Published online Aug 28, 2011. doi: 10.3748/wjg.v17.i32.3716
Revised: April 16, 2011
Accepted: April 23, 2011
Published online: August 28, 2011
AIM: To identify risk factors for nonalcoholic steatohepatitis following pancreaticoduodenectomy, with a focus on factors related to pancreatic secretions.
METHODS: The medical records of 228 patients who had a pancreaticoduodenectomy over a 16-mo period were reviewed retrospectively. The 193 patients who did not have fatty liver disease preoperatively were included in the final analysis. Hepatic steatosis was diagnosed using the differences between splenic and hepatic attenuation and liver-to-spleen attenuation as measured by non-enhanced computed tomography.
RESULTS: Fifteen patients (7.8%) who showed postoperative hepatic fatty changes were assigned to Group A, and the remaining patients were assigned to Group B. Patient demographics, preoperative laboratory findings (including levels of C-peptide, glucagon, insulin and glucose tolerance test results), operation types, and final pathological findings did not differ significantly between the two groups; however, the frequency of pancreatic fistula (P = 0.020) and the method of pancreatic duct stenting (P = 0.005) showed significant differences between the groups. A multivariate analysis identified pancreatic fistula (HR = 3.332, P = 0.037) and external pancreatic duct stenting (HR = 4.530, P = 0.017) as independent risk factors for the development of postoperative steatohepatitis.
CONCLUSION: Pancreatic fistula and external pancreatic duct stenting were identified as independent risk factors for the development of steatohepatitis following pancreaticoduodenectomy.
- Citation: Song SC, Choi SH, Choi DW, Heo JS, Kim WS, Kim MJ. Potential risk factors for nonalcoholic steatohepatitis related to pancreatic secretions following pancreaticoduodenectomy. World J Gastroenterol 2011; 17(32): 3716-3723
- URL: https://www.wjgnet.com/1007-9327/full/v17/i32/3716.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i32.3716
Steatohepatitis refers to a spectrum of nonalcoholic fatty liver diseases (NAFLD) ranging from simple triglyceride deposition and accumulation with or without fibrosis to the development of cirrhosis, end-stage liver failure, and even hepatocellular carcinoma[1-6]. Nonalcoholic steatohepatitis (NASH), first described by Ludwig et al[7] at the Mayo Clinic in 1980, refers to hepatic lobular or portal inflammation and focal necrosis with fatty changes in patients without a history of alcohol abuse[4,8-12]. Steatohepatitis is associated with lipodystrophy, metabolic syndrome (dyslipidemia, insulin resistance, and diabetes mellitus), genetic susceptibility, environmental factors, and hepatocyte apoptosis associated with mitochondrial dysfunction and the production of reactive oxygen species, which can lead to hepatic fibrogenesis and inflammation[10,13-15].
Fatty liver disease refers to either the accumulation of fat in hepatocytes in excess of 5% of the total liver weight or the fatty degeneration of more than one-third of the total number of cells in the liver[16]. In the general population, the prevalence of NAFLD is 6%-40% among asymptomatic patients, and the incidence of fatty liver disease is 60%-75% in obese patients and 84%-96% in morbidly obese patients who undergo bariatric surgery, with 25%-55% of these patients also having NASH[1,3-5,9,17].
Glucose intolerance in response to insulin resistance induces an elevation in blood levels of glucose and insulin and results in increased synthesis of hepatic free fatty acids[5]. The oxidation of free fatty acids can lead to the production of reactive oxygen free radicals that can be cytotoxic to DNA, mitochondria, and other cellular structures and can lead to the production of pro-inflammatory cytokines. Steatohepatitis reportedly develops after a “first hit” involving triglyceride accumulation and a “second hit” involving oxidative stress, lipid peroxidation, pro-inflammatory cytokines, and mitochondrial dysfunction[4,18].
Previous studies in humans or murine models have identified independent risk factors for hepatic fibrosis, including advanced age, obesity, hypertension, Type IIdiabetes, insulin resistance, dyslipidemia, an aspartate transaminase (AST)/alanine transaminase (ALT) ratio greater than 1, hyperinsulinemia, altered lipid homeostasis, and pancreatic steatosis[4,7-9,17-20]. Additional risk factors that might contribute to disease progression include increased transferrin saturation, long-term total parenteral nutrition leading to choline deficiency, jejunoileal bypass surgery for morbid obesity, environmental toxins, and drugs such as chemotherapeutic agents or glucocorticoids[3,5,18].
Ductal adenocarcinoma of the pancreatic head is the most predominant tumor in the pancreas, and pancreaticoduodenectomy is the treatment of choice[11]. Pancreaticoduodenectomy is also used to treat various other malignancies of the periampullary region, the bile duct, and the duodenum or the borderline diseases of the pancreas[21]. Side-effects associated with pancreaticoduodenectomy include weight loss, abdominal pain, fatigue, and exocrine and endocrine insufficiencies. Pancreaticoduodenectomy also has a high rate of morbidity and mortality, including the postoperative development of steatohepatitis. Only a few reports have explored the relationship between pancreaticoduodenectomy and the development of steatohepatitis[22]. Therefore, we aimed to identify the risk factors for steatohepatitis after pancreaticoduodenectomy, with a particular focus on factors related to pancreatic secretions.
