Published online Aug 28, 2011. doi: 10.3748/wjg.v17.i32.3724
Revised: April 23, 2011
Accepted: April 30, 2011
Published online: August 28, 2011
AIM: To characterize the effects of age on clinical presentations and endoscopic diagnoses and to determine outcomes after endoscopic therapy among patients aged ≥ 65 years admitted for acute upper gastrointestinal bleeding (UGIB) compared with those aged < 65 years.
METHODS: Medical records and an endoscopy data-base of 526 consecutive patients with overt UGIB ad-mitted during 2007-2009 were reviewed. The initial presentations and clinical course within 30 d after endoscopy were obtained.
RESULTS: A total of 235 patients aged ≥ 65 years constituted the elderly population (mean age of 74.2 ± 6.7 years, 63% male). Compared to young patients, the elderly patients were more likely to present with melena (53% vs 30%, respectively; P < 0.001), have comorbidities (69% vs 54%, respectively; P < 0.001), and receive antiplatelet agents (39% vs 10%, respectively; P < 0.001). Interestingly, hemodynamic instability was observed less in this group (49% vs 68%, respectively; P < 0.001). Peptic ulcer was the leading cause of UGIB in the elderly patients, followed by varices and gastropathy. The elderly and young patients had a similar clinical course with regard to the utilization of endoscopic therapy, requirement for transfusion, duration of hospital stay, need for surgery [relative risk (RR), 0.31; 95% confidence interval (CI), 0.03-2.75; P = 0.26], rebleeding (RR, 1.44; 95% CI, 0.92-2.25; P = 0.11), and mortality (RR, 1.10; 95% CI, 0.57-2.11; P = 0.77). In Cox’s regression analysis, hemodynamic instability at presentation, background of liver cirrhosis or disseminated malignancy, transfusion requirement, and development of rebleeding were significantly associated with 30-d mortality.
CONCLUSION: Despite multiple comorbidities and the concomitant use of antiplatelets in the elderly patients, advanced age does not appear to influence adverse outcomes of acute UGIB after therapeutic endoscopy.
- Citation: Charatcharoenwitthaya P, Pausawasdi N, Laosanguaneak N, Bubthamala J, Tanwandee T, Leelakusolvong S. Characteristics and outcomes of acute upper gastrointestinal bleeding after therapeutic endoscopy in the elderly. World J Gastroenterol 2011; 17(32): 3724-3732
- URL: https://www.wjgnet.com/1007-9327/full/v17/i32/3724.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i32.3724
The 2003 World Health Report highlighted the accelerated aging of the global population, as the number of elderly people will double in the next few decades[1]. Upper gastrointestinal bleeding (UGIB) affects a substantial number of elderly people and is a potentially life-threatening clinical event. Age has been considered as a significant prognostic factor for adverse outcomes, including rebleeding and mortality, from acute UGIB in numerous clinical risk models[2-6]. However, it is unclear if the role of age in UGIB is due to a more severe disease or differences in the treatment received. Generally, the elderly have often been treated less aggressively than younger patients because of an assumption of increased risk of any therapeutic procedures, secondary to comorbid conditions. However, recent studies of gastrointestinal endoscopy conducted in elderly patients reported an overall procedural success and morbidity similar to that reported for the general population, even in patients undergoing upper endoscopy for the evaluation of acute gastrointestinal bleeding[7,8].
Over the last decades, clinical considerations related to the diagnosis and treatment of UGIB has changed dramatically. Paramount to these changes have been the increased involvement of acute care specialists during resuscitation, advances in diagnostic and therapeutic endoscopy, the use of powerful acid suppressive and vasoactive agents, and more selective and less invasive surgical approaches that may offer a promising outcome for patients. Hence, outcome studies on the appropriate approach to UGIB in the elderly are needed. We therefore conducted the current study to characterize the effects of age on clinical presentations and endoscopic diagnoses and to determine outcomes after pharmacologic and endoscopic therapy with regard to the transfusion requirement, duration of hospital stay, need for surgical intervention, rate of rebleeding, and 30-d mortality among a large cohort of patients aged ≥ 65 years who were hospitalized for acute UGIB compared with those aged < 65 years.
This retrospective study was approved by the Institutional Review Board of the Hospital and was conducted at Siriraj Hospital, a tertiary academic medical center for the Bangkok metropolitan area and surrounding communities. All consecutive adult patients who were hospitalized for acute UGIB (e.g., hematochezia, melena, coffee-ground vomiting or hematemesis with or without hypotension) and who underwent endoscopy between January 2007 and December 2009 were included in the study. For the purposes of this study, we defined “elderly” as those older than 65 years of age.
At our institution, patients who presented with acute UGIB were given appropriate initial resuscitation followed by diagnostic and therapeutic measures. Empiric therapy using either an intravenous proton pump inhibitor (PPI) or somatostatin analogue infusion was given before the endoscopy for suspicion of peptic ulcer and varices, respectively. Urgent endoscopy within the first 12 h after admission was performed in patients with signs of ongoing bleeding as determined by gastroenterologists. Endoscopic treatment was given in the form of injection therapy with epinephrine, coaptive thermocoagulation, hemostatic clip or combination therapy in patients with active bleeding, nonbleeding visible vessels or adherent clots. High-dose PPI was administered by infusion for 72 h after endoscopy in patients who required endoscopic intervention. Bleeding esophageal and gastric varices were treated with band ligation and cyanoacrylate injection, respectively, in addition to the use of vasoactive drugs and intravenous antibiotics. After the procedure, the patients were subsequently transferred to a medical ward for monitoring. Endoscopy was repeated in the event of rebleeding. Patients underwent surgery if bleeding persisted or if rebleeding occurred after two therapeutic endoscopies.
Medical records and an endoscopy database of all patients were reviewed. Patient demographics, clinical presentations, initial vital signs, the presence of comorbid conditions, drugs taken at the time of admission and initial laboratory tests were obtained. We abstracted data describing the endoscopic management, including endoscopic diagnosis and the presence of stigmata of recent bleeding, endoscopic hemostasis, and medication use following endoscopy. Outcome data describing the overall course within 30 d after the initial endoscopic treatment with specific attention to rebleeding, the need for surgery, a requirement for blood transfusion, the length of hospital stay, and mortality were gathered.
