and SG groups received a high fat diet for 30 d, whereas rats in the control group received normal chow. Body mass was checked in all rats prior to the operation and sacrifice. Thirty days after surgery, all rats were sacrificed and blood samples were collected to measure high-density lipoprotein (HDL) and low-density lipoprotein (LDL) using fast-phase liquid chromatography (FPLC) and their respective colorimetric assay kits.
Aortas were homogenized and centrifuged at 15 000 rpm at 4 °C for 15 min. Protein concentrations were determined with a protein assay (Thermo Fisher Scientific Inc., IL, United States). Forty micrograms of protein were separated by electrophoresis via a 12.5% sodium dodecyl sulfate polyacrylamide gel electrophoresis gel. Gels were then blotted onto nitrocellulose membranes, which were blocked with 5% skimmed milk for 1 h and then blotted overnight at 4 °C with Rbt polyclonal primary antibody (ab60178, Abcam, Unit 225A and 225B, 2/F Core Building 2, No. 1 Science Park West Avenue, Hong Kong Science Park, Shatin, N.T., Hong Kong). After blotting with goat anti-Rbt secondary antibody, immune-complexes were visualized using an electrochemiluminescence Western blotting analysis system (FUJI film, United States).
Real-time quantitative polymerase chain reaction (PCR) analysis was carried out using an iQ5 Real-Time PCR Detection System (Bio-rad, CA, United States). The total amount of RNA used in reverse transcription was 1 μg. The following steps were performed to synthesize cDNA: samples were placed at 25 °C for 10 min, then 42 °C for 50 min, then 85 °C for 5 min, then chilled on ice, then 1 μL of Escherichia coli RNase H was added, and lastly the samples were incubated at 37 °C for 20 min. LOX-1 primers were as follows: sense: 5'-GACTGGATCTGGCATAAAGA-3'; antisense: 5'-CCTTCTTCTGACATATGCTG-3'.
GAPDH sequences were as follows: sense 5'-CAC-CCTGTGCTGCTCACCGAGGCC-3'; antisense 5'-CCACACAGATGACTTGCGCTCAGG-3'.
Real-time PCR parameters were as follows: 2 min at 50 °C, 2 min at 95 °C, followed by 40 cycles of 15 s at 95 °C, 30 s at 55 °C and 30 s at 60 °C. All measurements were performed in triplicate and each series of experiments was repeated twice. All quantifications were standardized to the amount of GAPDH amplification.
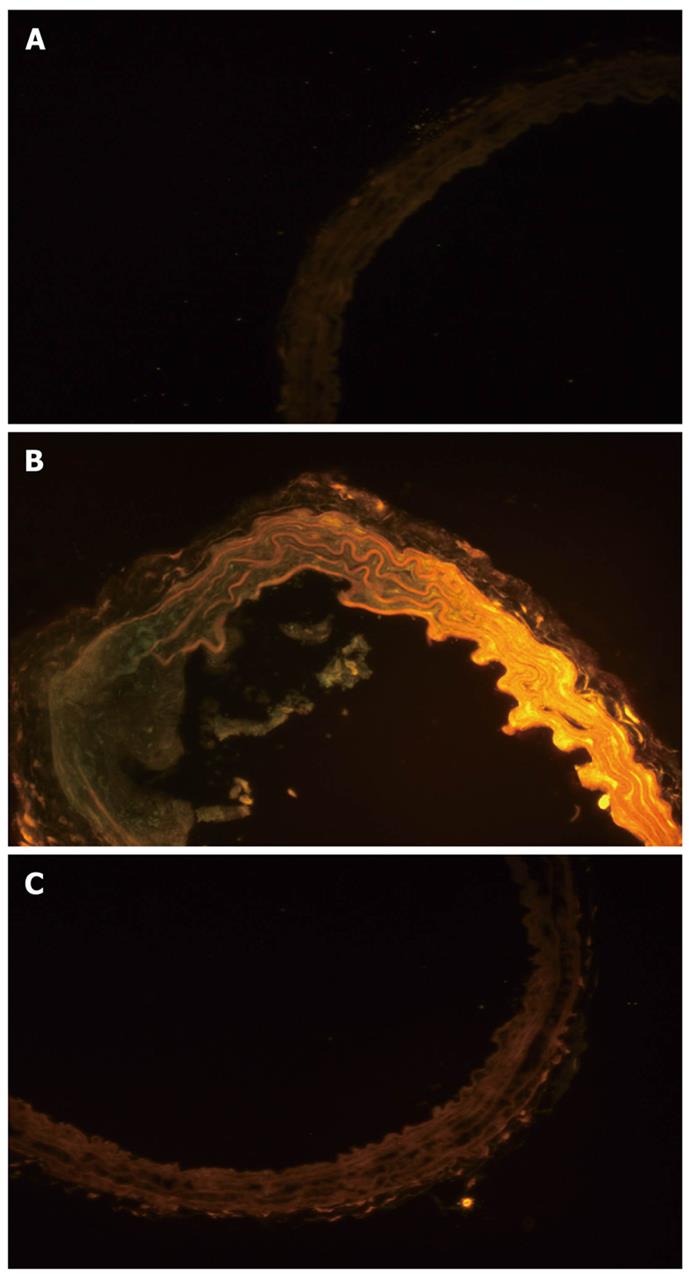
Aortas were taken out and frozen (Leica CM1850, Germany). A frozen slide (5 μm) was made, and stained immediately with 0.5 mL of Nile red (1 mg/mL) (Sigma Co, St Louis, MO, United States) for 5 min in the dark[15]. Fluorescence microscopy (Olympus IX51 10X10, Japan) was used to visualize adipose tissue in the slides.