All study procedures were approved by the Institutional Review Board (No. 2010-09-082, Samsung Medical Center). The study included 228 patients who had pylorus-preserving pancreaticoduodenectomy (PPPD), Whipple’s procedure, or hepato-pancreato-duodenectomy (HPD) between January 2009 and April 2010. Electronic medical records and data were retrospectively reviewed. Exclusion criteria were: (1) patients without non-enhanced computed tomography (CT) findings (n = 12); (2) a preoperative diagnosis of fatty liver disease by non-enhanced CT (n = 19); and (3) mortalities resulting from postoperative pseudoaneurysm (n = 4). Thirty-five patients who consumed more than 150 g of alcohol per week were not excluded from the study because they did not have a diagnosis of preoperative fatty liver disease by non-enhanced CT. The final study group therefore consisted of 193 patients that were divided into two groups: Group A consisted of 15 patients who developed postoperative steatohepatitis, and Group B consisted of 178 patients who did not develop postoperative steatohepatitis.
Data were collected on patient demographics, operative procedures, pathologies, and perioperative clinical variables, including levels of insulin, C-peptide, and glucagon, and results from an oral glucose tolerance test conducted preoperatively. Data were also collected on postoperative liver function and the postoperative attenuation ratios for the liver and spleen.
Data on pancreatic enzyme levels in serum on postoperative day 7, pancreatic duct size, pancreatic fistula, pancreatic duct stenting, and type of stenting were collected and considered as potential parameters associated with pancreatic secretions. Pancreatic fistula was diagnosed according to the International study group pancreatic fistula (ISGPF) definition[23]. External pancreatic duct stenting was usually placed during the first postoperative month. Post-discharge pancreatic enzyme supplementation was administered routinely to all patients who had a pancreaticoduodenectomy.
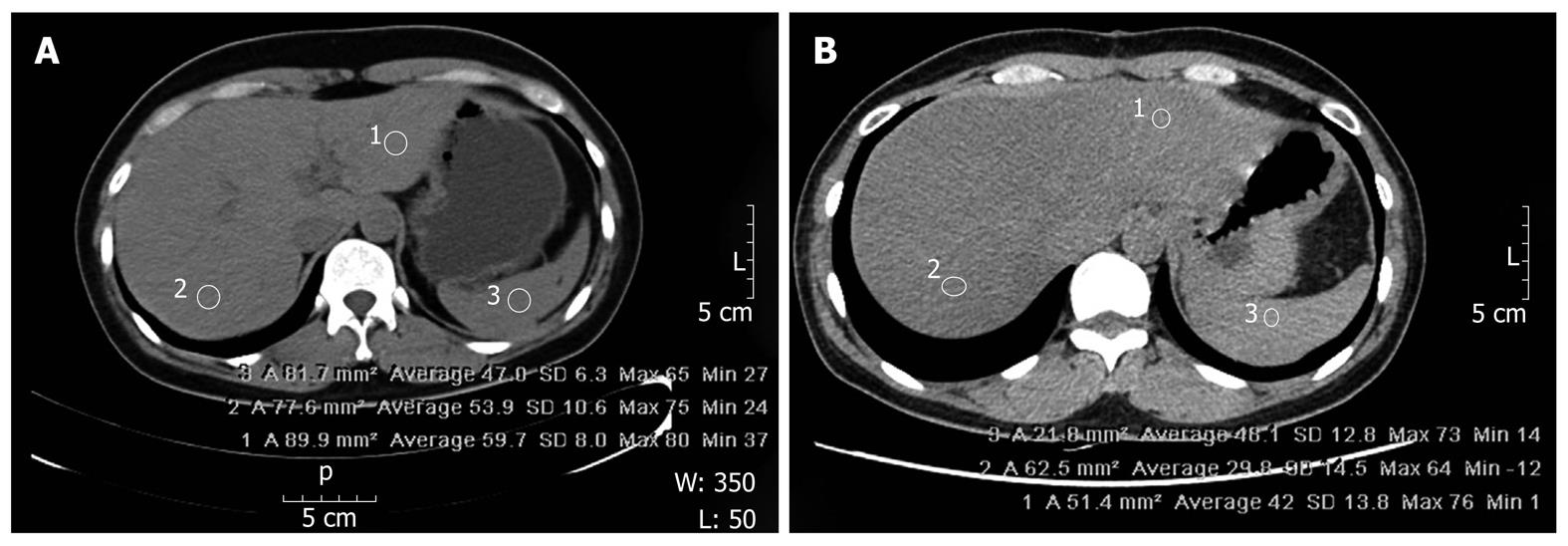
Fatty liver disease was defined according to the difference between the splenic and hepatic attenuation ratios (CTS-L) and the liver-to-spleen attenuation ratio (CTL/S). To minimize sampling error, we used two CT images from the liver, one from the right lobe and one from the left lobe, and we excluded images from the periphery of the liver. Perioperative steatohepatitis was presumed when CTS-L was equal to or greater than 10 Housefield Units (HU) or when CTL/S was equal to or less than 0.9 HU (Figure 1).
