Published online Aug 28, 2011. doi: 10.3748/wjg.v17.i32.3691
Revised: March 1, 2011
Accepted: March 8, 2011
Published online: August 28, 2011
AIM: To investigate and elucidate the molecular mechanism that regulates inducible expression of CD69 by Helicobacter pylori (H. pylori) infection.
METHODS: The expression levels of CD69 in a T-cell line, Jurkat, primary human peripheral blood mononuclear cells (PBMCs), and CD4+ T cells, were assessed by immunohistochemistry, reverse transcription polymerase chain reaction, and flow cytometry. Activation of CD69 promoter was detected by reporter gene. Nuclear factor (NF)-κB activation in Jurkat cells infected with H. pylori was evaluated by electrophoretic mobility shift assay. The role of NF-κB signaling in H. pylori-induced CD69 expression was analyzed using inhibitors of NF-κB and dominant-negative mutants. The isogenic mutants with disrupted cag pathogenicity island (cagPAI) and virD4 were used to elucidate the role of cagPAI-encoding type IV secretion system and CagA in CD69 expression.
RESULTS: CD69 staining was detected in mucosal lymphocytes and macrophages in specimens of patients with H. pylori-positive gastritis. Although cagPAI-positive H. pylori and an isogenic mutant of virD4 induced CD69 expression, an isogenic mutant of cagPAI failed to induce this in Jurkat cells. H. pylori also induced CD69 expression in PBMCs and CD4+ T cells. The activation of the CD69 promoter by H. pylori was mediated through NF-κB. Transfection of dominant-negative mutants of IκBs, IκB kinases, and NF-κB-inducing kinase inhibited H. pylori-induced CD69 activation. Inhibitors of NF-κB suppressed H. pylori-induced CD69 mRNA expression.
CONCLUSION: The results suggest that H. pylori induces CD69 expression through the activation of NF-κB. cagPAI might be relevant in the induction of CD69 expression in T cells. CD69 in T cells may play a role in H. pylori-induced gastritis.
-
Citation: Mori N, Ishikawa C, Senba M. Induction of CD69 expression by
cag PAI-positiveHelicobacter pylori infection. World J Gastroenterol 2011; 17(32): 3691-3699 - URL: https://www.wjgnet.com/1007-9327/full/v17/i32/3691.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i32.3691
The leukocyte receptor CD69 is a C-type lectin, disulfide-linked homodimer, type II protein that can be induced after activation[1,2]. In healthy subjects, CD69 is not detected in peripheral blood lymphocytes, but is expressed on small subsets of T and B cells in peripheral lymphoid tissues[3]. In addition, CD69 is selectively expressed in chronic inflammatory infiltrates at the sites of active immune responses in vivo[4,5]. However, the biological significance of CD69-induced cell activation is poorly understood.
Helicobacter pylori (H. pylori) is a Gram-negative bacterium that colonizes the human stomach, as well as areas of gastric metaplasia in the duodenal bulb[6]. The precise role of H. pylori in gastric pathology, especially the mechanism responsible for the transition of chronic active gastritis to gastric carcinoma, has been studied by many researchers. The infection triggers a local cellular immune response resulting in chronic cellular infiltration with or without an active component of neutrophils, as well as the development of lymphoid follicles in the lamina propria[7]. Although the exact mechanisms of the induction of various diseases by H. pylori infection have not been elucidated, one factor strongly associated with H. pylori virulence and the development of peptic ulcers and gastric cancer is the cag pathogenicity island (PAI), which constitutes a gene cluster encoding a type IV secretion system (T4SS)[8].
Enarsson et al[9] examined the transendothelial migration of human lymphocytes in response to H. pylori with the use of the Transwell system, employing a monolayer of human umbilical vein endothelial cells. H. pylori induced a significant T-cell migration and the presence of the H. pylori cagPAI increased T-cell transendothelial migration. Overexpression of CD69 was noted on migrating T cells[9]. These results suggest that H. pylori infection induces the expression of CD69 on T cells.
The present study was designed to test the hypothesis that H. pylori can induce both the surface expression of CD69 antigen and the promoter activity of the CD69 gene in human T cells, and to investigate whether such induction involves the cagPAI-coding T4SS and the nuclear factor (NF)-κB pathway. The presence of NF-κB motifs within the proximal promoter region of the CD69 gene may account for the H. pylori-inducible promoter activity.
N-acetyl-L-leucyl-L-leucyl-L-norleucinal (LLnL) and Bay 11-7082 were purchased from Sigma-Aldrich (St Louis, MO) and Calbiochem (La Jolla, CA), respectively. H. pylori ATCC 49503 (American Type Culture Collection, Rockville, MD) was used in most experiments described in this study. An isogenic H. pylori mutant lacking the cagPAI[9] or virD4 also was employed, together with the parental wild-type strain (26695). H. pylori strains were plated on blood agar plates and incubated at 37 °C for 2 d under microaerophilic conditions. Using inoculating needles, bacteria harvested from the plates were suspended in 50 mL of brucella broth containing 5% fetal bovine serum (FBS) and then cultured in a liquid medium at 37 °C for 1 d in a controlled microaerophilic environment. Bacteria were harvested from the broth culture by centrifugation and then resuspended at the concentrations indicated below in antibiotic-free medium. All procedures were performed with the approval of the appropriate institutional biosafety review committee and in compliance with the guidelines for biohazards.
The human T-cell line, Jurkat, was maintained in RPMI 1640 medium containing 10% FBS, 100 U/mL penicillin G, and 100 μg/mL streptomycin. Human peripheral blood mononuclear cells (PBMCs) were isolated from the peripheral blood of a healthy donor using Ficoll-Hypaque gradients. PBMCs then were further purified using positive selection with immunomagnetic beads specific for CD4 (Miltenyi Biotec, Auburn, CA). On the day of the experiment, cells were refed with fresh antibiotic-free medium and cocultured with H. pylori for the time intervals indicated below.
Stomach biopsy specimens from ten patients with H. pylori gastritis were examined histopathologically for CD69. The presence of H. pylori infection was confirmed by culture, serological analysis (with anti-H. pylori immunoglobulin G antibody), rapid urease test, and histological examination with Giemsa staining. Patients with H. pylori gastritis showed polymorphonuclear neutrophil infiltration in the gastric epithelium in conjunction with the presence of bacterial forms, which is consistent with H. pylori infection. All samples were collected after obtaining informed consent from each patient.