All of the rats survived and recovered from the gastrectomy. Body weights were significantly higher in the HD group compared to the control and SG groups (P < 0.05) (Figure 1). HDL levels in control, HD and SG groups were 32.9 ± 6.2 mg/dL, 43.4 ± 4.0 mg/dL and 37.5 ± 4.3 mg/dL, respectively. However, there were no statistical differences between the HD and SG groups (P > 0.05). LDL levels in the control, HD and SG groups were 31.8 ± 4.5 mg/dL, 53.3 ± 5.1 mg/dL and 40.5 ± 3.7 mg/dL, respectively (Figure 2). There was a statistically significant difference in LDL between the HD and SG groups (P < 0.01). LOX-1 protein expression in the HD and SG groups was 3.0 ± 1.6-fold and 1.5 ± 0.4-fold higher compared to control (Figure 3). There was a statistically significant difference between the HD and SG groups (P < 0.05). Furthermore, LOX-1 mRNA expression was 1.9 ± 0.6-fold and 1.3 ± 0.3-fold greater in the HD and SG groups versus control (Figure 4). There was a statistically significant difference between the SG and HD groups (P < 0.05). Nile red staining of control, HD and SG aortas is illustrated in Figures 5.
Obesity is associated with low-grade inflammation[16,17], which has been shown to be an initiating factor in endothelial dysfunction and atherosclerosis, and thus may cause arterial stiffness[17,18].
A direct association between the amount of weight loss and blood pressure reduction has been reported in some clinical trials[4,5]. Prospective cohort studies have also found that the prevalence of atherosclerosis and hypertension decreases with weight loss[6-8]. Additionally, several researchers have found a positive association between weight gain and obesity with atherosclerosis and hypertension[6,9-12].
Pro-inflammatory cytokines may play a role in the development of insulin resistance, which can be reversed by anti-inflammatory agents. These findings suggest that inflammation may be directly involved in the pathogenic properties of cytokines. Evidence suggests that both macronutrient intake and obesity may activate inflammatory signaling pathways in cells[19].
In the present study, LOX-1 protein expression in the aorta was upregulated in the HD group, and was prevented by gastrectomy in the SG group. Furthermore, LOX-1 mRNA expression was downregulated in the SG group versus the HD group. LOX-1 is a major receptor of ox-LDL and LDL in the vascular endothelium. The role of LOX-1 in atherogenesis is supported by several lines of evidence. LOX-1 demonstrates a strong affinity for binding, internalizing and degrading OX-LDL[20]. The oxidized form of LDL (OX-LDL) is thought to be more important in atherogenesis than the native LDL form[21]. OX-LDL injures the endothelium and is an important mediator in atherogenesis[22]. OX-LDL activates LOX-1 and induces endothelial dysfunction and apoptosis[23,24]. There are other mediators of atherosclerosis, such as angiotensin II, cytokines, sheer stress and advanced glycation end-products, that upregulate LOX-1. Furthermore, LOX-1 is dynamically upregulated by pro-atherogenic conditions, such as diabetes, hypertension and dyslipidemia. LOX-1 is present in atheroma-derived cells, and in human and animal atherosclerotic lesions[25-28].