CT images were obtained with a 64-channel, 4-multi-detector, CT scanner (General Electric®, NY, United States). The parameters for non-enhanced CT were: 100-300 mAs; rotation speed of 0.6 s; table speed of 3 mm; noise index of 11.57; detector coverage of 40 mm; pitch-to-speed ratio (mm/rot) of 0.984:1; and helical thickness of 5 mm. CT images were reviewed on a Picture Archiving Communication System workstation (General Electric®).
For continuous variables, the paired-sample Student’s t test was used to compare the two patient groups. For categorical variables, Chi Square analysis or Fisher’s exact test was used to compare the two groups. Two-way Analysis of Variance was used to analyze group differences in repeated measures of the levels of glucose, insulin, C-peptide, and glucagon. Pearson’s correlation coefficient test was used to determine the correlation between the postoperative difference of CTS-L and the postoperative liver function test results. Multivariate analysis of risk factors was conducted using multivariate Cox proportional hazards modeling. Statistical analyses were performed using SPSS, version 16.0 (SPSS Inc., Chicago, IL, United States), and P values < 0.05 were considered statistically significant.
The mean period between the operation and the patient’s postoperative follow-up appointment was 3.2 ± 2.0 mo (range: 1-11). For each group, the average period was 2.4 mo in Group A and 3.3 mo in Group B (P = 0.106). Fifteen patients (7.8%) who showed postoperative hepatic fatty changes were included in Group A, and the remaining 178 patients (92.2%) were included in Group B (Table 1). Seventy-eight patients (40.4%) had preoperative biliary drainage, including percutaneous transhepatic biliary drainage, endoscopic retrograde biliary drainage, endoscopic nasobiliary drainage, and biliary stenting. None of the patients had undergone bariatric surgery (data not shown).
| Group A(n = 15) | Group B (n = 178) | P | |
| Gender (M:F), n | 7:8 | 112:66 | 0.270 |
| Age in years, mean (range) | 58.7 (40-74) | 61.8 (15-81) | 0.295 |
| BMI (kg/m2) ± SD | 20.6 ± 2.9 | 22.0 ± 3.3 | 0.082 |
| Hepatitis B viral infection (+), n (%) | 0 (0) | 6 (3.4) | 1.000 |
| Type II diabetes, n (%) | 2 (13.3) | 34 (19.1) | 0.582 |
| Alcohol consumption > 150 g/wk, n (%) | 3 (20.0) | 32 (18.0) | 0.738 |
| Biliary drainage, n (%) | 4 (26.7) | 74 (41.6) | 0.245 |
| Albumin/globulin ratio (range) | 1.79 (1.2-4.1) | 1.53 (0.8-2.4) | 0.234 |
| Total cholesterol (mg/dL), mean (range) | 209.3 (136-309) | 200.6 (73-470) | 0.641 |
| Serum amylase (U/L), mean (range) | 91.3 (22-263) | 129.8 (15-1361) | 0.388 |
| Serum lipase (U/L), mean (range) | 170.4 (24-707) | 336.7 (7-13562) | 0.593 |
| AST (U/L), mean (range) | 134.3 (11-547) | 111.7 (12-1230) | 0.621 |
| ALT (U/L), mean (range) | 156.1 (11-551) | 144.0 (9-1371) | 0.826 |
| ALP (U/L), mean (range) | 315.9 (64-913) | 267.3 (34-2236) | 0.518 |
| INR, mean (range) | 0.99 (0.85-1.11) | 1.07 (0.81-8.78) | 0.585 |