Total RNA was extracted with Trizol (Invitrogen, Carlsbad, CA) according to the protocol provided by the manufacturer. First-strand complementary DNA was synthesized from 1 μg total cellular RNA using a RNA-polymerase chain reaction (PCR) kit (Takara Bio, Otsu, Japan) with random primers. The specific primers used were as follows: for CD69, 5'-CATAGCTCTCATTGCCTTATCAGT-3'(forward primer) and 5'-CCTCTCTACCTGCGTATCGTTT-3'(reverse primer); for β-actin, 5'-GTGGGGCGCCCCAGGCACCA-3'(forward primer) and 5'-CTCCTTAATGTCACGCACGATTTC-3'(reverse primer). Thereafter, cDNA was amplified using 30 and 28 cycles for CD69 and β-actin, respectively. The product sizes were 254 bp for CD69 and 548 bp for β-actin. The PCR products were fractionated on 2% agarose gels and visualized by ethidium bromide staining.
The IκBαΔN- and IκBβΔN-dominant-negative mutants are IκBα and IκBβ deletion mutants lacking the N-terminal 36 and 23 amino acids, respectively[10,11]. The dominant-negative mutants of IκB kinase (IKK)α, IKKα (K44M), IKKβ, IKKβ (K44A), IKKγ, IKKγ(1-305), and NF-κB-inducing kinase (NIK), NIK (KK429/430AA) have been described previously[12,13]. The CD69 promoter pXP2 luciferase reporter plasmid containing the wild-type sequence (position -255 to position +16), pAIM255-LUC, was described previously[14]. The internal deletion mutants of the NF-κB sites were constructed by deletion of the NF-κB sites of pAIM255-LUC. Jurkat cells were transfected with the appropriate reporter and effector plasmids by electroporation. After 24 h, H. pylori was added and incubated for 6 h. The cells were washed in phosphate buffered saline and lysed in reporter lysis buffer (Promega, Madison, WI). Lysates were assayed for reporter gene activity with the dual-luciferase assay system (Promega). Luciferase activities were normalized relative to the Renilla luciferase activity from phRL-TK.
Nuclear proteins were extracted and transcription factors bound to specific DNA sequences were examined by electrophoretic mobility shift assay (EMSA) as described previously[15]. The top strand sequence of the oligonucleotide probes or competitors are as follows: for the NF-κB element (κB1) of the CD69 gene, 5'-GATCCAGACAACAGGGAAAACCCATACTTC-3'; for the NF-κB element (κB2) of the CD69 gene, 5'-GATCCAGAGTCTGGGAAAATCCCACTTTCC-3'; for the NF-κB element of the interleukin-2 receptor α chain (IL-2Rα) gene, 5'-GATCCGGCAGGGGAATCTCCCTCTC-3'; and for the AP-1 element of the IL-8 gene, 5'-GATCGTGATGACTCAGGTT-3'. The oligonucleotide 5'-GATCTGTCGAATGCAAATCACTAGAA-3', containing the consensus sequence of the octamer binding motif, was used to identify specific binding of the transcription factor Oct-1. The above underlined sequences are the NF-κB, AP-1, and Oct-1 binding sites, respectively. To identify NF-κB proteins in the DNA-protein complex shown by EMSA, we used antibodies specific for various NF-κB family proteins, including p50, p65, c-Rel, p52, and RelB (Santa Cruz Biotechnology, Santa Cruz, CA).
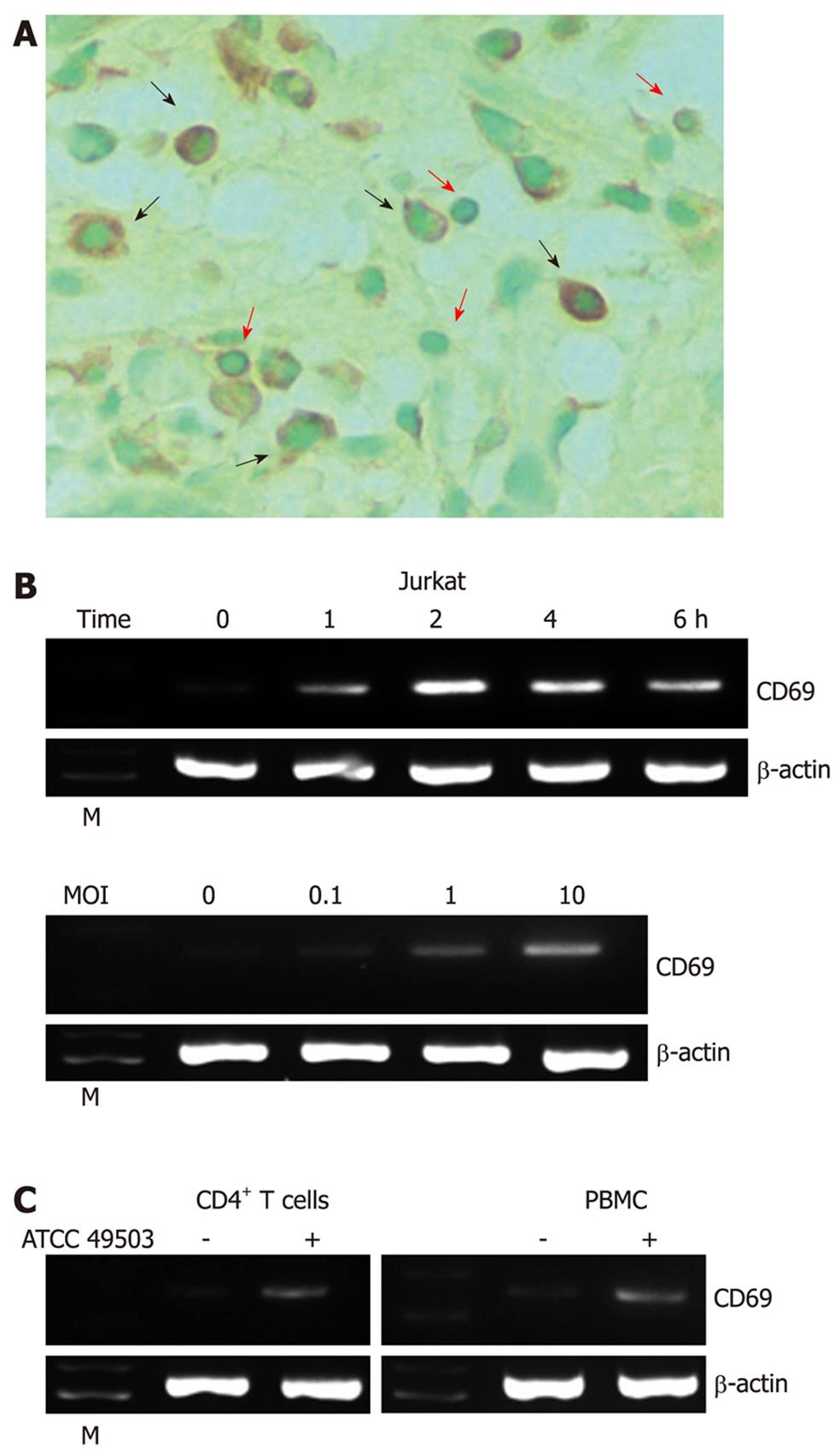
CD69 immunohistochemistry was performed using a mouse monoclonal antibody (clone FN50) to CD69 (BioLegend, San Diego, CA) after pretreatment of the deparaffinized tissue sections with ready-to-use proteinase K (Dako, Carpinteria, CA). The sections were counterstained with methyl green for 10 min, hydrated in ethanol, cleaned in xylene, and mounted. The stained cells were examined under a light microscope (Axioskop 2plus; Zeiss, Jena, Germany) with an Achroplan × 40/0.65 lens (Zeiss). Images were acquired with an AxioCam MRC camera and AxioVision 3.1 software (Zeiss). Gastric lymphocytes and macrophages were identified based on their morphological features.