To date, surgery has been proven to be the only effective method for treating morbid obesity[29,30]. Observational studies suggest that weight-loss surgery is associated with a 60% to 80% diabetes remission rate in severely obese individuals, and that earlier interventions are more likely to provide remission[31]. Additionally, there are concerns regarding the lack of evidence, as well as the safety, invasiveness, and cost-effectiveness of such surgical weight-loss procedures. Providing appropriate evidence has been problematic due to the invasive nature of the surgery, which makes recruitment difficult. However, with the advent of safer, less invasive surgical weight-loss procedures, randomized clinical trials are now feasible.
Sleeve gastrectomy, a type of bariatric surgery, was performed in this experiment. In the SG group, body weights were significantly lower than those of the HD group. As a result, LOX-1 protein and mRNA expression levels, as well as LDL levels, were significantly lower in the SG group versus the HD group. SG is a type of purely restrictive surgery, where a moderate restriction is created, while the integrity of the duodenum, pylorus, antrum, lesser curvature and vagal nerve, and a relatively normal eating behavior, are maintained. Recent findings also suggest that SG might be a safe, beneficial, and effective stand-alone approach[32-34]. Moon et al reported that SG resolves all comorbidities of obesity in over 90% of subjects over a 12-mo period, with the exception of dyslipidemia, which is resolved in 65% of subjects[33]. Moreover, there was a dramatic loss of appetite in more than half of the patients postoperatively[33]. Karamanakos et al[35] found that SG preserved the integrity of the pylorus and did not induce an intestinal bypass. Furthermore, LDL levels, as well as liver enzymes, were decreased significantly in SG patients.
In summary, a high fat diet elevates LOX-1 protein and mRNA expression in the aorta. Sleeve gastrectomy can prevent increases in plasma LDL levels, as well as an upregulation in LOX-1 protein and mRNA expression associated with a high fat diet.
The authors wish to thank Professor Yun Yu for revising this manuscript.
Morbid obesity is a serious health problem worldwide. Atherosclerosis is an important comorbidity of obesity. Diseases associated with atherosclerosis, such as myocardial infarction and stroke, account for the majority of deaths in industrialized countries. Studies have found a direct association between the amount of weight loss and the prevalence of atherosclerosis. Lectin-like oxidized low density lipoprotein receptor-1 (LOX-1) is a type of oxidized low density lipoprotein (OX-LDL) receptor. After binding with OX-LDL, LOX-1 can affect the formation and progress of atherosclerotic disease. Thus, the authors hypothesized that sleeve gastrectomy would result in weight loss, and thereby prevent LOX-1 protein and LOX-1 mRNA expression, as well as adipose tissue infiltration in the aorta.
The hotspot about this paper is the treatment of atherosclerosis in obese animals after bariatric surgery.
It was difficult to control morbid obesity and its comorbidities, such as atherosclerosis and non-alcoholic steatohepatitis, before bariatric surgery was used in the clinic. This kind of surgery can decrease body weight and reverse many comorbidities caused by obesity.
These results could expand the indication of bariatric surgery in the clinic and many obese patients with atherosclerosis could undergo surgery in order to decrease body weight and cure atherosclerosis.
Bariatric surgery: Bariatric surgery, or weight loss surgery, includes a variety of procedures performed on people who are obese. Weight loss is achieved by reducing the size of the stomach with an implanted medical device (gastric banding) or through removal of a portion of the stomach (sleeve gastrectomy or biliopancreatic diversion with duodenal switch) or by resecting and re-routing the small intestines to a small stomach pouch (gastric bypass surgery); Atherosclerosis: Atherosclerosis (also known as arteriosclerotic vascular disease) is a condition in which an artery wall thickens as the result of a build-up of fatty materials such as cholesterol. It is a syndrome affecting arterial blood vessels; a chronic inflammatory response in the walls of arteries, in large part due to the accumulation of macrophage white blood cells and promoted by low-density lipoproteins (plasma proteins that carry cholesterol and triglycerides) without adequate removal of fats and cholesterol from the macrophages by functional high density lipoproteins. It is commonly referred to as a hardening or furring of the arteries.
With great interest I have read the article entitled: “Sleeve gastrectomy prevents LOX-1 expression of aortas in obese rats”. This is a well-performed study with some interesting findings concerning the influence of obesity surgery on atherosclerosis.