| Total bilirubin (mg/dL), mean (range) | 5.7 (0.3-18.8) | 6.2 (0.2-44.3) | 0.799 |
| Fasting glucose (mg/dL), mean (range) | 140 (93-150) | 135 (47-458) | 0.735 |
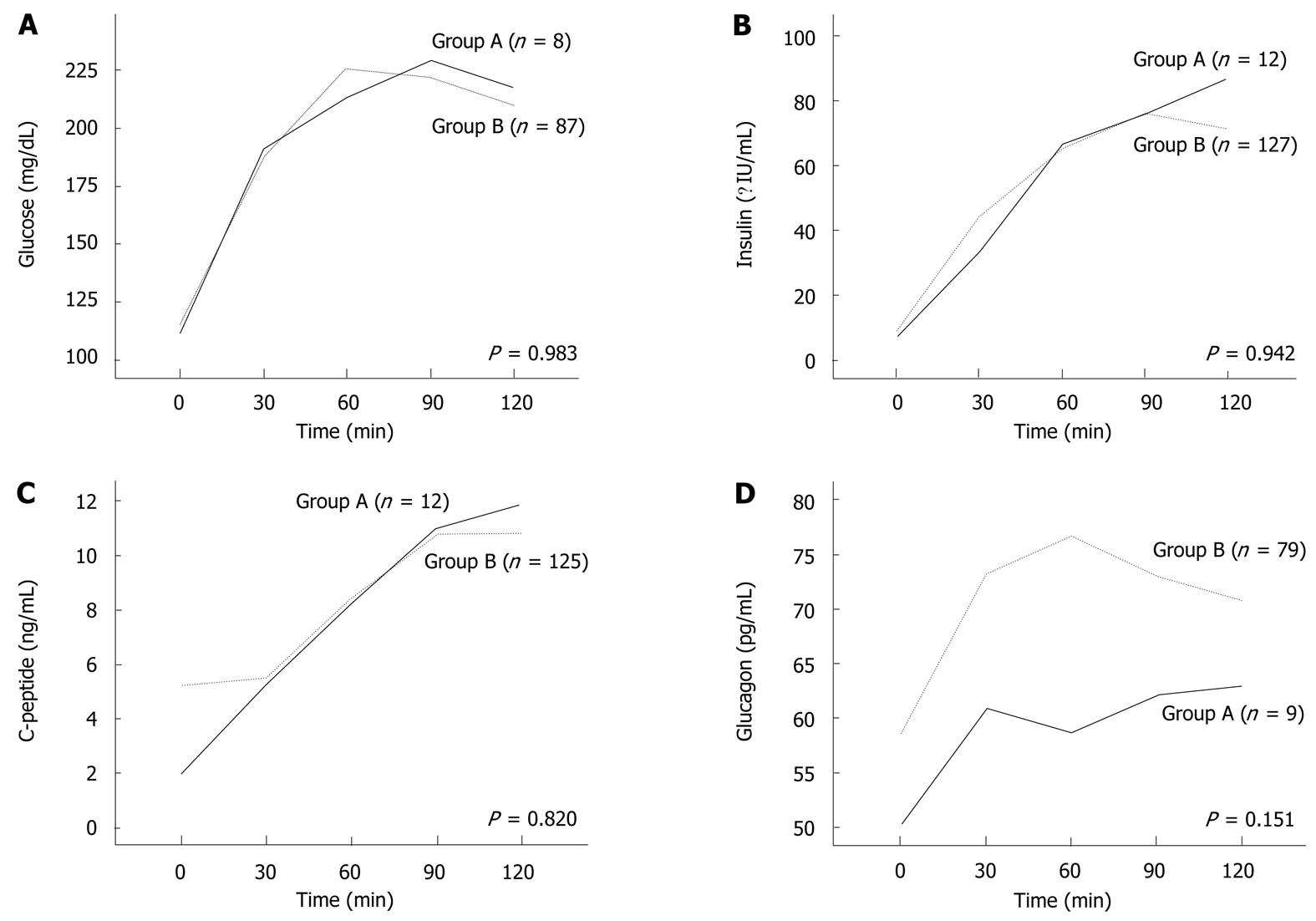
Preoperative patient characteristics and laboratory findings, including liver function test results and levels of pancreatic enzymes, were similar in the two groups (Table 1). PPPD or Whipple’s procedure was performed on 15 patients (100%) in Group A and 173 patients (97.2%) in Group B (P = 0.842) (Table 2). HPD was performed on 5 patients (2.8%) in Group B. Based on final pathologic reports, all patients (100%) in Group A were diagnosed with malignant disease, whereas patients in Group B were diagnosed with a variety of diseases (Table 2). Preoperative oral glucose tolerance test results, continuous stimulation test results for insulin, C-peptide and glucagon levels were not significantly different between the two groups (Figure 2). For patients with malignant disease, the two groups were similar in terms of cancer stage (P = 0.190), perineural invasion (P = 0.259), and vessel invasion (P = 1.000). The liver function test for all patients showed that postoperative CTL/S values correlated with postoperative ALT levels (γ = -0.149, P = 0.039) but not with postoperative AST or ALP levels (Table 3).
| Group A(n = 15) | Group B(n = 178) | P | |
| PPPD | 12 | 126 | 0.482 |
| Whipple’s procedure | 3 | 47 | |
| HPD | 0 | 5 | |
| Pancreatic cancer | 7 | 50 | 0.665 |
| Common bile duct cancer | 3 | 45 | |
| Ampulla of vater cancer | 5 | 27 | |
| Duodenal cancer | 0 | 11 | |
| IPMN of pancreas | 0 | 23 | |
| NET of pancreas or duodenum | 0 | 3 | |
| MCN or SPT of pancreas | 0 | 5 | |
| Duodenal GIST | 0 | 2 | |
| Hilar cholangiocarcinoma | 0 | 4 | |
| Colon cancer with duodenal invasion | 0 | 4 | |
| Gallbladder cancer with duodenal invasion | 0 | 1 | |
| Pancreatitis | 0 | 3 |
| Postoperative liver function test result | γ | P |
| AST | -0.138 | 0.056 |
| ALT | -0.149 | 0.039 |
| ALP | -0.023 | 0.755 |
Serum levels of pancreatic enzymes on postoperative day 7, pancreatic duct size, and the proportion of patients that received a pancreatic duct stent were similar in the two groups (Table 4); however, the proportion of patients that developed a pancreatic fistula postoperatively (P = 0.020) or had an external pancreatic duct stent placed (P = 0.005) was higher in Group A than in Group B (Table 4). A multivariate analysis of risk factors identified the postoperative development of a pancreatic fistula (HR = 3.332, P = 0.037) and the postoperative placement of an external pancreatic duct stent (HR = 4.530, P = 0.017) as independent risk factors for postoperative steatohepatitis (Table 5). Among the 41 patients who showed postoperative pancreatic fistula in the two groups, 32 and 9 patients showed grade A and B fistulas guided by ISGPF definition, respectively. Grade A fistula was observed in 6 patients (85.7%) in group A, and in 26 patients (76.5%) in group B (P = 1.000).