Cells were washed with cell WASH (Becton Dickinson Immunocytometry Systems, San Jose, CA) and incubated for 30 min with phycoerythrin-labeled mouse monoclonal antibody against CD69 (clone TP1.55.3) or control mouse IgG2b, which were purchased from Beckman Coulter (Fullerton, CA). Cells were analyzed on an Epics XL flow cytometer.
We investigated the expression of CD69 by immunostaining in H. pylori-positive gastric tissues (n = 10). CD69 staining was detected in mucosal lymphocytes and macrophages (Figure 1A). In contrast, only a faint staining for CD69 was detected in the normal mucosa, and the expression level was much weaker than in H. pylori-positive gastric tissues (data not shown).
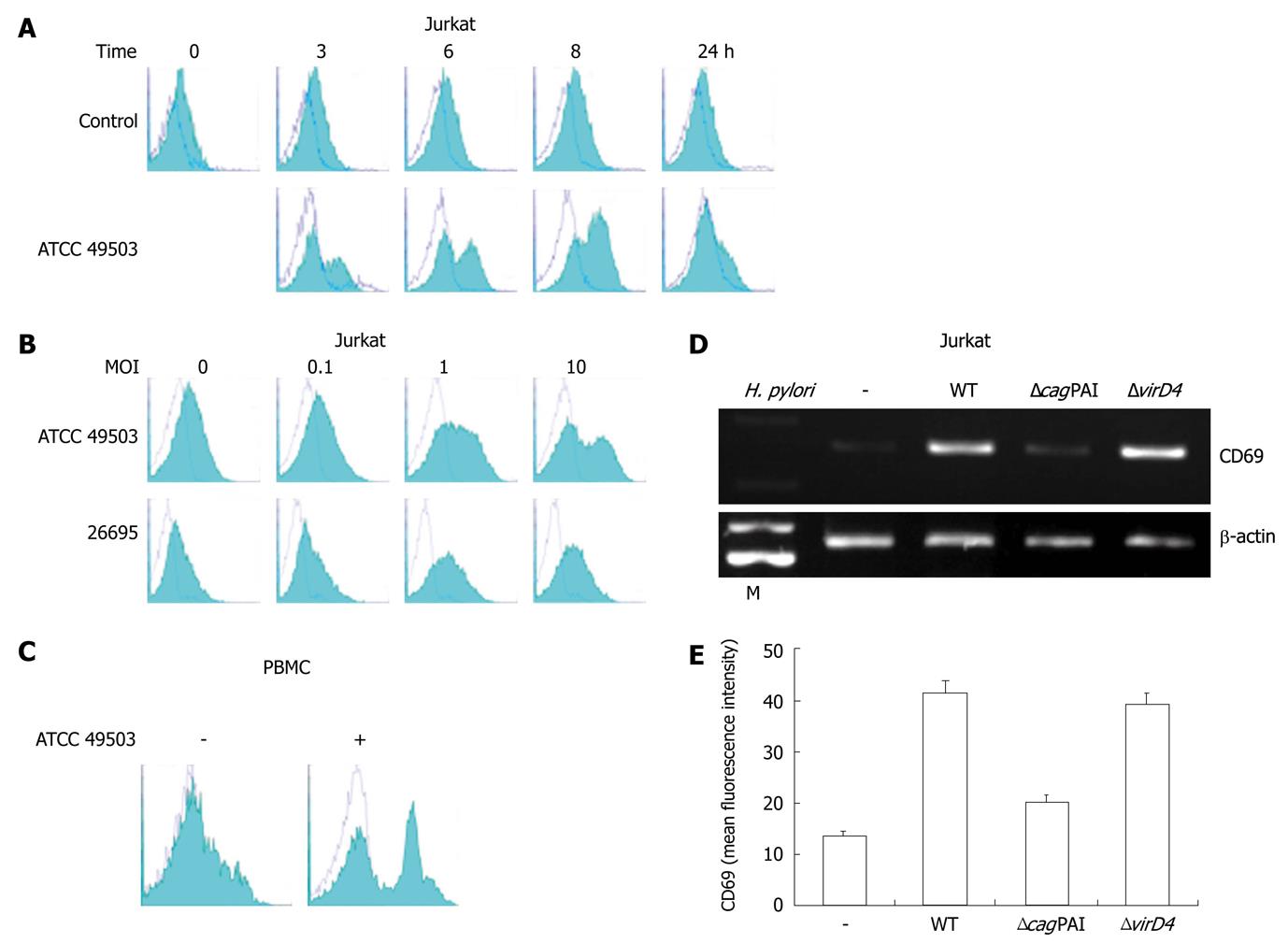
Using reverse transcription (RT)-PCR, we next examined the effect of coculture of Jurkat T cells (a transformed human T-cell line) with H. pylori ATCC 49503 on the induction of CD69 mRNA. Coculture with ATCC 49503 significantly enhanced the steady-state levels of CD69 mRNA in Jurkat cells. CD69 transcript levels clearly increased 1 h after the addition of ATCC 49503 to Jurkat cells (Figure 1B). In another series of experiments, in which Jurkat cells were infected with ATCC 49503 at different concentrations [i.e., the multiplicity of infection (MOI)] for 2 h (Figure 1B), H. pylori induced dose-dependent expression of CD69 mRNA. To characterize the effect of H. pylori infection on human T cells, we employed RT-PCR to examine CD69 mRNA expression in PBMCs and CD4+ T cells in response to ATCC 49503. After 2-h infection, H. pylori induced CD69 mRNA expression in PBMCs and CD4+ T cells, similar to the observation with Jurkat cells (Figure 1C).