The presence of hemodynamic instability was defined as systolic blood pressure < 100 mmHg, a heart rate > 100 beats/min and/or orthostatic changes in systolic blood pressure (a decrease of > 10%) or heart rate (an increase of > 10%) between a supine and seated position. Rebleeding was defined by the presence of hematemesis or melena with signs of hemodynamic instability or a decrease in hemoglobin level > 2 g/dL in a previously stable patient. Endoscopic grading of ulcer lesions was categorized according to the Forrest’s classification[9]. The stigmata of recent bleeding included arterial spurting or pulsatile bleeding from the ulcer base, a non-bleeding visible vessel, and an adherent clot covering the base of an ulcer. Grading of varices was carried out using the classification of the Italian Liver Cirrhosis Project[10].
Data were summarized using descriptive statistics. Continuous variables were compared using the t test or the Mann-Whitney test. Categorical variables were compared using the χ2 or Fisher exact test. The Kaplan-Meier method with the log-rank test was used to compare differences in the rates of rebleeding and death within 30 d after primary endoscopic treatment. Cox’s regression analysis was used to detect possible prognostic variables on recurrent bleeding and survival. All statistical testing was performed at the conventional 2-tailed α level of 0.05.
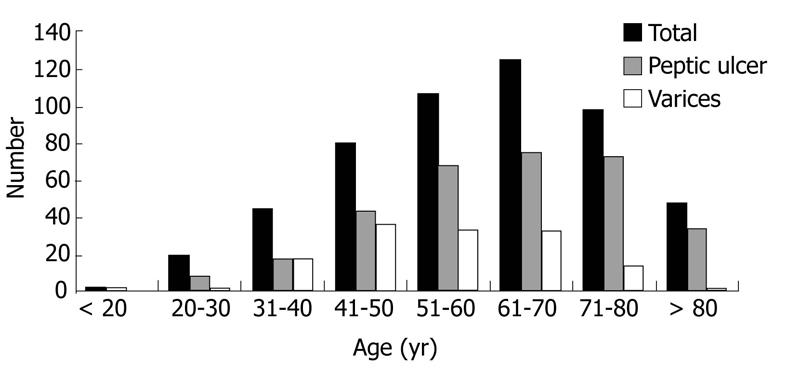
During a three-year period, a total of 526 patients (370 men, 156 women) with acute UGIB were identified. The age distribution at presentation is shown in Figure 1. Acute UGIB occurred in patients aged 18-40 years (13%), 41-50 years (15%), 51-60 years (20%), 61-70 years (24%), 71-80 years (19%) and > 80 years (9%). Two hundred thirty-five patients were at least 65 years of age and constituted the elderly population, with a mean age of 74.2± 6.7 years. Two hundred ninety-one patients were < 65 years old and constituted the young population, with a mean age of 48.4 ± 11.1 years. The patient demographics and clinical characteristics of the entire group and each of the cohorts are shown in Table 1.
| Total | Patients aged≥65 yr | Patients aged < 65 yr | P value | |
| (n = 526) | (n = 235) | (n = 291) | ||
| Age (yr) | 60 ± 15.9 | 74.2 ± 6.7 | 48.4 ± 11.1 | < 0.001 |
| Male: n (%) | 370 (70) | 148 (63) | 222 (76) | < 0.001 |
| Presenting symptoms: 1n (%) | ||||
| Hematemesis | 207 (39) | 65 (28) | 142 (49) | < 0.001 |
| Melena | 214 (41) | 126 (53) | 88 (30) | < 0.001 |
| “Coffee ground” vomiting | 86 (16) | 36 (15) | 50 (17) | 0.57 |
| Hematochezia | 19 (4) | 8 (4) | 11 (4) | 0.82 |
| Clinical findings: n (%) | ||||
| Red blood on nasogastric lavage | 97 (18) | 41 (17) | 56 (19) | 0.60 |
| Systolic blood pressure < 100 mmHg | 163 (31) | 62 (26) | 101 (35) | 0.04 |
| Heart rate > 100 beats/min | 192 (37) | 63 (27) | 129 (44) | < 0.001 |
| Presence of hemodynamic instability | 313 (60) | 114 (49) | 199 (68) | < 0.001 |
| Comorbid illness: 1n (%) | ||||
| Cardiovascular disease | 109 (21) | 81 (34) | 28 (10) | < 0.001 |
| Cerebrovascular disease | 49 (9) | 34 (14) | 15 (5) | < 0.001 |
| Chronic renal failure | 37 (7) | 17 (7) | 20 (7) | 0.87 |
| Liver cirrhosis | 140 (27) | 45 (19) | 95 (33) | < 0.001 |
| Cancer | 64 (12) | 33 (14) | 31 (11) | 0.24 |
| Diabetes mellitus | 126 (24) | 87 (37) | 39 (1) | < 0.001 |
| Hypertension | 186 (35) | 125 (53) | 61 (21) | < 0.001 |
| Alcohol drinking: n (%) | 246 (47) | 66 (28) | 180 (62) | < 0.001 |
| Previous use of medications: 1n (%) | ||||
| Low-dose aspirin | 111 (21) | 82 (35) | 29 (10) | < 0.001 |
| Clopidogrel | 33 (6) | 30 (13) | 3 (1) | < 0.001 |
| Warfarin | 38 (7) | 22 (9) | 16 (6) | 0.09 |
| NSAID other than aspirin | 123 (23) | 57 (24) | 66 (23) | 0.67 |
| Laboratory features at presentation: | ||||
| Hemoglobin (g/dL) | 8.7 ± 4.7 | 8.4 ± 2.3 | 9.0 ± 6.0 | 0.19 |
| White blood count (103/μL) | 11.8 ± 8.1 | 11.2 ± 5.3 | 12.3 ± 9.7 | 0.15 |
| Platelets (103/μL) | 232 ± 125 | 242 ± 116 | 223 ± 131 | 0.09 |
| Prothrombin time (s) | 13.4 (11.4-248) | 13.2 (11.4-160) | 13.9 (11.4-248) | 0.84 |
| Creatinine (mg/dL) | 1.1 (0.2-11.8) | 1.3 (0.4-10.8) | 0.9 (0.2-11.8) | 0.44 |
Patients ≥ 65 years of age were more likely to present with melena, receive antiplatelet agents, and have comorbid conditions including cardiovascular disease, cerebrovascular disease, cirrhosis, diabetes, and hypertension compared with the young population (Table 1). The rates of antiplatelet use were increased with older age (P < 0.001). In contrast, patients < 65 years old presented with hematemesis (49% vs 28%, respectively; P < 0.001) and hemodynamic instability (68% vs 49%, respectively; P < 0.001) more commonly than the elderly. There were no differences in terms of ‘coffee ground’ vomiting, hematochezia, red blood on the initial nasogastric lavage, the use of anticoagulants and non-steroidal anti-inflammatory drugs (NSAIDs), and laboratory indices at presentation between the two groups (Table 1).