| Group A (n = 15) | Group B (n = 178) | P | |
| Serum amylase1, mean (range) | 44.5 (9.0-119.0) | 51.4 (6.0-266.0) | 0.558 |
| Serum lipase1, mean (range) | 31.9 (6.0-266.0) | 30.0 (1.0-215.0) | 0.793 |
| Pancreatic duct size, mean (range) (mm) | 4.5 (2-18) | 3.5 (1-11) | 0.401 |
| Pancreatic fistula2, n | 7 | 34 | 0.020 |
| Placement of pancreatic duct stent, n | 13 | 131 | 0.363 |
| Internal, n | 4 | 95 | 0.005 |
| External, n | 9 | 36 |
| Variables | HR | 95% confidence interval | P |
| Serum amylase2 | 0.990 | 0.973-1.007 | 0.262 |
| Serum lipase2 | 1.014 | 0.986-1.041 | 0.332 |
| Pancreatic duct size3 | 0.882 | 0.635-1.224 | 0.452 |
| Pancreatic fistula4 (-) | 1.000 | - | - |
| Pancreatic fistula4 (+) | 3.332 | 1.075-10.321 | 0.037 |
| Internal pancreatic duct stenting | 1.000 | - | - |
| External pancreatic duct stenting | 4.530 | 1.312-15.643 | 0.017 |
Duct-to-mucosal anastomosis and pancreatic duct stenting are used to prevent pancreatic leakage. In our institution, pancreatic stenting is usually performed when the diameter of the pancreatic duct is 2-5 mm, although there is some variability according to the attending surgeon’s preferences. The methods chosen for pancreatic duct stenting in association with steatohepatitis involve several considerations. The remnant pancreas secretes several enzymes that are associated with digestion, including enterokinase, trypsinogen, chymotrypsin, amylase, lipase, cellulose, phospholipase, and esterase[24-26]. External pancreatic duct stenting induces an earlier impairment in the secretion of pancreatic enzymes into the bowel lumen. Phospholipases A1 and A2 cleave fatty acids from phospholipids, and esterase hydrolyzes cholesterol esters. We hypothesize that hepatic fibrosis can be prevented by inhibiting the entry of free fatty acids into hepatocytes. Furthermore, lipid absorption by bile acid denaturation, which can lead to fat accumulation in hepatocytes, might be prevented by impairing bicarbonate secretion within the acidic gastric environment.
Adipokines such as leptin, resistin, adiponectin, and tumor necrosis factor (TNF)-α are known to regulate hepatic and peripheral glucose levels and lipid metabolism[27]. Decreased serum levels of adiponectin and increased serum levels of leptin or TNF-α are associated with NASH. Also, the hydrolysis of starches and glycogen into disaccharides or trisaccharides by amylolytic pancreatic enzymes can be impaired by external pancreatic duct drainage. These endocrine abnormalities are accelerated by the loss of insulin and glucagon, and they can induce insulin insensitivity and abnormal glucose metabolism.
Reduced motilities of the stomach and duodenum after pancreaticoduodenectomy can lead to the development of diabetes (20%-40%) with hyperglycemia and delayed gastric emptying (15%-40%), and a reduction in the release of pancreas-stimulating hormones from the duodenum (100%)[28]. These effects can aggravate insulin resistance. Moreover, motility dysfunction can be induced by intestinal bacterial overgrowth as well as anatomic alterations that may result from anastomotic procedures. Wu et al[29] found that decreased small intestinal motility was associated with delayed intestinal transit followed by bacterial overgrowth (Escherichia coli) in a rat model of NASH. Furthermore, patients who have undergone a pancreaticoduodenectomy are at increased risk of developing an ascending infection through hepaticojejunostomy and jejunojejunostomy. The effectiveness of antibiotics for decreasing elevated liver enzymes in NASH needs to be further investigated, however.
A previous study[23] identified the duration of untreated jaundice, malignancy, small pancreatic duct size, and soft pancreatic texture as risk factors for pancreatic fistula. The relationship between pancreatic fistula and steatohepatitis is still questionable, but the use of long-term total parenteral nutrition or the development of a secondary infection or sepsis after a pancreatic fistula might influence hepatic function.
High levels of low density lipoprotein-cholesterol (LDL-C) and low levels of high density lipoprotein-cholesterol (HDL-C) are established risk factors for atherogenesis in patients with diabetic dyslipidemia[30]. Insulin resistance is a key factor in the development of metabolic syndrome involving dyslipidemia and Type II diabetes. Dyslipidemia is associated primarily with low levels of HDL-C, high levels of LDL-C, and hepatic overproduction of triglyceride-rich very low density lipoprotein-cholesterol (VLDL-C)[31]. In the present study, LDL-C levels tended to be higher in Group A than in Group B, but this apparent difference was not significant (data not shown); therefore, the relationship between LDL-C and steatohepatitis remains to be determined.