To analyze whether the increase of mRNA synthesis results in elevated expression on the cell surface, direct immunofluorescent staining and flow cytometry were performed. Consistent with the RT-PCR analysis, the expression was upregulated in a dose-dependent manner (Figure 2B). The peak expression level of cell surface CD69 was noted at 8 h after infection (Figure 2A). H. pylori ATCC 49503 also enhanced cell surface CD69 expression on PBMCs (Figure 2C).
The cagPAI, a cluster of about 28 genes, is one of the best known virulence factors; it encodes a T4SS that transports CagA protein, peptidoglycan, and possibly other molecules into host epithelial cells[16]. The cagPAI also encodes a homologue of the coupling protein virD4, which in Agrobacterium tumefaciens and conjugation systems is thought to deliver the T4SS substrates to the secretion machinery[17]. In H. pylori, virD4 is necessary for CagA translocation but dispensable for the induction of IL-8[18,19]. Accordingly, we compared the abilities of the wild-type H. pylori strain 26695, an isogenic cagPAI mutant (ΔcagPAI), and a virD4 mutant (ΔvirD4), with regard to the induction of CD69 transcripts and expression of CD69 on the cell surface. Infection with wild-type strain 26695 induced CD69 mRNA expression in Jurkat cells, while the isogenic mutant that lacked cagPAI expression did not induce CD69 mRNA expression (Figure 2D). In contrast, the virD4 mutant induced CD69 mRNA expression in Jurkat cells (Figure 2D). These results were confirmed by the cell surface expression of CD69 analyzed by flow cytometry (Figures 2B and E).
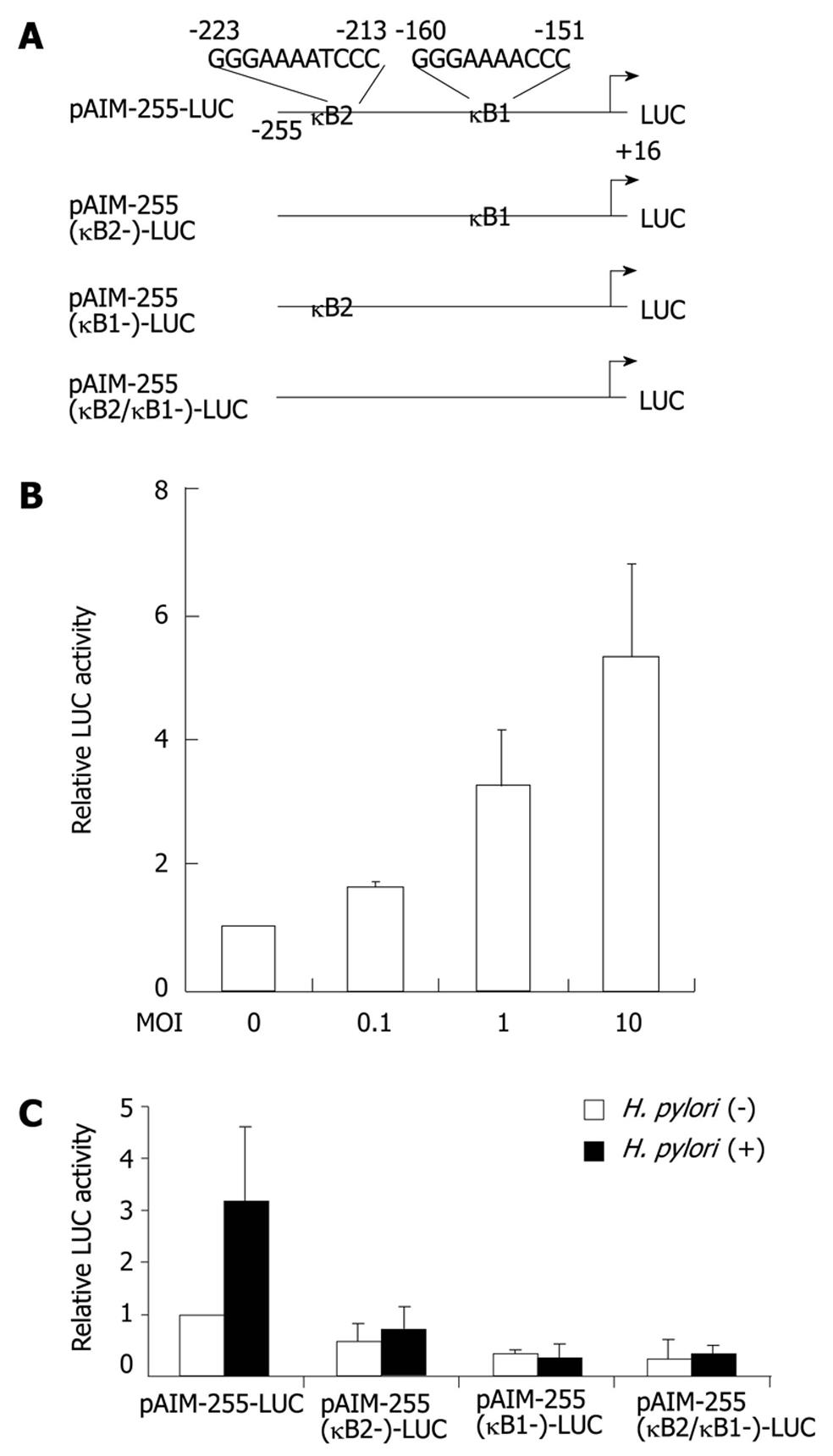
In the next series of experiments, we investigated whether the H. pylori-mediated upregulation of CD69 gene expression directly enhances the activity of its promoter. Jurkat cells were transiently transfected with a reporter gene construct containing a segment from position -255 to position +16 of the CD69 upstream regulatory sequences. Coculture of H. pylori strain ATCC 49503 resulted in a dose-dependent increase in the activity of this CD69-driven reporter construct (Figure 3B). The NF-κB signaling pathway is activated in epithelial cells infected with cagPAI-positive H. pylori but not in cells infected with cagPAI-negative strains of H. pylori[20-22]. Two potential NF-κB binding sequences were identified at positions -160 (κB1) and -223 (κB2) (Figure 3A). κB1 and κB2 were identical to those found in the gene promoters of c-myc and IL-6, respectively[23]. To test the relative contribution of the NF-κB binding sites to the H. pylori-mediated activation of CD69, plasmids with internal deletion mutants of these sites of the CD69 promoter were transfected (Figure 3C). After H. pylori infection, single deletion of the κB2 site resulted in marked reduction of the inducible activity. Single deletion of the κB1 site and the combination of double deletions abolished H. pylori-mediated activation of this reporter construct. These data clearly indicate that the two NF-κB binding sites in the CD69 promoter regulate CD69-enhanced expression after infection with H. pylori.