The endoscopic findings for the entire patient group and each of the cohorts are shown in Table 2. Distributions of peptic ulcer and varices as the source of bleeding among each age range at presentation are summarized in Figure 1. Bleeding peptic ulcers were identified more frequently in patients aged 60-80 years, suggesting that peptic ulcers are a more common source of bleeding in the elderly than in patients aged < 65 years (68% vs 56%, respectively; P = 0.006). Among those with peptic ulcer bleeding, clean base ulcers were seen more frequently in the elderly compared with young patients (48% vs 38%, respectively; P = 0.03). The numbers of active bleeding, non-bleeding visible vessels, clots, and flat pigmented spots did not differ significantly between the two groups (Table 2). Of these, 45 (28%) elderly patients required therapeutic endoscopy compared with 55 (34%) young patients (relative risk for the elderly, 0.84; 95% confidence interval (CI), 0.60 to 1.16; P = 0.29) (Table 3).
| Total | Patients aged≥65 yr | Patients aged < 65 yr | P value | |
| (n = 526) | (n = 235) | (n = 291) | ||
| Peptic ulcer as source of bleeding: 1n (%) | ||||
| Active bleeding | 19 (4) | 11 (5) | 8 (3) | 0.20 |
| Non-bleeding visible vessel | 59 (11) | 25 (11) | 34 (12) | 0.71 |
| Clot with underlying vessel | 16 (3) | 8 (3) | 8 (3) | 0.66 |
| Flat, pigmented spot | 41 (8) | 23 (10) | 18 (6) | 0.13 |
| Clean base | 224 (46) | 112 (48) | 112 (38) | 0.03 |
| Portal hypertensive related-lesions as source of bleeding: 1n (%) | ||||
| Esophageal varices | 137 (26) | 41 (17) | 96 (33) | < 0.001 |
| Gastric and duodenal varices | 17 (3) | 4 (2) | 14 (5) | 0.051 |
| Portal hypertensive gastropathy | 58 (11) | 17 (7) | 41 (14) | 0.01 |
| Other endoscopic findings: 1n (%) | ||||
| Esophageal ulcer | 19 (4) | 9 (4) | 10 (3) | 0.81 |
| Esophagitis | 36 (7) | 17 (7) | 19 (7) | 0.75 |
| Gastropathy, duodenitis, or erosions | 129 (25) | 61 (26) | 68 (23) | 0.49 |
| Mallory-Weiss tear | 26 (5) | 5 (2) | 21 (7) | 0.007 |
| Gastric cancer | 15 (3) | 6 (3) | 9 (3) | 0.71 |
| Dieulafoy’s lesion | 11 (2) | 6 (3) | 5 (2) | 0.51 |
| Angiodysplasia | 2 (0.004) | 1 (0.4) | 1 (0.3) | 0.88 |
| No clinically significant finding | 11 (2) | 3 (1) | 8 (3) | 0.24 |
| Patients aged≥65 yr | Patients aged < 65 yr | P value | Relative risk | |
| (n = 235) | (n = 291) | (95% CI) | ||
| Urgent Endoscopy: n (%) | 47 (20) | 64 (22) | 0.58 | 0.91 (0.65-1.27) |
| For peptic ulcers bleeding | 22 (14) | 29 (18) | 0.33 | 0.78 (0.47-1.29) |
| For variceal bleeding | 16 (46) | 30 (37) | 0.36 | 1.25 (0.79-1.98) |
| Endoscopic therapy for bleeding peptic ulcers: 1n (%) | 45 (28) | 55 (34) | 0.29 | 0.84 (0.60-1.16) |
| Epinephrine injection | 36 (80) | 50 (91) | 0.12 | |
| Coaptive thermocoagulation | 28 (62) | 39 (71) | 0.36 | |
| Hemostatic clip | 11 (24) | 14 (25) | 0.91 | |
| Combined therapy | 29 (64) | 42 (76) | 0.19 | |
| Endoscopic therapy for variceal bleeding: 1n (%) | 27 (66) | 72 (75) | 0.34 | 0.89 (0.69-1.14) |
| Esophageal band ligation | 25 (93) | 64 (89) | 0.59 | |
| Cyanoacrylate injection | 3 (11) | 10 (14) | 0.72 | |
| The 72-h infusion of PPI after endoscopic therapy for bleeding peptic ulcers: n (%) | 47 (29) | 49 (30) | 0.84 | 0.97 (0.69-1.35) |
| The 3-5 d infusion of vasoactive agent after endoscopic therapy for variceal bleeding: n (%) | 27 (66) | 73 (76) | 0.22 | 0.87 (0.68-1.11) |
| Units of blood transfused: | ||||
| Before endoscopy | 1.8 ± 1.4 | 1.8 ± 1.6 | 0.88 | |
| During hospitalization | 2.7 ± 0.2 | 2.9 ± 0.2 | 0.33 | |
| Hospital stay (d) | 5 (1-14) | 4 (1-13) | 0.84 | |
| Hospital stay < 3 d: n (%) | 85 (36) | 106 (36) | 0.95 | 0.99 (0.79-1.25) |
| Recurrent bleeding: n (%) | ||||
| Within 3 d | 11 (5) | 11 (4) | 0.61 | 1.24 (0.55-2.81) |
| Within 7 d | 22 (9) | 17 (6) | 0.13 | 1.60 (0.87-2.95) |
| Within 30 d | 36 (15) | 31 (11) | 0.11 | 1.44 (0.92-2.25) |
| Emergency surgery: n (%) | 1 (0.4) | 4 (1.4) | 0.26 | 0.31 (0.03-2.75) |
| Death within 30 d: n (%) | 16 (7) | 18 (6) | 0.77 | 1.10 (0.57-2.11) |
Variceal bleeding tended to decrease after the fourth decade of life. As a result of higher alcohol consumption, esophageal varices (33% vs 17%, respectively; P < 0.001) and Mallory-Weiss tears (7% vs 2%, respectively; P = 0.007) were noted more frequently in patients < 65 than in those > 65 years of age. Furthermore, young patients had a trend toward higher numbers of bleeding from gastric and duodenal varices compared to the elderly population (Table 2). Of those with variceal bleeding, 27 (66%) elderly patients received therapeutic endoscopy compared with 75 (75%) young patients (relative risk for the elderly, 0.89; 95% CI, 0.69 to 1.14; P = 0.34) (Table 3).