Bariatric surgery is often considered for patients who are morbidly obese. Roux-en-Y gastric bypass, gastroplasty, or adjustable gastric banding are commonly performed, and jejunoileal or ileoileal bypass surgeries are no longer preferred[4,5]. Biliopancreatic diversion that involves a small bowel bypass procedure to form a short common channel from the ileocecal valve can induce metabolic derangement and is associated with a high incidence of postoperative hepatic steatosis[3,32]. This is caused by a combination of malnutrition and malabsorption of vitamins, iron, ferritin, and calcium. Pancreaticoduodenectomy involving a Roux-en-Y jejunojejunal bypass appears to be associated with the same side-effects as small bowel bypass surgery. We also found that all patients with postoperative steatohepatitis had malignant pathological findings, but the pathogenesis remains uncertain.
Histological evaluation through liver biopsy remains the gold-standard method for distinguishing NASH from simple fatty liver disease and for estimating intrahepatic fat content, the extent of necroinflammatory lesions and fibrosis. However, liver biopsy is associated with sampling errors and the risk of bleeding, infection, and biliary leakage[1,6,8,20,33]. Kleiner et al[15] proposed a semi-quantitative scoring system (the NAFLD activity score) to assess the histological features of NAFLD and to discriminate between NASH and non-NASH fatty liver disease. Five features-steatosis, hepatocellular ballooning, lobular inflammation, fibrosis, and the absence of lipogranulomas-were independently associated with the accurate diagnosis of NASH using adult liver biopsies.
Ultrasonography, non-enhanced CT, magnetic resonance imaging, and proton magnetic resonance spectroscopy (1H MRS) are radiological, non-invasive methods to diagnose hepatic steatosis[1,4,16], but these methods cannot accurately distinguish NASH from simple fatty liver disease or objectively quantify fat content[1,6]. Recently, fatty infiltration of the liver was detected using chemical-shift imaging and a selective fat-suppression technique, acquired by the percentage of relative signal intensity loss on magnetic resonance T1- or T2-weighted images, the ratio of peak lipid to water by 1H MRS, dual-energy multi-slice spiral CT, and non-enhanced CT measuring tissue density as a radiographic attenuation that can be objectively measured in Housefield units[16,34]. In a study by Nomura et al[22], non-enhanced CT was found to be useful for diagnosing steatohepatitis with established accuracy and for evaluating CTL/S and CTS-L. Other studies identified a correlation between CTL/S and CTS-L and histological findings of steatohepatitis, and some reports defined steatohepatitis as CTL/S < 0.9 HU or CTS-L≥ 10 HU[16,35]. Unlike the study by Nomura et al[22], which identified a relationship between postoperative AST and CTS-L, our study found that CTS-L correlated significantly with postoperative ALT levels, not AST levels. Kato et al[36] proposed a NAFLD scoring system that was based on the development of pancreatic adenocarcinoma, the pancreatic resection line, and postoperative diarrhea. This group diagnosed NASH by percutaneous liver biopsy after pancreaticoduodenectomy and revealed a significant correlation between their scoring system and CT findings.
The present study has several limitations that should be considered when interpreting the results. First, the study was retrospective, and the period between the operation and the postoperative follow-up CT was not uniform and averaged 3.2 mo, which is short. Nevertheless, if we consider the prevalent period for the development of steatohepatitis postoperatively, the results could present useful information.
Second, there were only 15 patients (7.8%) who developed steatohepatitis after surgery in our study, whereas in the study by Nomura et al[22] 33% of asymptomatic patients without severe obesity had decreased hepatic attenuation meeting the criteria for steatohepatitis after pancreaticoduodenectomy. The reason for the difference in the incidence between our study and that of Nomura et al is not clear, but it might reflect differences in the rates of obesity and the timing between the operation and the follow-up CT scan. Our study had a very small number of obese patients (24 patients with a BMI ≥ 25 kg/m2 and 2 patients with a BMI ≥ 30 kg/m2).
Third, we used a radiological method to diagnose steatohepatitis or NAFLD without histopathological evidence. Non-enhanced CT was reported to have a sensitivity of 73%-100% and a specificity of 95%-100% for the detection of moderate or severe steatohepatitis, although hepatic iron overload might alter these rates[1,37].
Finally, the location and the extent of the pancreatic resection and postoperative patient-related factors such as steroid use, weight loss, and exercise were not included in our statistical analyses. In addition, the effect of adjuvant chemoradiotherapy, which was administered to 7 patients (46.7%) in Group A and 80 patients (44.9%) in Group B, was not included in our analysis.
Future prospective controlled studies with a larger sample size based on histopathological findings are needed to verify the relationships identified in the present study. Postoperative steatohepatitis might not be a significant problem, especially in late-stage malignant patients. Nevertheless, this preliminary report provides evidence for operation-related causes of steatohepatitis following pancreaticoduodenectomy, ruling out other factors causing hepatic fatty change or injury.
Only a limited number of reports have examined operation-related causes of postoperative steatohepatitis following pancreaticoduodenectomy.