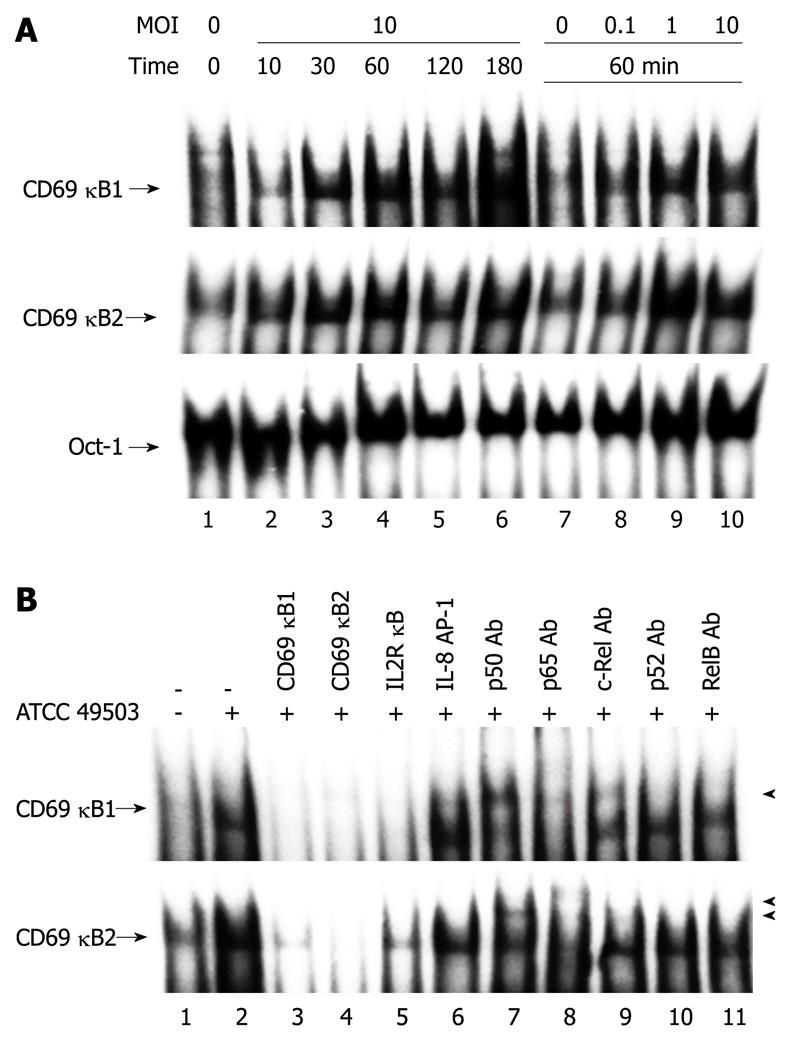
The data presented above indicate that H. pylori-induced CD69 expression is mediated by the κB1 and κB2 sites. To analyze whether these two putative NF-κB binding sites of the CD69 promoter could bind NF-κB family members, gel retardation assays were performed using as probes two double-stranded oligonucleotides (CD69 κB1 and CD69 κB2) containing these motifs. To characterize the NF-κB-related proteins that bind to the NF-κB sites of the CD69 promoter in CD69-expressing cells, the two oligonucleotide probes were incubated with nuclear extracts prepared from untreated Jurkat cells and from Jurkat cells infected with H. pylori. Jurkat cells were infected with H. pylori at different times after challenge, and nuclear protein extracts were prepared and analyzed to determine NF-κB DNA binding activity. As shown in Figure 4A, complexes were induced in these cells within 30 min after infection with H. pylori and were detected at 180 min after infection with both oligonucleotide probes. The amounts of these inducible DNA-protein complexes were H. pylori dose-dependent. In both probes, the addition of an excess of unlabeled κB1 and κB2 oligonucleotides to the binding reaction completely abolished the formation of the inducible DNA-protein complexes (Figure 4B, lanes 3 and 4). Similarly, an equal amount of the oligonucleotide IL-2R κB, which contained the NF-κB motif of the IL-2R α chain gene, efficiently competed with the specific complexes (Figure 4B, lane 5). In contrast, the formation of these DNA-protein complexes was not blocked by the addition of an excess of the unrelated oligonucleotide AP-1 (Figure 4B, lane 6).
To identify the NF-κB family members that bind to the NF-κB motifs of the CD69 gene promoter, the binding reactions were preincubated with antibodies specific to p50, p65, c-Rel, p52, and RelB (Figure 4B). The anti-p50 antibody induced the supershifted bands or reduced the intensity of complexes κB1 and κB2 (Figure 4B, lane 7). The anti-p65 antibody induced supershifted bands or blocked the formation of complexes κB1 and κB2 (Figure 4B, lane 8). The anti-c-Rel antibody induced the supershifted band and reduced the intensity of only complex κB1 (Figure 4B, lane 9). In contrast, the anti-p52 or anti- RelB antibody did not interfere with the formation of any of these complexes (Figure 4B, lanes 10 and 11). These results indicate that the complexes κB1 and κB2 correspond to p50/p65/c-Rel and p50/p65, respectively. These results suggest that H. pylori infection seems to induce CD69 gene expression at least in part through the induced binding of NF-κB family members to the NF-κB sites in the CD69 promoter region.
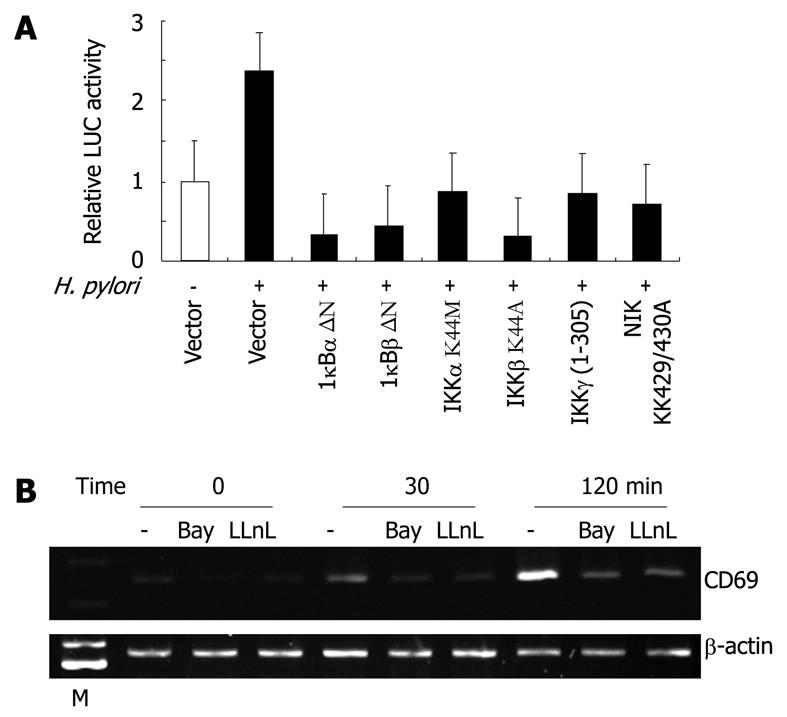
We also examined whether the H. pylori-mediated upregulation of CD69 gene expression involves signal transduction components in NF-κB activation. Activation of NF-κB requires the phosphorylation of two conserved serine residues of IκBα (Ser-32 and Ser-36) and IκBβ (Ser-19 and Ser-23) within the N-terminal domain[24]. Phosphorylation leads to the ubiquitination and 26S proteasome-mediated degradation of IκBs, thereby releasing NF-κB from the complex and its translocation to the nucleus and activation of various genes[24]. The IKK complex, which is composed of two catalytic subunits (IKKα and IKKβ) and a regulatory subunit (IKKγ), phosphorylates IκBs[24]. Previous studies indicated that members of the mitogen-activated protein kinase kinase kinase family mediate the physiologic activation of IKK[25]. These kinases include NIK[26]. IκBα-, IκBβ-, and IKKγ-dominant-interfering mutants and IKKα, IKKβ, and NIK kinase-deficient mutants were tested to determine their abilities to inhibit the H. pylori-mediated activation of the CD69-driven reporter gene. The expression of these various inhibitory mutants abolished H. pylori-induced CD69 expression (Figure 5A). These results emphasize the importance of signaling components involved in the activation of NF-κB in H. pylori-induced activation of the CD69 promoter.
Because activation of the CD69 promoter by H. pylori infection requires the activation of NF-κB, we blocked NF-κB activation with Bay 11-7082, an inhibitor of IκBα phosphorylation[27], or LLnL, a proteasome inhibitor[28]. The latter is known to inhibit the activation of NF-κB by blocking the degradation of the IκBα protein. Both Bay 11-7082 and LLnL markedly inhibited the H. pylori-induced expression of CD69 mRNA (Figure 5B).
Early studies showed that CD69 regulates the immune response by modulating the expression of various cytokines; CD69-deficient mice show increased anti-tumor and autoimmune responses caused at least in part by increased production of proinflammatory cytokines and chemokines[29,30]. Although the functions of CD69 have been studied extensively, there is little or no information on its role in the immune response against microbial pathogens. Recently, CD69 was reported to be a critical negative regulator of immune activation during intracellular bacterial infection, protecting infected mice against lethal tissue damage[31]. The present study explores the way in which H. pylori infection controls the expression of CD69 gene in T cells.
The main findings of the study were: (1) H. pylori deregulated the expression of CD69 in T cells; (2) CD69 protein was upregulated in gastric lymphocytes of patients with H. pylori gastritis; (3) the importance of H. pylori cagPAI in the induction of CD69 expression in T cells; and (4) H. pylori stimulates the NF-κB signaling pathway to activate CD69 gene expression and also to activate the CD69 promoter.
This is the first report to demonstrate that CD69 gene expression is regulated by H. pylori. Despite the development of immune responses against H. pylori infection, the bacteria are rarely eliminated, and colonization generally is persistent. Factors that contribute to the failure of the immune response to clear the organism remain elusive[31]. Recent studies have suggested that CD69 may downregulate the immune response through the production of the pleiotropic cytokine, transforming growth factor-β[32]. Thus, CD69 expressed on T cells may regulate the immune responses against H. pylori infection.
It has been reported that the inducible expression of CD69 gene is tightly regulated by transcription factors of the NF-κB, AP-1, EGR, and ATF/CREB families, which are rapidly activated through different signaling pathways[14,33]. However, nothing is known about the regulation of CD69 expression in T cells infected with H. pylori. We demonstrate herewith that cagPAI-positive H. pylori can induce the expression of the CD69 antigen and that this induction is mediated by an increase in the CD69 promoter activity. Deletion of the sequences that contain the κB1 and/or κB2 motifs abolished the response to H. pylori. Pharmacologic inhibition of NF-κB, as well as IκBα-, IκBβ-, IKKγ-dominant-interfering mutants and kinase-deficient IKKα, IKKβ, and NIK mutants, determined the role of NF-κB signaling molecules targeted by H. pylori to activate CD69 gene expression. Thus, our results suggest that NF-κB is essential for H. pylori cagPAI-mediated CD69 induction in T cells, and the two NF-κB sites (κB1 and κB2) appear to play an important role in this process.
Our results also demonstrated that the two NF-κB motifs of the CD69 promoter bind H. pylori-inducible NF-κB-related complexes. Antibodies directed against the different NF-κB proteins were used to identify the family members present in the DNA-protein complexes detected with the NF-κB motif-derived probes. These experiments demonstrated that the DNA-binding activities consisted of p50/p65/c-Rel and p50/p65 complexes binding to the κB1 and κB2 motifs, respectively. Although NF-κB clearly plays an important role in H. pylori-mediated induction of CD69, the role of CD69 in the control of immune responses against H. pylori infection needs to be further clarified. We are planning further studies using CD69-deficient mice to investigate the role of CD69 in the regulation of immune responses against H. pylori infection.
We thank Mr. Satoru Aida and Dr. Eriko Takeshima for their excellent technical assistance. We also thank Drs. Toshiya Hirayama, Chihiro Sasakawa, and Hitomi Mimuro for providing H. pylori strains. We acknowledge Drs. Francisco Sanchez-Madrid, Dean W. Ballard, Romas Geleziunas, and Kuan-Teh Jeang for providing CD69 promoter pXP2 luciferase reporter plasmid; expression vectors for IκBα and IκBβ-dominant-negative mutants; for NIK, IKKα, and IKKβ-dominant-negative mutants; and for IKKγ-dominant-negative mutant.
Helicobacter pylori (H. pylori) is regarded as the major cause of various gastric diseases. Despite the development of immune responses against H. pylori infection, the bacteria are rarely eliminated. Factors that contribute to the failure of the immune response to clear the organism remain elusive. Recently, the leukocyte receptor CD69 was reported to be a critical negative regulator of immune activation during bacterial infection.
CD69 is selectively expressed in chronic inflammatory infiltrates at the sites of active immune responses. However, whether and how H. pylori can induce the expression of CD69 has not been addressed in T cells. In this study, the authors demonstrate that H. pylori can induce CD69 expression through the activation of nuclear factor-κB and show that cag pathogenicity island (cagPAI) might be relevant in the induction of CD69 expression in T cells.
CD69 staining was detected in mucosal lymphocytes and macrophages in specimens of patients with H. pylori-positive gastritis. H. pylori also induced CD69 expression in peripheral blood mononuclear cells and CD4+ T cells in vitro. This is the first study to report the regulation of intracellular events leading to CD69 expression by H. pylori infection in T cells. The results also demonstrate that the two nuclear factor-κB motifs of the CD69 promoter are important in H. pylori-mediated induction of CD69.
By understanding how CD69 is induced and the role of CD69 in the control of immune responses against H. pylori infection, and by blocking its expression, this study may indicate a future strategy for elimination of H. pylori.
CD69 is a C-type lectin, disulfide-linked homodimer, type II protein that can be induced after lymphocyte activation. The cagPAI is responsible for the secretion of the CagA effector through a type IV secretion system apparatus as well as transport of peptidoglycan.
This article by Mori et al described induction of CD69 expression by cagPAI-positive H. pylori infection. According to my literature review, this might be the first study reporting to demonstrate that CD69 gene expression is regulated by H. pylori. This manuscript is scientific and well-written.
Peer reviewer: Jae J Kim, MD, PhD, Associate Professor, Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, 50, Irwon-dong, Gangnam-gu, Seoul 135-710, South Korea
S- Editor Tian L L- Editor Logan S E- Editor Li JY
| 1. | Cebrián M, Yagüe E, Rincón M, López-Botet M, de Landázuri MO, Sánchez-Madrid F. Triggering of T cell proliferation through AIM, an activation inducer molecule expressed on activated human lymphocytes. J Exp Med. 1988;168:1621-1637. [PubMed] |
| 2. | Sánchez-Mateos P, Sánchez-Madrid F. Structure-function relationship and immunochemical mapping of external and intracellular antigenic sites on the lymphocyte activation inducer molecule, AIM/CD69. Eur J Immunol. 1991;21:2317-2325. [PubMed] |
| 3. | Sánchez-Mateos P, Cebrián M, Acevedo A, López-Botet M, De Landázuri MO, Sánchez-Madrid F. Expression of a gp33/27,000 MW activation inducer molecule (AIM) on human lymphoid tissues. Induction of cell proliferation on thymocytes and B lymphocytes by anti-AIM antibodies. Immunology. 1989;68:72-79. [PubMed] |
| 4. | Laffón A, García-Vicuña R, Humbría A, Postigo AA, Corbí AL, de Landázuri MO, Sánchez-Madrid F. Upregulated expression and function of VLA-4 fibronectin receptors on human activated T cells in rheumatoid arthritis. J Clin Invest. 1991;88:546-552. [PubMed] |
| 5. | García-Monzón C, Moreno-Otero R, Pajares JM, García-Sánchez A, López-Botet M, de Landázuri MO, Sánchez-Madrid F. Expression of a novel activation antigen on intrahepatic CD8+ T lymphocytes in viral chronic active hepatitis. Gastroenterology. 1990;98:1029-1035. [PubMed] |
| 6. | Montecucco C, Rappuoli R. Living dangerously: how Helicobacter pylori survives in the human stomach. Nat Rev Mol Cell Biol. 2001;2:457-466. [PubMed] |
| 7. | Wotherspoon AC, Ortiz-Hidalgo C, Falzon MR, Isaacson PG. Helicobacter pylori-associated gastritis and primary B-cell gastric lymphoma. Lancet. 1991;338:1175-1176. [PubMed] |
| 8. | Rothenbacher D, Brenner H. Burden of Helicobacter pylori and H. pylori-related diseases in developed countries: recent developments and future implications. Microbes Infect. 2003;5:693-703. [PubMed] |
| 9. | Enarsson K, Brisslert M, Backert S, Quiding-Järbrink M. Helicobacter pylori induces transendothelial migration of activated memory T cells. Infect Immun. 2005;73:761-769. [PubMed] |
| 10. | Brockman JA, Scherer DC, McKinsey TA, Hall SM, Qi X, Lee WY, Ballard DW. Coupling of a signal response domain in I kappa B alpha to multiple pathways for NF-kappa B activation. Mol Cell Biol. 1995;15:2809-2818. [PubMed] |
| 11. | McKinsey TA, Brockman JA, Scherer DC, Al-Murrani SW, Green PL, Ballard DW. Inactivation of IkappaBbeta by the tax protein of human T-cell leukemia virus type 1: a potential mechanism for constitutive induction of NF-kappaB. Mol Cell Biol. 1996;16:2083-2090. [PubMed] |
| 12. | Geleziunas R, Ferrell S, Lin X, Mu Y, Cunningham ET, Grant M, Connelly MA, Hambor JE, Marcu KB, Greene WC. Human T-cell leukemia virus type 1 Tax induction of NF-kappaB involves activation of the IkappaB kinase alpha (IKKalpha) and IKKbeta cellular kinases. Mol Cell Biol. 1998;18:5157-5165. [PubMed] |
| 13. | Iha H, Kibler KV, Yedavalli VR, Peloponese JM, Haller K, Miyazato A, Kasai T, Jeang KT. Segregation of NF-kappaB activation through NEMO/IKKgamma by Tax and TNFalpha: implications for stimulus-specific interruption of oncogenic signaling. Oncogene. 2003;22:8912-8923. [PubMed] |
| 14. | López-Cabrera M, Muñoz E, Blázquez MV, Ursa MA, Santis AG, Sánchez-Madrid F. Transcriptional regulation of the gene encoding the human C-type lectin leukocyte receptor AIM/CD69 and functional characterization of its tumor necrosis factor-alpha-responsive elements. J Biol Chem. 1995;270:21545-21551. [PubMed] |
| 15. | Mori N, Prager D. Transactivation of the interleukin-1alpha promoter by human T-cell leukemia virus type I and type II Tax proteins. Blood. 1996;87:3410-3417. [PubMed] |
| 16. | Bourzac KM, Guillemin K. Helicobacter pylori-host cell interactions mediated by type IV secretion. Cell Microbiol. 2005;7:911-919. [PubMed] |
| 17. | Gomis-Rüth FX, Solà M, de la Cruz F, Coll M. Coupling factors in macromolecular type-IV secretion machineries. Curr Pharm Des. 2004;10:1551-1565. [PubMed] |
| 18. | Fischer W, Püls J, Buhrdorf R, Gebert B, Odenbreit S, Haas R. Systematic mutagenesis of the Helicobacter pylori cag pathogenicity island: essential genes for CagA translocation in host cells and induction of interleukin-8. Mol Microbiol. 2001;42:1337-1348. [PubMed] |
| 19. | Selbach M, Moese S, Meyer TF, Backert S. Functional analysis of the Helicobacter pylori cag pathogenicity island reveals both VirD4-CagA-dependent and VirD4-CagA-independent mechanisms. Infect Immun. 2002;70:665-671. [PubMed] |
| 20. | Foryst-Ludwig A, Naumann M. p21-activated kinase 1 activates the nuclear factor kappa B (NF-kappa B)-inducing kinase-Ikappa B kinases NF-kappa B pathway and proinflammatory cytokines in Helicobacter pylori infection. J Biol Chem. 2000;275:39779-39785. [PubMed] |
| 21. | Maeda S, Yoshida H, Ogura K, Mitsuno Y, Hirata Y, Yamaji Y, Akanuma M, Shiratori Y, Omata M. H. pylori activates NF-kappaB through a signaling pathway involving IkappaB kinases, NF-kappaB-inducing kinase, TRAF2, and TRAF6 in gastric cancer cells. Gastroenterology. 2000;119:97-108. [PubMed] |
| 22. | Sharma SA, Tummuru MK, Blaser MJ, Kerr LD. Activation of IL-8 gene expression by Helicobacter pylori is regulated by transcription factor nuclear factor-kappa B in gastric epithelial cells. J Immunol. 1998;160:2401-2407. [PubMed] |
| 23. | Baeuerle PA. The inducible transcription activator NF-kappa B: regulation by distinct protein subunits. Biochim Biophys Acta. 1991;1072:63-80. [PubMed] |
| 24. | Karin M, Ben-Neriah Y. Phosphorylation meets ubiquitination: the control of NF-[kappa]B activity. Annu Rev Immunol. 2000;18:621-663. [PubMed] |
| 25. | Zandi E, Karin M. Bridging the gap: composition, regulation, and physiological function of the IkappaB kinase complex. Mol Cell Biol. 1999;19:4547-4551. [PubMed] |
| 26. | Woronicz JD, Gao X, Cao Z, Rothe M, Goeddel DV. IkappaB kinase-beta: NF-kappaB activation and complex formation with IkappaB kinase-alpha and NIK. Science. 1997;278:866-869. [PubMed] |
| 27. | Pierce JW, Schoenleber R, Jesmok G, Best J, Moore SA, Collins T, Gerritsen ME. Novel inhibitors of cytokine-induced IkappaBalpha phosphorylation and endothelial cell adhesion molecule expression show anti-inflammatory effects in vivo. J Biol Chem. 1997;272:21096-21103. [PubMed] |
| 28. | Jeremias I, Kupatt C, Baumann B, Herr I, Wirth T, Debatin KM. Inhibition of nuclear factor kappaB activation attenuates apoptosis resistance in lymphoid cells. Blood. 1998;91:4624-4631. [PubMed] |
| 29. | Esplugues E, Sancho D, Vega-Ramos J, Martínez C, Syrbe U, Hamann A, Engel P, Sánchez-Madrid F, Lauzurica P. Enhanced antitumor immunity in mice deficient in CD69. J Exp Med. 2003;197:1093-1106. [PubMed] |
| 30. | Sancho D, Gómez M, Viedma F, Esplugues E, Gordón-Alonso M, García-López MA, de la Fuente H, Martínez-A C, Lauzurica P, Sánchez-Madrid F. CD69 downregulates autoimmune reactivity through active transforming growth factor-beta production in collagen-induced arthritis. J Clin Invest. 2003;112:872-882. [PubMed] |
| 31. | Baldari CT, Lanzavecchia A, Telford JL. Immune subversion by Helicobacter pylori. Trends Immunol. 2005;26:199-207. [PubMed] |
| 32. | Sancho D, Gómez M, Sánchez-Madrid F. CD69 is an immunoregulatory molecule induced following activation. Trends Immunol. 2005;26:136-140. [PubMed] |
| 33. | Castellanos Mdel C, López-Giral S, López-Cabrera M, de Landázuri MO. Multiple cis-acting elements regulate the expression of the early T cell activation antigen CD69. Eur J Immunol. 2002;32:3108-3117. [PubMed] |