Peer reviewers: Misha Luyer, MD, PhD, Department of Surgery, Orbis Medical Centre, Postbus 5500, Sittard, 6130 MB, The Netherlands; Hiroshi Yoshida, MD, First Department of Surgery, Nippon Medical School, 1-1-5 Sendagi, Bunkyo-ku, Tokyo 113-8603, Japan
S- Editor Sun H L- Editor Logan S E- Editor Zhang DN
| 1. | Guijarro A, Kirchner H, Meguid MM. Catabolic effects of gastric bypass in a diet-induced obese rat model. Curr Opin Clin Nutr Metab Care. 2006;9:423-435. [PubMed] |
| 2. | Zimmet P, Alberti KG, Shaw J. Global and societal implications of the diabetes epidemic. Nature. 2001;414:782-787. [PubMed] |
| 4. | Truesdale KP, Stevens J, Cai J. Effect of 3-year weight history on blood pressure: the atherosclerosis risk in communities study. Obesity (Silver Spring). 2008;16:1112-1119. [PubMed] |
| 5. | Stevens VJ, Obarzanek E, Cook NR, Lee IM, Appel LJ, Smith West D, Milas NC, Mattfeldt-Beman M, Belden L, Bragg C. Long-term weight loss and changes in blood pressure: results of the Trials of Hypertension Prevention, phase II. Ann Intern Med. 2001;134:1-11. [PubMed] |
| 6. | Drøyvold WB, Midthjell K, Nilsen TI, Holmen J. Change in body mass index and its impact on blood pressure: a prospective population study. Int J Obes (Lond). 2005;29:650-655. [PubMed] |
| 7. | Moore LL, Visioni AJ, Qureshi MM, Bradlee ML, Ellison RC, D'Agostino R. Weight loss in overweight adults and the long-term risk of hypertension: the Framingham study. Arch Intern Med. 2005;165:1298-1303. [PubMed] |
| 8. | Huang Z, Willett WC, Manson JE, Rosner B, Stampfer MJ, Speizer FE, Colditz GA. Body weight, weight change, and risk for hypertension in women. Ann Intern Med. 1998;128:81-88. [PubMed] |
| 9. | Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults-The Evidence Report. National Institutes of Health. Obes Res. 1998;6 Suppl 2:51S-209S. [PubMed] |
| 10. | Juhaeri J, Chambless LE, Tyroler HA, Rosamond W, Nieto FJ, Schreiner P, Jones DW, Arnett D. Associations between weight gain and incident hypertension in a bi-ethnic cohort: the Atherosclerosis Risk in Communities Study. Int J Obes Relat Metab Disord. 2002;26:58-64. [PubMed] |
| 11. | Field AE, Byers T, Hunter DJ, Laird NM, Manson JE, Williamson DF, Willett WC, Colditz GA. Weight cycling, weight gain, and risk of hypertension in women. Am J Epidemiol. 1999;150:573-579. [PubMed] |
| 12. | French SA, Jeffery RW, Folsom AR, McGovern P, Williamson DF. Weight loss maintenance in young adulthood: prevalence and correlations with health behavior and disease in a population-based sample of women aged 55-69 years. Int J Obes Relat Metab Disord. 1996;20:303-310. [PubMed] |
| 13. | Honjo M, Nakamura K, Yamashiro K, Kiryu J, Tanihara H, McEvoy LM, Honda Y, Butcher EC, Masaki T, Sawamura T. Lectin-like oxidized LDL receptor-1 is a cell-adhesion molecule involved in endotoxin-induced inflammation. Proc Natl Acad Sci USA. 2003;100:1274-1279. [PubMed] |
| 14. | Hinagata J, Kakutani M, Fujii T, Naruko T, Inoue N, Fujita Y, Mehta JL, Ueda M, Sawamura T. Oxidized LDL receptor LOX-1 is involved in neointimal hyperplasia after balloon arterial injury in a rat model. Cardiovasc Res. 2006;69:263-271. [PubMed] |
| 15. | Greenspan P, Mayer EP, Fowler SD. Nile red: a selective fluorescent stain for intracellular lipid droplets. J Cell Biol. 1985;100:965-973. [PubMed] |
| 16. | Visser M, Bouter LM, McQuillan GM, Wener MH, Harris TB. Elevated C-reactive protein levels in overweight and obese adults. JAMA. 1999;282:2131-2135. [PubMed] |
| 17. | Ziccardi P, Nappo F, Giugliano G, Esposito K, Marfella R, Cioffi M, D'Andrea F, Molinari AM, Giugliano D. Reduction of inflammatory cytokine concentrations and improvement of endothelial functions in obese women after weight loss over one year. Circulation. 2002;105:804-809. [PubMed] |
| 18. | Nigam A, Mitchell GF, Lambert J, Tardif JC. Relation between conduit vessel stiffness (assessed by tonometry) and endothelial function (assessed by flow-mediated dilatation) in patients with and without coronary heart disease. Am J Cardiol. 2003;92:395-399. [PubMed] |
| 19. | Shoelson SE, Herrero L, Naaz A. Obesity, inflammation, and insulin resistance. Gastroenterology. 2007;132:2169-2180. [PubMed] |
| 20. | Moriwaki H, Kume N, Sawamura T, Aoyama T, Hoshikawa H, Ochi H, Nishi E, Masaki T, Kita T. Ligand specificity of LOX-1, a novel endothelial receptor for oxidized low density lipoprotein. Arterioscler Thromb Vasc Biol. 1998;18:1541-1547. [PubMed] |
| 21. | Fraley AE, Tsimikas S. Clinical applications of circulating oxidized low-density lipoprotein biomarkers in cardiovascular disease. Curr Opin Lipidol. 2006;17:502-509. [PubMed] |
| 22. | Matsuura E, Kobayashi K, Tabuchi M, Lopez LR. Oxidative modification of low-density lipoprotein and immune regulation of atherosclerosis. Prog Lipid Res. 2006;45:466-486. [PubMed] |
| 23. | Chen J, Mehta JL, Haider N, Zhang X, Narula J, Li D. Role of caspases in Ox-LDL-induced apoptotic cascade in human coronary artery endothelial cells. Circ Res. 2004;94:370-376. [PubMed] |
| 24. | Imanishi T, Hano T, Sawamura T, Takarada S, Nishio I. Oxidized low density lipoprotein potentiation of Fas-induced apoptosis through lectin-like oxidized-low density lipoprotein receptor-1 in human umbilical vascular endothelial cells. Circ J. 2002;66:1060-1064. [PubMed] |
| 25. | Li DY, Zhang YC, Philips MI, Sawamura T, Mehta JL. Upregulation of endothelial receptor for oxidized low-density lipoprotein (LOX-1) in cultured human coronary artery endothelial cells by angiotensin II type 1 receptor activation. Circ Res. 1999;84:1043-1049. [PubMed] |
| 26. | Kume N, Murase T, Moriwaki H, Aoyama T, Sawamura T, Masaki T, Kita T. Inducible expression of lectin-like oxidized LDL receptor-1 in vascular endothelial cells. Circ Res. 1998;83:322-327. [PubMed] |
| 27. | Murase T, Kume N, Korenaga R, Ando J, Sawamura T, Masaki T, Kita T. Fluid shear stress transcriptionally induces lectin-like oxidized LDL receptor-1 in vascular endothelial cells. Circ Res. 1998;83:328-333. [PubMed] |
| 28. | Chen M, Nagase M, Fujita T, Narumiya S, Masaki T, Sawamura T. Diabetes enhances lectin-like oxidized LDL receptor-1 (LOX-1) expression in the vascular endothelium: possible role of LOX-1 ligand and AGE. Biochem Biophys Res Commun. 2001;287:962-968. [PubMed] |
| 29. | Steinbrook R. Surgery for severe obesity. N Engl J Med. 2004;350:1075-1079. [PubMed] |
| 30. | Pinkney JH, Sjöström CD, Gale EA. Should surgeons treat diabetes in severely obese people? Lancet. 2001;357:1357-1359. [PubMed] |
| 31. | Dixon JB, Pories WJ, O'Brien PE, Schauer PR, Zimmet P. Surgery as an effective early intervention for diabesity: why the reluctance? Diabetes Care. 2005;28:472-474. [PubMed] |
| 32. | Himpens J, Dapri G, Cadière GB. A prospective randomized study between laparoscopic gastric banding and laparoscopic isolated sleeve gastrectomy: results after 1 and 3 years. Obes Surg. 2006;16:1450-1456. [PubMed] |
| 33. | Moon Han S, Kim WW, Oh JH. Results of laparoscopic sleeve gastrectomy (LSG) at 1 year in morbidly obese Korean patients. Obes Surg. 2005;15:1469-1475. [PubMed] |