To identify the risk factors for steatohepatitis after pancreaticoduodenectomy, with a particular focus on factors related to pancreatic secretions.
This preliminary report helps to identify operation-related causes of steatohepatitis following pancreaticoduodenectomy, and it is the first study to identify potential risk factors related to pancreatic secretions.
In this study, pancreatic fistula and external pancreatic duct stenting significantly influenced the development of steatohepatitis following pancreaticoduodenectomy. These findings have clinical implications and could be used to design future clinical trials.
This is very interesting clinical research about the mechanism of post-operative steatohepatitis development following pancreaticoduodenectomy.
Peer reviewers: Shoichiro Sumi, MD, PhD, Associate Professor, Department of Organ Reconstruction, Institute for Frontier Medical Sciences, Kyoto University, Sakyo-ku, Kyoto, 606-8507, Japan; Deliang Fu, MD, PhD, Professor, Department of Surgery, Pancreatic Disease Institute, Fudan University, 12 Wulumqi Road (M), Shanghai 200040, China
S- Editor Tian L L- Editor Webster JR E- Editor Zhang DN
| 1. | Fabbrini E, Conte C, Magkos F. Methods for assessing intrahepatic fat content and steatosis. Curr Opin Clin Nutr Metab Care. 2009;12:474-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 41] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 2. | Comporti M, Signorini C, Leoncini S, Gardi C, Ciccoli L, Giardini A, Vecchio D, Arezzini B. Ethanol-induced oxidative stress: basic knowledge. Genes Nutr. 2010;5:101-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 128] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 3. | Angulo P. Treatment of nonalcoholic fatty liver disease. Ann Hepatol. 2002;1:12-19. [PubMed] |
| 4. | Tevar AD, Clarke C, Wang J, Rudich SM, Woodle ES, Lentsch AB, Edwards ML. Clinical review of nonalcoholic steatohepatitis in liver surgery and transplantation. J Am Coll Surg. 2010;210:515-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 30] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 5. | Patrick L. Nonalcoholic fatty liver disease: relationship to insulin sensitivity and oxidative stress. Treatment approaches using vitamin E, magnesium, and betaine. Altern Med Rev. 2002;7:276-291. [PubMed] |
| 6. | Mehta SR, Thomas EL, Bell JD, Johnston DG, Taylor-Robinson SD. Non-invasive means of measuring hepatic fat content. World J Gastroenterol. 2008;14:3476-3483. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 201] [Cited by in RCA: 196] [Article Influence: 11.5] [Reference Citation Analysis (2)] |
| 7. | Ludwig J, Viggiano TR, McGill DB, Oh BJ. Nonalcoholic steatohepatitis: Mayo Clinic experiences with a hitherto unnamed disease. Mayo Clin Proc. 1980;55:434-438. [PubMed] |
| 8. | Kohli R, Kirby M, Xanthakos SA, Softic S, Feldstein AE, Saxena V, Tang PH, Miles L, Miles MV, Balistreri WF. High-fructose, medium chain trans fat diet induces liver fibrosis and elevates plasma coenzyme Q9 in a novel murine model of obesity and nonalcoholic steatohepatitis. Hepatology. 2010;52:934-944. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 309] [Cited by in RCA: 282] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 9. | Clark JM. The epidemiology of nonalcoholic fatty liver disease in adults. J Clin Gastroenterol. 2006;40 Suppl 1:S5-S10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 234] [Reference Citation Analysis (0)] |
| 10. | Feldstein AE, Canbay A, Angulo P, Taniai M, Burgart LJ, Lindor KD, Gores GJ. Hepatocyte apoptosis and fas expression are prominent features of human nonalcoholic steatohepatitis. Gastroenterology. 2003;125:437-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 775] [Cited by in RCA: 796] [Article Influence: 36.2] [Reference Citation Analysis (0)] |
| 11. | Harrison SA, Kadakia S, Lang KA, Schenker S. Nonalcoholic steatohepatitis: what we know in the new millennium. Am J Gastroenterol. 2002;97:2714-2724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 42] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 12. | Conlon KC, Klimstra DS, Brennan MF. Long-term survival after curative resection for pancreatic ductal adenocarcinoma. Clinicopathologic analysis of 5-year survivors. Ann Surg. 1996;223:273-279. [PubMed] |
| 13. | Hall D, Poussin C, Velagapudi VR, Empsen C, Joffraud M, Beckmann JS, Geerts AE, Ravussin Y, Ibberson M, Oresic M. Peroxisomal and microsomal lipid pathways associated with resistance to hepatic steatosis and reduced pro-inflammatory state. J Biol Chem. 2010;285:31011-31023. [PubMed] |
| 14. | Lupsa BC, Sachdev V, Lungu AO, Rosing DR, Gorden P. Cardiomyopathy in congenital and acquired generalized lipodystrophy: a clinical assessment. Medicine (Baltimore). 2010;89:245-250. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 66] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 15. | Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, Ferrell LD, Liu YC, Torbenson MS, Unalp-Arida A. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313-1321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6807] [Cited by in RCA: 8212] [Article Influence: 410.6] [Reference Citation Analysis (5)] |