Urgent endoscopy was performed in 47 patients aged ≥ 65 years and in 64 patients aged < 65 years. Hemodynamic instability at presentation was less frequent in the elderly patients compared to the young patients (49% vs 68%, respectively; P < 0.001). One of the elderly patients and four of the young patients underwent emergency surgery because of failure to achieve hemostasis during endoscopy. The mean number of units of packed erythrocytes transfused prior to endoscopy and during hospitalization was similar in both groups. In addition, the length of hospital stay was not significantly different between the two groups.
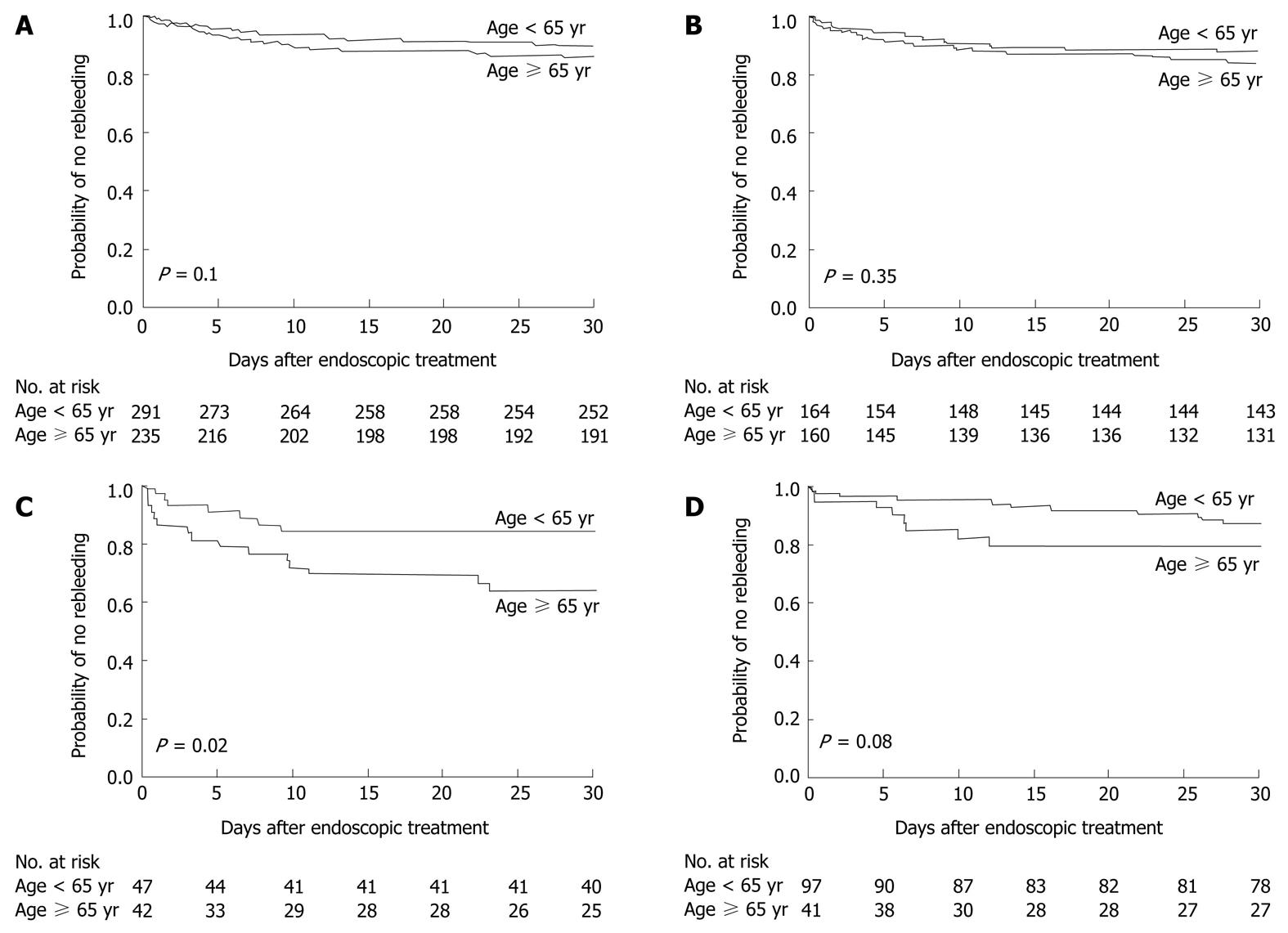
Rebleeding occurred within 30 d after the initial endoscopic therapy in 36 patients (15%) aged ≥ 65 years and in 31 patients (11%) aged < 65 years (P = 0.1) (Figure 2A). When we analyzed the rebleeding rate according to the source of bleeding, the probability of rebleeding within 30 d after endoscopic therapy among patients with peptic ulcer bleeding was similar in both groups (P = 0.35) (Figure 2B), but the rebleeding rate among patients who had ulcers with stigmata of recent bleeding was higher in the elderly patients (P = 0.02) (Figure 2C). The rate of recurrent variceal bleeding was also higher in the elderly patients than in the young patients, although the difference was not statistically significant (P = 0.08) (Figure 2D).
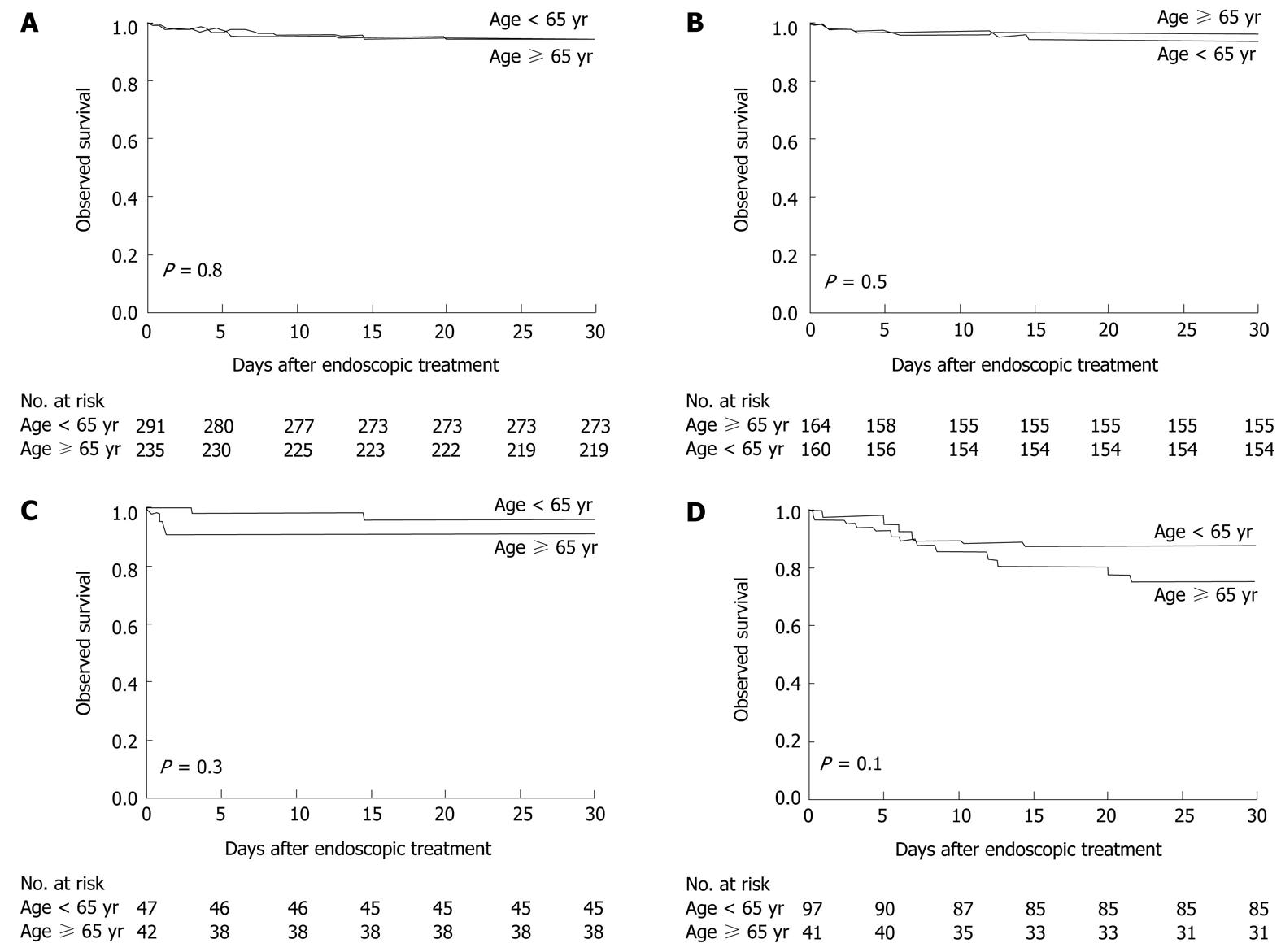
Sixteen patients (7%) aged ≥ 65 years and 18 patients (6%) aged < 65 years died within 30 d after the initial endoscopic treatment. The observed survival was virtually identical for both groups (P = 0.8) (Figure 3A). However, all deaths tended to occur in a greater proportion of elderly patients who had ulcers with stigmata of recent bleeding (P = 0.3) (Figure 3C) and varices (P = 0.1) (Figure 3D). The cause of death in the elderly patients was profound shock at presentation caused by a spurting hemorrhage from the ulcer that failed endoscopic therapy (two) and refractory variceal bleeding after endoscopic band ligation (one). The deaths in the remaining 13 patients were related to their comorbid illnesses: nosocomial pneumonia (five), septicemia (two), spontaneous bacterial peritonitis (one), myocardial infarction (one), congestive heart failure (one), liver failure (two), and primary liver cancer (one). The causes of death in the young patients were profound shock at presentation caused by active variceal bleeding (five) and ulcer bleeding (one), which failed endoscopic intervention. Twelve patients died of their comorbid illnesses: nosocomial pneumonia (four), septicemia (three), renal failure as a consequence of massive gastrointestinal bleeding (four), and metastatic biliary cancer (one).
By univariate analysis, an increased risk of rebleeding after endoscopic hemostasis was associated with the presence of endoscopic stigmata of recent bleeding (P < 0.001) and high blood transfusion requirement before the endoscopy (P = 0.003). The risk of rebleeding was not associated with age ≥ 65 years (P = 0.11) or even with age ≥ 85 years (P = 0.53), male gender (P = 0.75), the presence of comorbid illness (P = 0.23), the use of antiplatelet agents (P = 0.78), the presence of hemodynamic instability at presentation (P = 0.19), hematemesis (P = 0.27), hematochezia (P = 0.75), peptic ulcer bleeding (P = 0.33), variceal bleeding (P = 0.14), and medium to large variceal size (P = 0.57). In the multivariable Cox regression model, the number of blood transfusions before endoscopy and stigmata of recent bleeding remained significantly associated with rebleeding (Table 4).
| Variable | Parameter estimate | Standard error | Hazard ratio | P value |
| (95% CI) | ||||
| Prediction for Recurrent bleeding | ||||
| Number of blood transfusions | ||||
| before endoscopy | 0.22 | 0.07 | 1.25 (1.08-1.42) | 0.003 |
| Stigmata of recent bleeding | 0.99 | 0.26 | 2.68 (1.58-4.41) | < 0.001 |
| Prediction for Mortality | ||||
| Hemodynamic instability | 0.95 | 0.50 | 2.57 (1.05-7.76) | 0.04 |
| Liver cirrhosis | 1.33 | 0.62 | 3.77 (1.10-12.2) | 0.03 |
| Disseminated malignancy | 1.8 | 0.65 | 6.06 (1.37-18.8) | 0.02 |
| Number of blood transfusions | 0.11 | 0.03 | 1.12 (1.03-1.19) | 0.004 |
| Recurrent bleeding | 1.62 | 0.39 | 5.07 (2.30-10.9) | < 0.001 |
| Variceal bleeding | 0.51 | 0.61 | 1.66 (0.55-5.79) | 0.39 |
In the univariate analysis, the following variables had a significant influence on patient survival within 30 d: the presence of hemodynamic instability at presentation (P < 0.001), liver cirrhosis (P < 0.001), disseminated malignancy (P < 0.001), variceal bleeding (P < 0.001), the total number of blood transfusions (P = 0.02), and the occurrence of rebleeding (P < 0.001). The risk of death was not significantly associated with age ≥ 65 years (P = 0.80) or even with age ≥ 85 years (P = 0.13), male gender (P = 0.74), hematemesis (P = 0.09), hematochezia (P = 0.51), and the endoscopic stigmata of recent bleeding (P = 0.89). In the multivariate analysis, the presence of hemodynamic instability at presentation, a background of liver cirrhosis or disseminated malignancy, a transfusion requirement during admission, and the development of rebleeding remained significantly associated with 30-d mortality (Table 4).
The current study shows that age is associated with a steep rise in the incidence of acute UGIB. The elderly patients had different sources and clinical presentations of acute UGIB compared with the young patients. The risk for rebleeding correlated with endoscopic stigmata of recent bleeding and the severity of bleeding, as reflected by blood transfusion requirement, but not with advanced age. Furthermore, the elderly patients did not show a significant difference in clinical course from the young patients with regard to the utilization of endoscopic therapy, transfusion requirement, the duration of hospital stay, the need for surgery, the rate of rebleeding, and mortality.
Aging may result in various physiologic changes in the gastrointestinal tract[11], which may increase the risk for the development of acid-related disorders. Our study confirms that the incidence of acid-related bleeding increases with increasing age[12-14]. Consistent with previous studies, we found that approximately 80% of UGIB occurring in patients aged ≥ 65 years is derived from acid-related disorders[12-15]. One of the major factors that might explain this feature in the elderly population is the increased prescribing of gastroduodenal-damaging drugs, including aspirin, clopidogrel, and NSAIDs. As expected, 60% of our elderly population used either antiplatelet agents or a prescription NSAID, which was 2 times greater than that seen in the young population. Furthermore, some investigators have hypothesized that a mechanism underlying various manifestations of gastrointestinal bleeding in the elderly may involve ischemic damage to gastrointestinal mucosa[16,17]. In the current study, while direct measurements of visceral atherosclerosis were not available, we examined whether clinically recognized cardiovascular or cerebrovascular diseases were associated with the risk of ulcer-related bleeding. We found no association between clinical atherosclerotic disease and the risk of ulcer-related bleeding (data not shown).
Patients with acute UGIB typically present with vomiting of fresh blood or coffee ground-like material or the rectal passage of blood. Compared with young individuals, our elderly patients commonly presented with mild symptoms or subtle bleeding. One possible explanation could be that these patients were more likely to be hospitalized for non-life-threatening bleeding for close medical attention given their vulnerability and multiple comorbid diseases. Therefore, it is possible that patient comorbidity may result in the early recognition or management of UGIB rather than reflecting the etiology of the bleeding. Our experiences are in agreement with prior evidence that suggests an association between hospitalized gastrointestinal bleeding and poor health[16,18,19]. Moreover, we cannot exclude the possibility that the incidence of hemodynamic instability from UGIB was underestimated in this study because the use of beta blockers, which is common in the elderly who have multiple comorbid conditions, can mask tachycardia in patients with UGIB.
Patient risk stratification can be performed based on predictive factors for rebleeding, and resources can be allocated accordingly. There are numerous studies that have reported the predictive factors for rebleeding[2-6]. The current study confirms that endoscopic stigmata of recent bleeding is the most important predictor of rebleeding and influences other important end points such as transfusion requirement[20-23]. In contrast to prior studies, our data do not show a significant association between older age and the risk of rebleeding. It is possible that the current study included all patients with UGIB regardless of etiology in the analysis, and thus, the effect of stigmata of recent bleeding in elderly patients with peptic ulcers could have been diluted by the inclusion of a large number of patients with clean-based ulcers that are at low risk of rebleeding. When we restricted our analysis according to the endoscopic findings, we found that age ≥ 65 years was associated with an increase in the risk of ulcer rebleeding among those with high-risk ulcer stigmata. Although the reason for this observation was unclear, we hypothesize that it may be related to impaired hemostasis caused by platelet dysfunction because elderly patients are more likely to have received antiplatelet therapy or NSAIDs before admission. The late rebleeding seen in our elderly patients also suggests that there may be an unknown pathogenic process that adversely affects the healing of peptic ulcers (Figure 2C). In addition, there was a trend toward a higher rate of variceal rebleeding in elderly patients. A larger study size may be required to clarify the possible prognostic factor of older age for variceal rebleeding.
Despite advances in the management of UGIB during the past decade, the reported mortality for patients over 60 years of age with UGIB is 12%-25% and nearly 35% in those over 80 years of age[24,25]. The lack of change is probably explained by the associated comorbidities with increasing age. These patients are also more vulnerable to a physiological challenge from an acute bleeding episode. However, the mortality of our elderly cohort for acute UGIB was 7%, which was lower than those of previous reports. The leading cause of death in the elderly is sepsis followed by multiorgan failure. This study also reports a low rate of surgical intervention, which was seen in approximately 1% of all patients. The decrease in surgical requirement and mortality in our patients could reflect the increasing use of endoscopic hemostasis and likely underlines the systematic use of potent antisecretory agents for acid-related bleeding and vasoactive agents for variceal bleeding after therapeutic endoscopy. These therapeutic measures have been reported to improve the outcome of patients with UGIB[26,27].
Several bleeding scoring systems have been developed to predict the outcomes for patients with UGIB[2-6] and have shown that the risk for adverse outcomes increases when the patients are older. However, our study showed that age ≥ 65 years did not influence the transfusion requirement, duration of hospital stay, need for surgery, and mortality. Furthermore, multivariate analysis showed that comorbid illnesses with liver cirrhosis or disseminated malignancy, severe bleeding represented by significant hemodynamic change requiring multiple blood transfusions and the development of rebleeding were significant predictive factors for mortality. These findings are consistent with the reported predictive models on mortality for UGIB in the literature[2-6].
Some factors may limit the generalizability of our findings. First, our patients awaiting endoscopy, who were suspected to have a high risk of ulcer or variceal rebleeding, received the preemptive use of high-dose intravenous PPI or vasoactive agents, respectively. It is possible that this management could influence endoscopic findings and the course of UGIB. Second, we cannot exclude referral bias, which may select patients with severe diseases. However, we would not expect the age-related differences in clinical presentation and the source of UGIB to be a large artifact of this bias. The similar outcome in young and elderly patients after endoscopic therapy indicates that a selection bias for severe disease does not have an adverse impact on treatment outcome.
In conclusion, the etiology of UGIB in the elderly has changed little in recent years. Despite multiple comorbidities and the concomitant use of antiplatelet therapy in elderly patients, advanced age does not appear to influence adverse outcomes of acute UGIB after therapeutic endoscopy. Morbidity and mortality from UGIB in the elderly are determined by the nature of the bleeding lesions and the presence of comorbid conditions. With the growth of older populations, a coordinated approach to diagnosis and management of acute UGIB should optimize favorable outcomes in this vulnerable patient population similar to those in younger people.
Upper gastrointestinal bleeding (UGIB) affects a substantial number of elderly individuals and is a potentially life-threatening clinical event. Advanced age has been considered a significant prognostic factor for adverse outcomes from acute UGIB; however, it remains unclear if this is due to the severity of disease or differences in the treatment received. A better understanding of the prognostic significance of age should enhance the accuracy during triage and could lead to the more efficient use of critical care resources for the management of acute UGIB.
The aim of the research was to characterize the effects of age on clinical presentations and endoscopic diagnoses and to determine outcomes after pharmacologic and endoscopic therapy with regard to transfusion requirement, the duration of hospital stay, the need for surgical intervention, the rate of rebleeding, and 30-d mortality among a large cohort of patients aged ≥ 65 years who were hospitalized for acute UGIB compared with those aged < 65 years.
In the current study, the authors demonstrated that the elderly patients had different sources and clinical presentations of acute UGIB compared with the young patients. The risk for rebleeding correlated significantly with endoscopic stigmata of recent bleeding and the severity of bleeding but not with advanced age. Furthermore, the clinical course did not significantly differ between the elderly and young patients with regard to the utilization of endoscopic therapy, transfusion requirement, the duration of hospital stay, the need for surgery, the rate of rebleeding, and mortality.
In conclusion, advanced age does not appear to influence adverse outcomes of acute UGIB after therapeutic endoscopy. The promising outcomes of acute UGIB in the elderly may be due in part to the increased involvement of acute care specialists during resuscitation, advances in diagnostic and therapeutic endoscopy, the use of powerful acid suppressive and vasoactive agents, and more selective and less invasive surgical approaches. Therefore, the authors recommend a coordinated approach to manage acute UGIB, which should serve to optimize favorable outcomes in this vulnerable patient population similar to those in young people.
The authors did not discuss beta blockers usages in the patients. Beta blocker usages are common in the elderly who have multiple comorbid conditions. They select heart rate as one of a predictor of hemodynamic instability. Use of beta blockers can mask tachycardia in the patients with UGIB. This would underestimate the incidence of hemodynamic instability from UGIB in the study.
Peer reviewer: Weekitt Kittisupamongkol, MD, Hua Chiew Hospital, 665 Bumrungmuang Road, Bangkok 10100, Thailand; Kevin Cheng-Wen Hsiao, MD, Assistant Professor, Colon and Rectal Surgery, Tri-Service General Hospital, No. 325, Sec. 2, Cheng-Kung Rd, Nei-Hu district, Taipei 114, Taiwan, China
S- Editor Tian L L- Editor Webster JR E- Editor Li JY
| 1. | World Health Organization. The World Health Report 2003. Available from: http://www.who.int/whr/2003/chapter1. |
| 2. | Rockall TA, Logan RF, Devlin HB, Northfield TC. Risk assessment after acute upper gastrointestinal haemorrhage. Gut. 1996;38:316-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 888] [Cited by in RCA: 896] [Article Influence: 30.9] [Reference Citation Analysis (0)] |
| 3. | Blatchford O, Davidson LA, Murray WR, Blatchford M, Pell J. Acute upper gastrointestinal haemorrhage in west of Scotland: case ascertainment study. BMJ. 1997;315:510-514. [PubMed] |
| 4. | Zimmerman J, Siguencia J, Tsvang E, Beeri R, Arnon R. Predictors of mortality in patients admitted to hospital for acute upper gastrointestinal hemorrhage. Scand J Gastroenterol. 1995;30:327-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 58] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 5. | Marmo R, Koch M, Cipolletta L, Capurso L, Pera A, Bianco MA, Rocca R, Dezi A, Fasoli R, Brunati S. Predictive factors of mortality from nonvariceal upper gastrointestinal hemorrhage: a multicenter study. Am J Gastroenterol. 2008;103:1639-1647; quiz 1648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 105] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 6. | Chiu PW, Ng EK, Cheung FK, Chan FK, Leung WK, Wu JC, Wong VW, Yung MY, Tsoi K, Lau JY. Predicting mortality in patients with bleeding peptic ulcers after therapeutic endoscopy. Clin Gastroenterol Hepatol. 2009;7:311-316; quiz 253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 71] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 7. | Gilbert DA, Silverstein FE, Tedesco FJ. National ASGE survey on upper gastrointestinal bleeding: complications of endoscopy. Dig Dis Sci. 1981;26:55S-59S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 45] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 8. | Clarke GA, Jacobson BC, Hammett RJ, Carr-Locke DL. The indications, utilization and safety of gastrointestinal endoscopy in an extremely elderly patient cohort. Endoscopy. 2001;33:580-584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 105] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 9. | Forrest JA, Finlayson ND, Shearman DJ. Endoscopy in gastrointestinal bleeding. Lancet. 1974;2:394-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 594] [Cited by in RCA: 525] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 10. | Prediction of the first variceal hemorrhage in patients with cirrhosis of the liver and esophageal varices. A prospective multicenter study. N Engl J Med. 1988;319:983-989. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 996] [Cited by in RCA: 841] [Article Influence: 22.7] [Reference Citation Analysis (1)] |
| 11. | Greenwald DA. Aging, the gastrointestinal tract, and risk of acid-related disease. Am J Med. 2004;117 Suppl 5A:8S-13S. [PubMed] |
| 12. | Cooper BT, Weston CF, Neumann CS. Acute upper gastrointestinal haemorrhage in patients aged 80 years or more. Q J Med. 1988;68:765-774. [PubMed] |
| 13. | Nankhonya JM, Datta-Chaudhuri ML, Bhan GL. Acute upper gastrointestinal hemorrhage in older people: a prospective study in two neighboring districts. J Am Geriatr Soc. 1997;45:752-754. [PubMed] |
| 14. | Segal WN, Cello JP. Hemorrhage in the upper gastrointestinal tract in the older patient. Am J Gastroenterol. 1997;92:42-46. [PubMed] |
| 15. | Kaplan RC, Heckbert SR, Koepsell TD, Furberg CD, Polak JF, Schoen RE, Psaty BM. Risk factors for hospitalized gastrointestinal bleeding among older persons. Cardiovascular Health Study Investigators. J Am Geriatr Soc. 2001;49:126-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 60] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 16. | Pahor M, Guralnik JM, Salive ME, Chrischilles EA, Manto A, Wallace RB. Disability and severe gastrointestinal hemorrhage. A prospective study of community-dwelling older persons. J Am Geriatr Soc. 1994;42:816-825. [PubMed] |
| 17. | Rogers BH. Endoscopic diagnosis and therapy of mucosal vascular abnormalities of the gastrointestinal tract occurring in elderly patients and associated with cardiac, vascular, and pulmonary disease. Gastrointest Endosc. 1980;26:134-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 76] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 18. | Smalley WE, Ray WA, Daugherty JR, Griffin MR. No association between calcium channel blocker use and confirmed bleeding peptic ulcer disease. Am J Epidemiol. 1998;148:350-354. [PubMed] |
| 19. | García Rodríguez LA, Cattaruzzi C, Troncon MG, Agostinis L. Risk of hospitalization for upper gastrointestinal tract bleeding associated with ketorolac, other nonsteroidal anti-inflammatory drugs, calcium antagonists, and other antihypertensive drugs. Arch Intern Med. 1998;158:33-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 224] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 20. | Brullet E, Calvet X, Campo R, Rue M, Catot L, Donoso L. Factors predicting failure of endoscopic injection therapy in bleeding duodenal ulcer. Gastrointest Endosc. 1996;43:111-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 60] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 21. | Chung IK, Kim EJ, Lee MS, Kim HS, Park SH, Lee MH, Kim SJ, Cho MS, Hwang KY. Endoscopic factors predisposing to rebleeding following endoscopic hemostasis in bleeding peptic ulcers. Endoscopy. 2001;33:969-975. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 50] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 22. | Thomopoulos KC, Mitropoulos JA, Katsakoulis EC, Vagianos CE, Mimidis KP, Hatziargiriou MN, Nikolopoulou VN. Factors associated with failure of endoscopic injection haemostasis in bleeding peptic ulcers. Scand J Gastroenterol. 2001;36:664-668. [PubMed] [DOI] [Full Text] |
| 23. | Guglielmi A, Ruzzenente A, Sandri M, Kind R, Lombardo F, Rodella L, Catalano F, de Manzoni G, Cordiano C. Risk assessment and prediction of rebleeding in bleeding gastroduodenal ulcer. Endoscopy. 2002;34:778-786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 82] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 24. | Peter DJ, Dougherty JM. Evaluation of the patient with gastrointestinal bleeding: an evidenc DOI: 10.1016/S0733-8627(05)70055-9]e based approach. Emerg Med Clin North Am. 1999;17:239-261, x. [PubMed] |
| 25. | Chow LW, Gertsch P, Poon RT, Branicki FJ. Risk factors for rebleeding and death from peptic ulcer in the very elderly. Br J Surg. 1998;85:121-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 35] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 26. | Leontiadis GI, Sharma VK, Howden CW. Proton pump inhibitor therapy for peptic ulcer bleeding: Cochrane collaboration meta-analysis of randomized controlled trials. Mayo Clin Proc. 2007;82:286-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 37] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 27. | Bañares R, Albillos A, Rincón D, Alonso S, González M, Ruiz-del-Arbol L, Salcedo M, Molinero LM. Endoscopic treatment versus endoscopic plus pharmacologic treatment for acute variceal bleeding: a meta-analysis. Hepatology. 2002;35:609-615. [PubMed] |