| 16. | Zhong L, Chen JJ, Chen J, Li L, Lin ZQ, Wang WJ, Xu JR. Nonalcoholic fatty liver disease: quantitative assessment of liver fat content by computed tomography, magnetic resonance imaging and proton magnetic resonance spectroscopy. J Dig Dis. 2009;10:315-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 34] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 17. | Park SH. Current status of liver disease in Korea: nonalcoholic fatty liver disease. Korean J Hepatol. 2009;15 Suppl 6:S34-S39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 18. | Siebler J, Galle PR. Treatment of nonalcoholic fatty liver disease. World J Gastroenterol. 2006;12:2161-2167. [PubMed] |
| 19. | van Geenen EJ, Smits MM, Schreuder TC, van der Peet DL, Bloemena E, Mulder CJ. Nonalcoholic fatty liver disease is related to nonalcoholic fatty pancreas disease. Pancreas. 2010;39:1185-1190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 137] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 20. | Angulo P, Keach JC, Batts KP, Lindor KD. Independent predictors of liver fibrosis in patients with nonalcoholic steatohepatitis. Hepatology. 1999;30:1356-1362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1099] [Cited by in RCA: 1090] [Article Influence: 41.9] [Reference Citation Analysis (1)] |
| 21. | Huang JJ, Yeo CJ, Sohn TA, Lillemoe KD, Sauter PK, Coleman J, Hruban RH, Cameron JL. Quality of life and outcomes after pancreaticoduodenectomy. Ann Surg. 2000;231:890-898. [PubMed] |
| 22. | Nomura R, Ishizaki Y, Suzuki K, Kawasaki S. Development of hepatic steatosis after pancreatoduodenectomy. AJR Am J Roentgenol. 2007;189:1484-1488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 50] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 23. | Liang TB, Bai XL, Zheng SS. Pancreatic fistula after pancreaticoduodenectomy: diagnosed according to International Study Group Pancreatic Fistula (ISGPF) definition. Pancreatology. 2007;7:325-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 37] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 24. | Roxas M. The role of enzyme supplementation in digestive disorders. Altern Med Rev. 2008;13:307-314. [PubMed] |
| 25. | Layer P, Holtmann G. Pancreatic enzymes in chronic pancreatitis. Int J Pancreatol. 1994;15:1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 16] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 26. | Tran TC, van Lanschot JJ, Bruno MJ, van Eijck CH. Functional changes after pancreatoduodenectomy: diagnosis and treatment. Pancreatology. 2009;9:729-737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 49] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 27. | Kawamoto S, Soyer PA, Fishman EK, Bluemke DA. Nonneoplastic liver disease: evaluation with CT and MR imaging. Radiographics. 1998;18:827-848. [PubMed] |
| 28. | Diepenhorst GM, van Ruler O, Besselink MG, van Santvoort HC, Wijnandts PR, Renooij W, Gouma DJ, Gooszen HG, Boermeester MA. Influence of prophylactic probiotics and selective decontamination on bacterial translocation in patients undergoing pancreatic surgery: a randomized controlled trial. Shock. 2011;35:9-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 29. | Wu WC, Zhao W, Li S. Small intestinal bacteria overgrowth decreases small intestinal motility in the NASH rats. World J Gastroenterol. 2008;14:313-317. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 53] [Cited by in RCA: 59] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 30. | Merkel M. [Diabetic dyslipoproteinemia: beyond LDL]. Dtsch Med Wochenschr. 2009;134:1067-1073. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 31. | Therond P. Catabolism of lipoproteins and metabolic syndrome. Curr Opin Clin Nutr Metab Care. 2009;12:366-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 32. | Doliba NM, Qin W, Vinogradov SA, Wilson DF, Matschinsky FM. Palmitic acid acutely inhibits acetylcholine- but not GLP-1-stimulated insulin secretion in mouse pancreatic islets. Am J Physiol Endocrinol Metab. 2010;299:E475-E485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 33. | Thampanitchawong P, Piratvisuth T. Liver biopsy: complications and risk factors. World J Gastroenterol. 1999;5:301-304. [PubMed] |
| 34. | Salama IA, Dessouky BA, Korayem EM, Aal SA. Impact of multislice spiral computed tomography on donor selection and surgical planning in living-related liver transplant. Exp Clin Transplant. 2010;8:111-124. [PubMed] |
| 35. | Park SH, Kim PN, Kim KW, Lee SW, Yoon SE, Park SW, Ha HK, Lee MG, Hwang S, Lee SG. Macrovesicular hepatic steatosis in living liver donors: use of CT for quantitative and qualitative assessment. Radiology. 2006;239:105-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 364] [Cited by in RCA: 406] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 36. | Kato H, Isaji S, Azumi Y, Kishiwada M, Hamada T, Mizuno S, Usui M, Sakurai H, Tabata M. Development of nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH) after pancreaticoduodenectomy: proposal of a postoperative NAFLD scoring system. J Hepatobiliary Pancreat Sci. 2010;17:296-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 86] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 37. | Han R. Plasma lipoproteins are important components of the immune system. Microbiol Immunol. 2010;54:246-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |