Published online Aug 14, 2011. doi: 10.3748/wjg.v17.i30.3487
Revised: February 15, 2011
Accepted: February 22, 2011
Published online: August 14, 2011
AIM: To investigate the effects of integrin-linked kinase (ILK) on gastric cancer cells both in vitro and in vivo.
METHODS: ILK small interfering RNA (siRNA) was transfected into human gastric cancer BGC-823 cells and ILK expression was monitored by real-time quantitative polymerase chain reaction, Western blotting analysis and immunocytochemistry. Cell attachment, proliferation, invasion, microfilament dynamics and the secretion of vascular endothelial growth factor (VEGF) were also measured. Gastric cancer cells treated with ILK siRNA were subcutaneously transplanted into nude mice and tumor growth was assessed.
RESULTS: Both ILK mRNA and protein levels were significantly down-regulated by ILK siRNA in human gastric cancer cells. This significantly inhibited cell attachment, proliferation and invasion. The knockdown of ILK also disturbed F-actin assembly and reduced VEGF secretion in conditioned medium by 40% (P < 0.05). Four weeks after injection of ILK siRNA-transfected gastric cancer cells into nude mice, tumor volume and weight were significantly reduced compared with that of tumors induced by cells treated with non-silencing siRNA or by untreated cells (P < 0.05).
CONCLUSION: Targeting ILK with siRNA suppresses the growth of gastric cancer cells both in vitro and in vivo. ILK plays an important role in gastric cancer progression.
- Citation: Zhao G, Guo LL, Xu JY, Yang H, Huang MX, Xiao G. Integrin-linked kinase in gastric cancer cell attachment, invasion and tumor growth. World J Gastroenterol 2011; 17(30): 3487-3496
- URL: https://www.wjgnet.com/1007-9327/full/v17/i30/3487.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i30.3487
Gastric cancer is one of the most commonly diagnosed malignant tumors, and is also one of the most frequent causes of cancer mortality worldwide[1]. Its incidence is highest in Japan, China, Eastern Europe and Latin America. In 2002, 934 000 new cases were diagnosed, making it the fourth most common cancer, causing approximately 700 000 deaths[2]. The 5-year survival rate of gastric cancer is poor, being approximately 20%, even in patients treated with surgical resection, chemotherapy, radiotherapy and other approaches. However, in Japan, according to a systematic screening program, this figure reached 60%[3-5]. Therefore, an understanding of the molecular mechanisms involved in gastric cancer formation and progression, and the identification of specific targets for gene therapy should be helpful in developing more effective strategies. Integrin-linked kinase (ILK) is an ankyrin repeat-containing serine/threonine protein kinase[6] that interacts with the cytoplasmic domain of β1 and β3 integrins[7] and regulates integrin dependent functions. It mediates a diversity of cell functions by coupling integrins and growth factors to cascades of downstream signaling events. ILK is a downstream substrate of phosphoinositide 3-kinase, and is an important upstream kinase for the regulation of protein kinase B (PKB/Akt) and glycogen synthase kinase 3 (GSK-3)[8,9]. ILK is now recognized to play an important role in linking extracellular signaling to the regulation of survival, cell cycle progression, migration, and invasion. The expression and activity of ILK are increased in a range of tumors, and small-molecule inhibitors of ILK activity have been identified and shown to inhibit tumor growth, invasion and angiogenesis[10-12], although certain tumors have decreased or no ILK expression. In gastric cancer, there is no ILK expression in non-neoplastic gastric epithelia, while the number of cells expressing ILK increased to 69% in neoplastic gastric epithelia, which was associated with tumor cell invasion and nodal metastasis[13]. To further investigate the role of ILK in gastric cancer progression and to determine if ILK can be used as a therapeutic target, we specifically knocked down ILK expression using small interfering RNA (siRNA) in gastric cancer cell line, BGC-823. We also analyzed these cancer cells’ spontaneous attachment, proliferation, invasion and cell morphology in vitro and their tumor growth in vivo.
Human gastric cancer cell line, BGC-823, was obtained from the Institute of Geriatrics, Ministry of Health (Beijing, China), and was cultured in RPMI-1640 medium (Gibco BRL, Grand Island, United States) supplemented with 10% heat-inactivated fetal bovine serum (FBS, Gibco) in a 5% CO2 humidified atmosphere at 37 °C. HiPer-Fect Transfection Reagent was purchased from Qiagen (Hilden, Germany). A First-Strand cDNA Synthesis kit and SYBR-green real-time polymerase chain reaction (PCR) Mastermix were purchased from Toyobo (Osaka, Japan). For Western blotting analysis, an anti-ILK antibody, purchased from Abcam (Cambridge, United Kingdom) was used at a dilution of 1:2000, and an HRP-goat anti-rabbit secondary antibody obtained from Rockland Immunochemicals (Gilbertsville, PA, United States) was used at a dilution of 1:3000. For immunocytochemistry, the ILK antibody was used at a dilution of 1:1000, and a goat anti-rabbit TRITC conjugated antibody was used at a dilution of 1:400. Anti-β-actin-HRP antibody was obtained from Santa Cruz Biotechnology (Santa Cruz, CA, United States), and SuperSignal West Pico Chemiluminescent Substrate was purchased from Pierce (Rockford, IL, United States). Matrigel was obtained from BD Biosciences (Bedford, MA, United States), while Alexa Fluor 488 phalloidin was purchased from Molecular Probes (Invitrogen, Carlsbad, CA, United States). All other reagents were purchased from Sigma (St. Louis, MO, United States).
The ILK-specific (GenBank accession No. NM004517) siRNAs (ILK siRNA, forward: 5'-GAAUCUCAACCGUAUUCCATT-3'; reverse: 5'-UGGAAUACGGUUGAGAUUCTG-3') were chemically synthesized by Qiagen. BGC-823 cells were transfected with siRNA using HiPer-Fect Transfection Reagent according to the manufacturer’s instructions. Briefly, the original stock of siRNA was suspended in siRNA suspension buffer provided by the manufacturer. The resulting suspension was aliquoted in a required amount for each experiment and stored at -20 °C until use. On the day of transfection, cells were seeded in plates at the recommended density according to the manufacturer’s instructions. The siRNA was then gently introduced onto the cells by mixing with the required amount of HiPer-Fect Transfection Reagent as recommended by the manufacturer. In our study, the final concentration of siRNA was 10 nmol/L. Non-silencing siRNA (NS siRNA, forward: 5'-UUCUCCGAACGUGUCACGUTT-3'; reverse: 5'-ACGUGACACGUUCGGAGAATT-3')-treated cells were used to control any effects of the transfection reagent and the non-specific siRNA effects. The in vitro assays described here were performed 48 h after transfection. Chemically modified siRNAs used in animal models were synthesized by Qiagen according to the sequences described above.
Isolation of total RNA was performed using Trizol solution according to the manufacturer’s protocol. Reverse transcription was then performed using 100 ng RNA and the First-Strand cDNA Synthesis kit. Real-time quantitative PCR analysis was performed with the DNA Engine Opticon 2 System (Bio-Rad, Richmond, CA, United States) using the SYBR® green Real-time PCR Mastermix. We used the following primers: for ILK, forward 5'-TTTGCAGTGCTTCTGTGGGAA-3' and reverse 5'-CTACTTGTCCTGCATCTTCTC-3'; for GAPDH, forward 5'-GAAGGTGAAGGTCGGAGTC-3' and reverse 5'-GAAGATGGTGATGGGATTTC-3'. After initial denaturation at 95 °C for 3 min, reactions were cycled 40 times. Each cycle consisted of denaturation at 95 °C for 15 s, primer annealing at 60 °C for 15 s and primer extension at 72 °C for 45 s[14,15]. Results were collected and analyzed using MJ Opticon Monitor Analysis software (Bio-Rad). The quantity data of mRNA input was controlled by measuring the reference gene, GAPDH. Experiments were performed in triplicate and repeated three times.
Cells were washed with ice-cold phosphate buffered saline (PBS), and whole-cell extracts were prepared using cell lysis buffer [20 mmol/L Tris (pH 7.5), 0.1% Triton X, 0.5% deoxycholate, 1 mmol/L phenylmethylsulfonyl fluoride, 10 μg/mL aprotinin and 10 μg/mL leupeptin] and cleared by centrifugation at 12 000 ×g at 4 °C. Total protein concentration was measured using the bicinchoninic acid assay with bovine serum albumin (BSA) as a standard. Equal amounts of protein were loaded and analyzed by immunoblotting. Enhanced chemiluminescence detection was performed in accordance with the manufacturer’s instructions[16,17]. The ILK signal was quantified using BandScan software version 5.1 (Glyko, Novato, Calif., United States) and normalized to that of β-actin. Experiments were performed in triplicate and repeated 3 times.
Immunocytochemical assays were performed as previously described[18]. Briefly, cells were grown on fibronectin coated coverslips, washed in PBS, and fixed for 15 min in 4% paraformaldehyde. Cell monolayers were permeabilized in 0.1% Triton X-100, washed, and blocked in 10% normal goat serum. Cells were incubated with the anti-ILK antibody overnight at 4 °C. Cells were then washed and incubated with fluorescently labeled secondary antibodies for 1 h at room temperature in the dark. Cells were washed and coverslips were mounted using Kaiser’s glycerin gelatin (Merck, Darmstadt, Germany). Fluorescence signals were visualized and acquired using an epifluorescence microscope (Leica, Heidelberg, Germany) with appropriate excitation and emission filters under 40 × magnification. Pictures of observed fields were recorded digitally. Experiments were performed in triplicate and repeated three times.
Plates of 96 wells were coated with 1.25 mg/mL fibronectin in 100 mL PBS overnight at 4 °C. The plates were blocked with 2.5 mg/mL BSA for 2 h in DMEM at 37 °C. Transfected cells were trypsinized and 1.5 × 104 cells were seeded in each well for 1 h at 37 °C. Cells were then washed twice with PBS and the unattached cells were discarded. After the washing step, the number of attached cells was determined by the MTT assay in accordance with the manufacturer’s instructions. Absorbance was measured using an enzyme-linked immunosorbent assay (ELISA) plate reader at 570 nm[19]. Experiments were performed in triplicate and repeated three times.
Cell proliferation was assessed using the MTT assay[20]. Gastric cancer BGC-823 cells were plated at 5 × 103 cells/well in 96-well plates in RPMI-1640 medium containing 10% FBS. After 24 h, the culture medium was replaced by fresh medium containing ILK siRNA or non-silencing siRNA. Six duplicate wells were set up for each group. Untreated cells served as control. After 4, 24, 48 or 72 h of incubation, 20 μL MTT (5 g/L, Sigma) was added to each well and incubation continued for 4 h. Cells were collected by centrifugation at 1000 ×g for 5 min at room temperature. The reaction was stopped by the addition of 150 μL dimethyl sulfoxide. The absorbance of samples was measured at 570 nm. Each assay was performed in triplicate and repeated three times. Cell proliferation inhibition rate [proliferation inhibition rate = (1-A570 experiment group/A570 control group) × 100%] was plotted vs time.
Polycarbonate membranes (8.0 μm pore size) of the upper compartment of 24-well Transwell culture chambers were coated with 18 μL of 5 mg/mL Matrigel (BD Biosciences) in serum-free medium. Cells (5 × 104) suspended in 250 μL of serum-free medium were applied on the upper compartment, and the lower compartment was filled with 750 μL of DMEM containing 10% fetal bovine serum. After incubation for 24 h, cells were fixed with 10% trichloroacetic acid at 4 °C for 1 h. Non-invaded cells on the upper surface of the filter were removed carefully with a cotton swab. Invading cells on the lower side of the filter were stained with 0.5% crystal violet for 2 h and the stained filters were photographed. The crystal violet dye retained on the filters was extracted with 30% acetic acid and cell invasion was measured by reading the absorbance at 590 nm[19]. Each assay was performed in triplicate and repeated three times.
Microfilament organization of RF/6A cells was assessed by a modification of an immunofluorescence protocol using rhodamine-phalloidin[21]. After transfection, cells were trypsinized and seeded onto coverslips for 6 h at 37 °C in 5% CO2. Following this, the medium was aspirated, and adherent cells were fixed with 4% paraformaldehyde in PBS for 20 min. After they had been washed with PBS (pH 7.4) 3 times, cells were permeabilized with 0.1% Triton X-100 for 20 min and blocked with 1% BSA in PBS for 5 min. Cells were then incubated with rhodamine-phalloidin (200 U/mL) for 30 min and diamidinophenylindole (0.1 μg/mL) for 1 min in the dark. PBS was used as the base of all solutions and intervening rinses, and incubations were performed at room temperature. After mounting (Kaiser’s glycerin gelatin; Merck, Darmstadt, Germany), slides were examined under an epifluorescence microscope (Leica, Heidelberg, Germany) with appropriate excitation and emission filters under a magnification × 40. Pictures of observed fields were recorded digitally. Experiments were performed in triplicate and repeated 3 times.
siRNA-transfected cells were seeded in 6-well plates (3 × 105 cells/well) and incubated at 37 °C. After 24 h, the cell culture supernatant was harvested, and cell counts were made after trypsinization. After collection, the medium was spun at 800 ×g for 3 min at 4 °C to remove cellular debris[17]. The supernatants were frozen and stored at -80 °C until use. The levels of vascular endothelial growth factor (VEGF) were measured in culture medium samples with a VEGF ELISA kit according to the manufacturer’s instructions. Experiments were performed in triplicate and repeated 3 times.
An equal number (1 × 107) of BGC-823 cells transfected with ILK siRNA, non-silencing siRNA, or untreated cells was harvested 48 h after transfection, washed twice with 1 × PBS, and resuspended in 0.2 mL of saline. Three groups (each group with 5 mice) of 4-6 wk old male BALB/c nude mice (Institute of Zoology, Chinese Academy of Sciences) were housed in a specific pathogen-free environment at the Animal Laboratory, then given subcutaneous injections with either untransfected cells, cells transfected with non-silencing siRNA, or cells transfected with ILK siRNA. The mice were monitored every 3 d for tumor formation. The date at which a palpable tumor first arose and the volume of the tumor (V = L × W2×π/6) were recorded[22]. At week 4 after injection of the cells, the mice were killed and the weights of tumors were recorded. The animal experiments performed on nude mice were approved by the Animal Ethics Committee of Peking University.
Statistical analysis was performed using SPSS software (SPSS V 14.0; SPSS). All results were expressed as mean ± SD. To determine the significance of differences, ANOVA was performed. Differences with P < 0.05 were considered statistically significant.
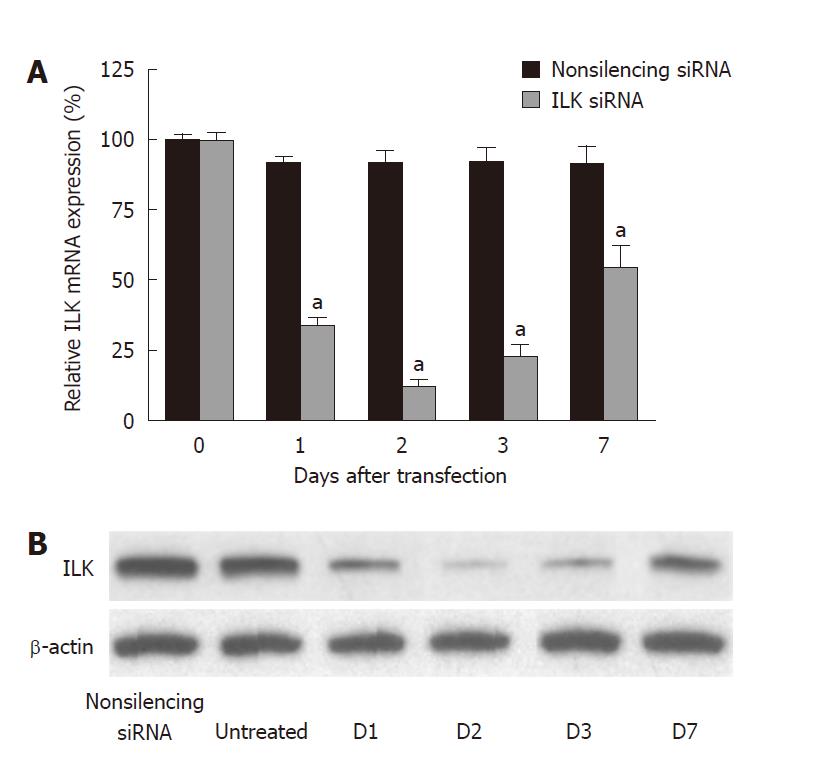
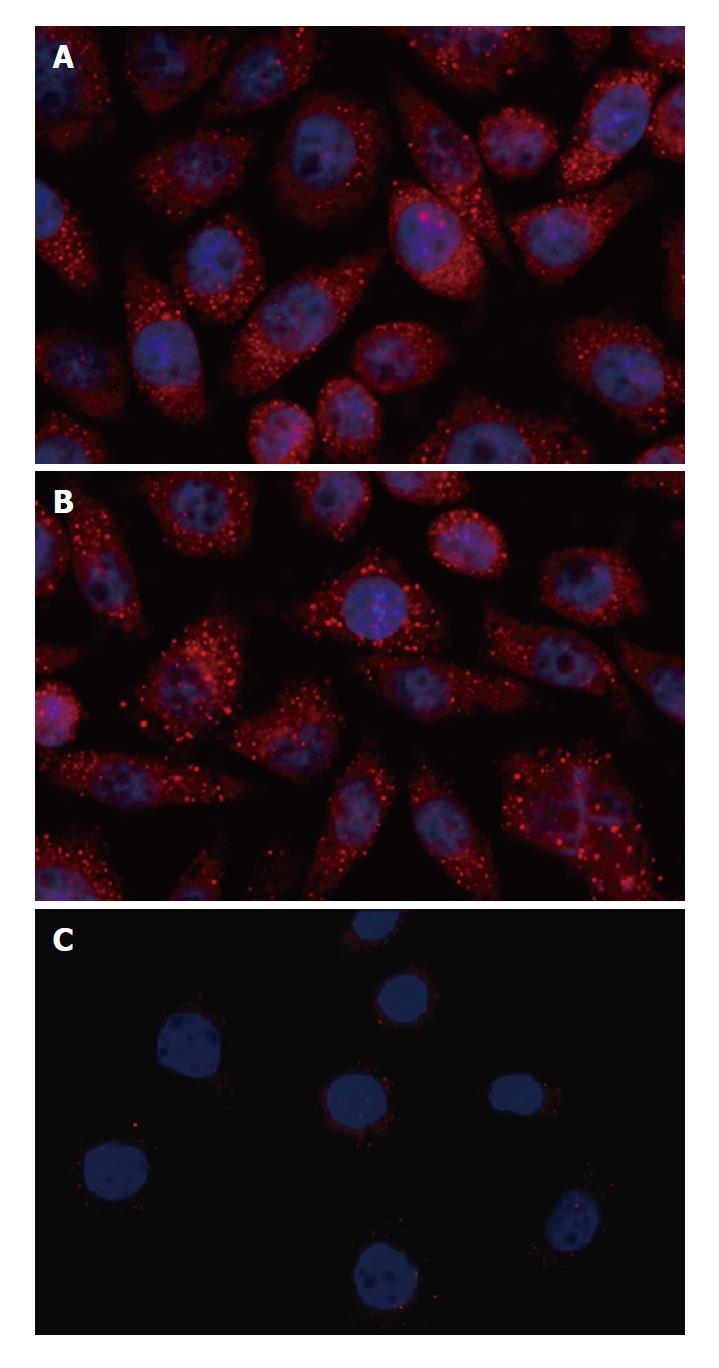
Expression of ILK was significantly suppressed in gastric cancer BGC-823 cells transfected with ILK siRNA. The suppression of ILK occurred within 24 h after transfection and lasted a week. ILK siRNA caused a reduction of ILK mRNA of more than 85% after 48 h (88.2% ± 9.3% inhibition, P < 0.01) (Figure 1A), and a reduction of ILK protein of more than 85% after 48 h (86.8% ± 8.2% inhibition, P < 0.01) (Figure 1B) compared with non-silencing siRNA. Down-regulation of ILK expression in BGC-823 cells was further confirmed by immunocytochemistry (Figure 2): the fluorescence intensity representing the expression of ILK in untreated or non-silencing siRNA-treated cells was very strong, but that in ILK siRNA-transfected cells was barely detectable. Control cells also showed a higher degree of cell spreading when compared with the ILK-knockdown cells.
We investigated the role of ILK in attachment of gastric cancer cells. In the cell attachment assay, we found that down-regulation of ILK lowered the ability of cells to attach to fibronectin compared with the non-silencing siRNA group (P < 0.01, Figure 3). The non-silencing siRNA and untreated groups had no significant difference in cell attachment ability (P > 0.05).
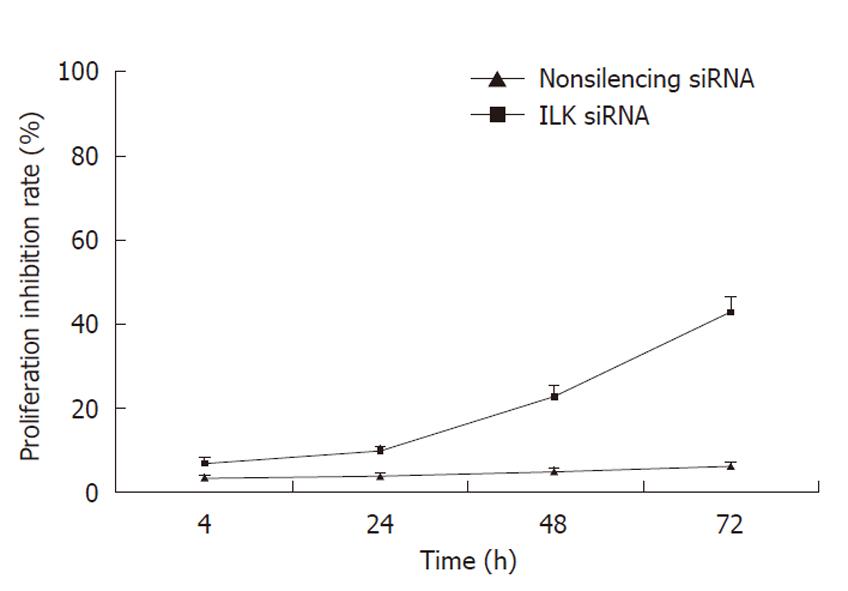
MTT assay was performed to observe whether down-regulation of ILK had an inhibitory effect on BGC-823 cell proliferation. We found that treatment of BGC-823 cells with ILK siRNA was associated with a time-dependent inhibition of cell proliferation, whereas no significant inhibitory effect was observed in cells treated with non-silencing siRNA (Figure 4).
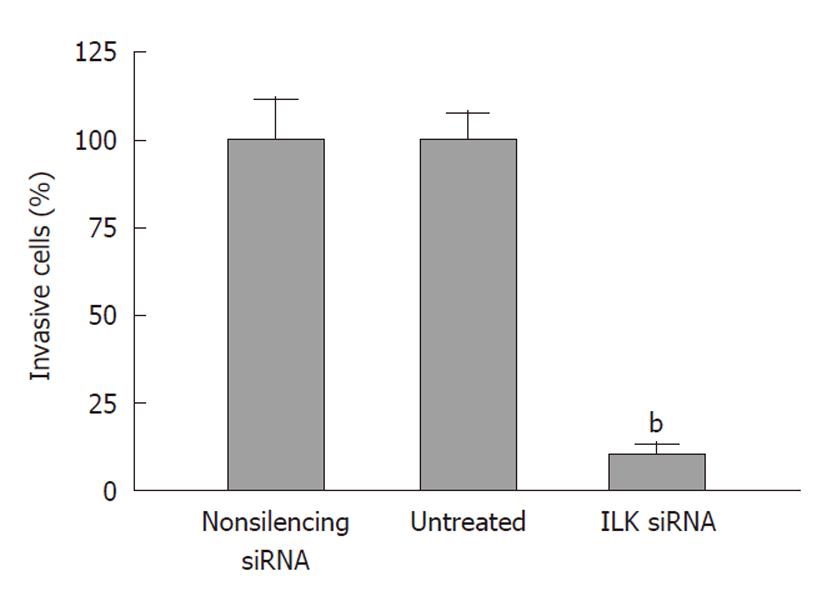
Next, we investigated the role of ILK in the invasion of gastric cancer cells. We found that down-regulation of ILK reduced the ability of cells to invade through Matrigel-coated Boyden chambers to 10.7% ± 1.5% of that achieved by the non-silencing siRNA group (P < 0.01, Figure 5). The non-silencing siRNA and untreated groups had no significant difference in cell invasion ability (P > 0.05).
In the microfilament dynamics assay, ILK siRNA-transfected gastric cancer cells displayed different patterns of F-actin assembly and cell morphologies compared with the non-silencing siRNA group (Figure 6). F-actin assembly in the ILK siRNA group was significantly disturbed. There was less lamellipodia and filopodia formation in ILK-knockdown cells. However, cells in the non-silencing siRNA group displayed a well-organized actin skeleton with fibers extending throughout the cytoplasm into the cell membrane. Non-silencing siRNA-treated cells also showed a higher degree of cell spreading compared with the ILK siRNA-treated cells.
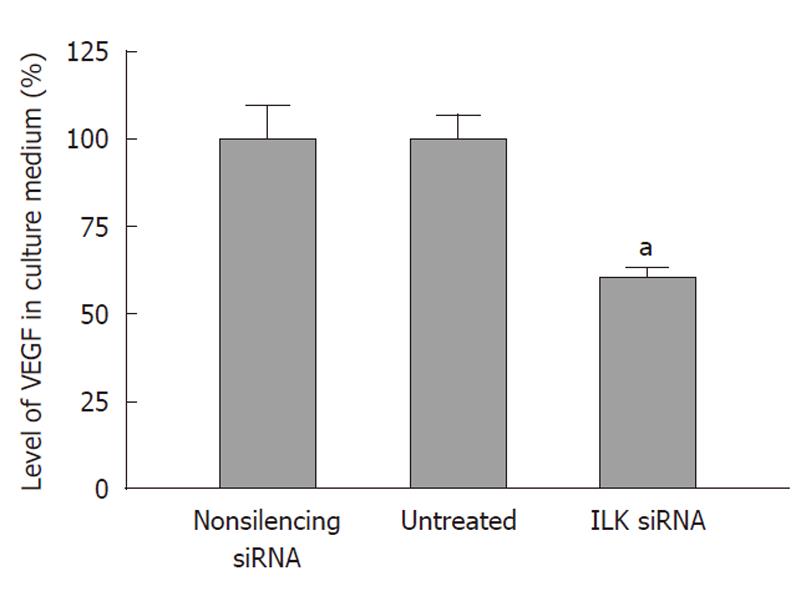
VEGF plays an important role in tumor angiogenesis. To explore whether down-regulation of ILK can reduce VEGF activity in BGC-823 cells, we examined the levels of VEGF secreted into the culture medium by ELISA. We found that ILK knockdown led to a decrease in the level of VEGF secreted into the culture medium. As shown in Figure 7, the level of VEGF in the culture medium was decreased by 40% compared with non-silencing siRNA treated cells (P < 0.05). In contrast, there was no significant difference in the level of VEGF secretion between the non-silencing siRNA treated and untreated cells (P > 0.05).
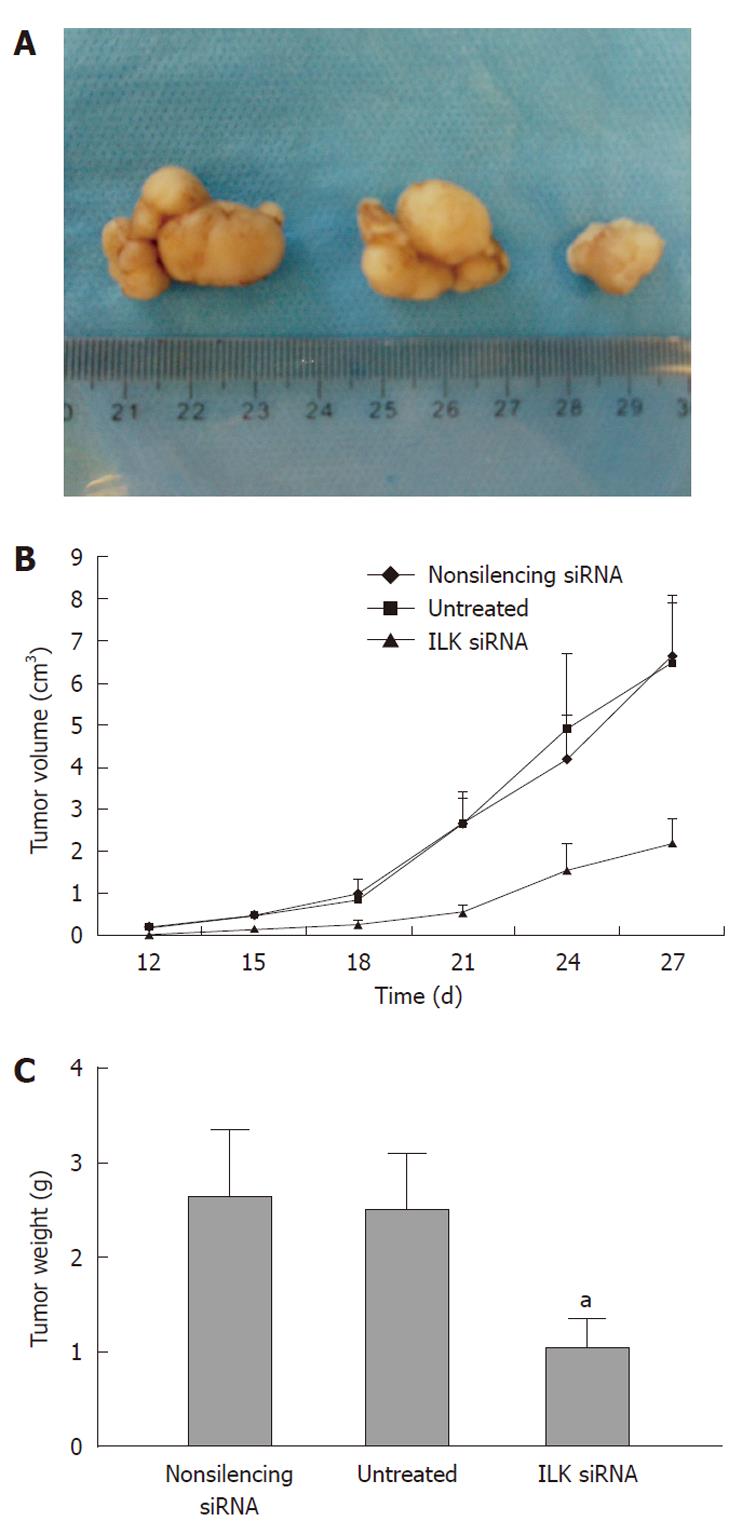
To further investigate the role of ILK in gastric cancer tumorigenesis, equal numbers (1 × 107) of BGC-823 cells transfected with ILK siRNA, non-silencing siRNA, or untreated cells were subcutaneously injected into nude mice. The growth of tumors was measured every 3 d. Four weeks after injection of the cells, mice were sacrificed and the weights of tumors were recorded. As shown in Figure 8, cells with down-regulation of ILK produced significantly smaller tumors in nude mice compared with untreated cells and cells treated with non-silencing siRNA (volume: 2.19 g ± 0.58 g vs 6.52 g ± 1.42 g, 6.68 g ± 1.40 g, P < 0.05; weight: 1.0 cm3± 0.2 cm3vs 2.5 cm3± 0.4 cm3, 2.6 cm3± 0.4 cm3, P < 0.05, respectively) indicating that targeting ILK by siRNA may exert a strong anti-tumor effect on BGC-823 cells in vivo. All the tumors were analyzed by H&E staining and were verified to have similar cell morphologies that were consistent with gastric cancer.
Gastric cancer is a life-threatening disease with a high mortality worldwide, especially in Asia. Therefore, it is important to understand the molecular mechanisms of gastric cancer progression to discover useful targets to treat advanced gastric cancer. Previous findings showed that ILK has an increased expression and an association with tumor cell invasion and nodal metastasis in gastric cancer[13]. We, therefore, investigated whether ILK was important in different processes involved in gastric cancer progression, including cell attachment, growth, invasion, microfilament dynamics, angiogenesis and tumor growth in vivo by targeted siRNA knockdown of ILK.
Although RNA interference mediated by siRNAs is a powerful technology allowing the silencing of mammalian genes with high specificity and potency, non-specific effects both at the messenger RNA and protein levels can result from siRNA mediated mechanisms, and may represent one of the limitations of this technology[23]. Therefore, we used non-silencing siRNA-transfected cells to control these non-specific effects. We observed significant differences between the ILK-specific siRNA treated group and the non-silencing siRNA treated group in all assays. However, some studies indicated that the inhibition effect of synthetic siRNA may only last a short time. Therefore, following tumorigenesis, ILK expression may be released from RNAi inhibition in tumor tissues. Thus, to get a more stable inhibitory effect, we used a long-acting siRNA whose stability was improved by chemical modification. The expression of ILK was inhibited for about 7 d by this technique[24]. We observed that the suppression of ILK lasted a week and reached a peak 48 h after transfection. Thereafter, the expression of ILK gradually recovered, because of the remaining instability of the ILK siRNA.
We first showed that ILK is important for gastric cancer cells to attach to fibronectin-coated plates. Upon engagement with the extra cellular matrix (ECM), numerous signaling proteins are recruited to the adhesion sites, where cell-matrix contact is established[25]. Fibronectin is one of the major components in the ECM that connect cells through the extracellular domain of integrins. Here, we demonstrate that ILK plays a central role in the transduction of signals when gastric cancer cells engage in ECM-regulated cell attachment. The mechanism of how ILK regulates the dynamic rearrangement of cell-matrix adhesions and cell spreading is not well understood. Some scholars have suggested that ILK regulates cell-matrix adhesion dynamics through Rac-1[26]. In addition, inhibition of PI3K-dependent ILK activity in PTEN-null PC3 prostate cancer cells disrupted the localization of ILK/α-parvin/paxillin complex to focal adhesions, leading to decreased cell adhesion and migration[27]. However, others found that knock-down of human ILK by siRNA increased cell adhesion in diverse gastric carcinoma cell variants, including SNU16, integrin-α5-expressing SNU16, and integrin-α5-expressing SNU620 cells[28]. It appears that the correlation between ILK expression and cell adhesion is very sophisticated, and may depend on cell types and the signaling contexts. The epigenetic control of ILK expression and adhesion properties of gastric carcinoma cells appear to affect each other, through a bidirectional regulatory linkage[28].
Inhibition of ILK kinase activity by an ILK inhibitor is known to impede cell attachment and filamentous actin organization[27]. ILK over-expression induced the distribution of actin filaments onto the cell membrane to form cell motility structures[29]. We found that formation of the actin cytoskeleton and motility structures, which are important for the early events in cell spreading and migration, were severely affected in ILK knockdown gastric cancer cells. ILK may modulate cell spreading, migration and cytoskeletal organization by activating PAK-interactive exchange factor (PIX, also known as ARHGEF6), a guanine-nucleotide exchange factor for Rac1 and Cdc42[28], and by activating cofilin through an interaction with phosphorylated Scr[30]. This study suggests that targeting ILK seems to be linked with microfilament assembly, resulting in a decreased ability of the gastric cancer cells to attach, spread and migrate.
Tumor cell proliferation is another important event in tumor progression. Knockdown of ILK using siRNA inhibited the proliferation of gastric cancer cells and impaired the growth of gastric cancer xenografts in vivo. ILK over-expression or constitutive activation leads to the stimulation of cell-cycle progression, and inhibition of ILK activity in some cancer cells results in inhibition of cyclin D1 expression and G1/S cell-cycle arrest[31-33]. ILK-mediated phosphorylation and consequent inhibition of the activity of GSK-3 may regulate several pathways, leading to stimulation if cyclin D1 expression. Inhibition of GSK-3 activity leads to activation of the AP1 transcription factor, cyclic-AMP-responsive-element-binding protein, and the β-catenin/TCF transcription factor[32,34,35], both of which can stimulate the expression of cyclin D1 and promote cell proliferation.
We also found that ILK is important for gastric cancer cell invasion; knockdown of ILK using siRNA inhibited gastric cancer cell invasion. Increased ILK expression was shown to stimulate the expression and activity of the matrix metallopeptidase 9 (MMP9), through activation of the AP1 transcription factor[36]. Inhibition of ILK activity in highly invasive human glioblastoma cells, resulted in substantial inhibition of invasion into matrigel, and pharmacological inhibition of MMP9 activity also inhibited invasion[36], demonstrating that ILK can promote invasion through up-regulation and activation of MMP9. In gastric cancer, the T allele of the 1562 C/T polymorphism in the MMP9 gene is associated with an invasive tumor phenotype[37] and elevated plasma MMP-9 correlates significantly with lymph node metastasis, lymphatic invasion, venous invasion and poor survival rates[38]. Therefore, it is reasonable to predict that down-regulating ILK and then inhibiting MMP9 hold promise for the treatment of gastric cancer.
Tumor angiogenesis is promoted by the expression and secretion of VEGF from tumor cells, which then binds to the VEGF receptor on the nearby endothelial cells, stimulating their survival, proliferation and migration, which are events required for the formation of new blood vessels. We observed that silencing ILK with siRNA significantly reduced VEGF secretion from gastric cancer cells. What is the mechanism by which ILK regulates VEGF? ILK has been reported to be essential for the regulation of hypoxia inducible factor (HIF)-1α expression, and for the consequent production of VEGF in a PKB/Akt- and mTOR/FRAP-dependent manner[10]. And HIF-1α is a major transcriptional activator of the VEGF gene[39]. A model has been proposed to explain this regulation[10]: phosphorylation of serine 473 of Akt/PKB by activated ILK results in the full activation of PKB/Akt, which promotes the phosphorylation of serine 2448 of mTOR/FRAP. This activates mTOR/FRAP, which raises the levels of HIF-1α protein translation. HIF-1α protein combines with HIF-1β to form an active transcription factor. This heterodimer binds to the VEGF promoter and activates VEGF transcription, translation, and secretion. VEGF binds to its receptor on the nearby endothelial cells and stimulates ILK activity. Furthermore, down-regulation of VEGF expression with siRNA not only impaired tube formation, but also inhibited the synthesis of multiple angiogenic proteins, such as angiogenin, interleukin (IL)-6, IL-8, transforming growth factor β1 and monocyte chemoattractant protein 1[40]. Collectively, these studies demonstrate a crucial role of ILK in the regulation of vascular morphogenesis, and indicate that ILK should be considered as a promising target for anti-angiogenic therapy.
In summary, our results indicate that knockdown of ILK with siRNA is able to inhibit not only gastric cancer cell attachment, proliferation, invasion and tumor angiogenesis in vitro, but also tumor growth in vivo. These findings also suggest that ILK could be a valid therapeutic target in gastric cancer.
Gastric cancer is one of the most commonly diagnosed malignant tumors and also is one of the most frequent causes of cancer mortality worldwide. Therefore, an understanding of the molecular mechanisms involved in gastric cancer formation and progression, and the identification of specific gene therapy targets are important for developing more effective approaches for gastric cancer treatment.
Integrin-linked kinase (ILK), an ankyrin repeat-containing serine/threonine protein kinase, mediates a diversity of cell functions by coupling integrins and growth factors to cascades of downstream signaling events. The expression and activity of ILK are increased in a range of tumors, and small-molecule inhibitors of ILK activity have been identified, and shown to inhibit tumor growth, invasion and angiogenesis. ILK has become a hot topic in tumor research.
To investigate the role of ILK in gastric cancer, the authors specifically knocked down ILK expression using small interfering RNA (siRNA) in the gastric cancer cell line, BGC-823. The authors verified that knockdown of ILK significantly inhibited human gastric cancer cell attachment, proliferation, and invasion, and also disturbed F-actin assembly and reduced vascular endothelial growth factor secretion. Knockdown of ILK also suppressed the growth of gastric cancer cells in vivo. This research attempts to systematically understand the role of ILK in gastric cancer progression. The results could improve our understanding of gastric cancer progression.
The study provides the first evidence that ILK plays an important role in gastric cancer progression. The results indicate that ILK should be considered as a promising target for anti-gastric cancer treatment.
The expression and activity of ILK are increased in a range of tumors, including gastric cancer. This study shows that targeting ILK with siRNA suppressed the growth of gastric cancer cells. The results indicate that ILK plays an important role in gastric cancer progression and that ILK could be a therapeutic target for gastric cancer.
Peer reviewer: Hoon Jai Chun, MD, PhD, AGAF, Professor, Department of Internal Medicine, Institute of Digestive Disease and Nutrition, Korea University College of Medicine, 126-1, Anam-dong 5-ga, Seongbuk-gu, 136-705 Seoul, South Korea
S- Editor Sun H L- Editor Ma JY E- Editor Zheng XM
| 1. | Jemal A, Thomas A, Murray T, Thun M. Cancer statistics, 2002. CA Cancer J Clin. 2002;52:23-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2235] [Cited by in RCA: 2115] [Article Influence: 92.0] [Reference Citation Analysis (0)] |
| 2. | Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13286] [Cited by in RCA: 13549] [Article Influence: 677.5] [Reference Citation Analysis (1)] |
| 3. | Roukos DH, Kappas AM. Perspectives in the treatment of gastric cancer. Nat Clin Pract Oncol. 2005;2:98-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 127] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 4. | Kamangar F, Dores GM, Anderson WF. Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol. 2006;24:2137-2150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2591] [Cited by in RCA: 2640] [Article Influence: 138.9] [Reference Citation Analysis (0)] |
| 5. | Tsubono Y, Hisamichi S. Screening for gastric cancer in Japan. Gastric Cancer. 2000;3:9-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 96] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 6. | Hannigan GE, Leung-Hagesteijn C, Fitz-Gibbon L, Coppolino MG, Radeva G, Filmus J, Bell JC, Dedhar S. Regulation of cell adhesion and anchorage-dependent growth by a new beta 1-integrin-linked protein kinase. Nature. 1996;379:91-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 851] [Cited by in RCA: 859] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 7. | Li F, Zhang Y, Wu C. Integrin-linked kinase is localized to cell-matrix focal adhesions but not cell-cell adhesion sites and the focal adhesion localization of integrin-linked kinase is regulated by the PINCH-binding ANK repeats. J Cell Sci. 1999;112:4589-4599. [PubMed] |
| 8. | Delcommenne M, Tan C, Gray V, Rue L, Woodgett J, Dedhar S. Phosphoinositide-3-OH kinase-dependent regulation of glycogen synthase kinase 3 and protein kinase B/AKT by the integrin-linked kinase. Proc Natl Acad Sci U S A. 1998;95:11211-11216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 829] [Cited by in RCA: 837] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 9. | Yoganathan N, Yee A, Zhang Z, Leung D, Yan J, Fazli L, Kojic DL, Costello PC, Jabali M, Dedhar S. Integrin-linked kinase, a promising cancer therapeutic target: biochemical and biological properties. Pharmacol Ther. 2002;93:233-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 10. | Tan C, Cruet-Hennequart S, Troussard A, Fazli L, Costello P, Sutton K, Wheeler J, Gleave M, Sanghera J, Dedhar S. Regulation of tumor angiogenesis by integrin-linked kinase (ILK). Cancer Cell. 2004;5:79-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 210] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 11. | Persad S, Attwell S, Gray V, Mawji N, Deng JT, Leung D, Yan J, Sanghera J, Walsh MP, Dedhar S. Regulation of protein kinase B/Akt-serine 473 phosphorylation by integrin-linked kinase: critical roles for kinase activity and amino acids arginine 211 and serine 343. J Biol Chem. 2001;276:27462-27469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 377] [Cited by in RCA: 391] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 12. | Edwards LA, Thiessen B, Dragowska WH, Daynard T, Bally MB, Dedhar S. Inhibition of ILK in PTEN-mutant human glioblastomas inhibits PKB/Akt activation, induces apoptosis, and delays tumor growth. Oncogene. 2005;24:3596-3605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 80] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 13. | Ito R, Oue N, Zhu X, Yoshida K, Nakayama H, Yokozaki H, Yasui W. Expression of integrin-linked kinase is closely correlated with invasion and metastasis of gastric carcinoma. Virchows Arch. 2003;442:118-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 63] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 14. | Morissette MC, Parent J, Milot J. Perforin, granzyme B, and FasL expression by peripheral blood T lymphocytes in emphysema. Respir Res. 2007;8:62. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 15. | Ohnishi M, Hasegawa G, Yamasaki M, Obayashi H, Fukui M, Nakajima T, Ichida Y, Ohse H, Mogami S, Yoshikawa T. Integrin-linked kinase acts as a pro-survival factor against high glucose-associated osmotic stress in human mesangial cells. Nephrol Dial Transplant. 2006;21:1786-1793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 16. | Duxbury MS, Ito H, Benoit E, Waseem T, Ashley SW, Whang EE. RNA interference demonstrates a novel role for integrin-linked kinase as a determinant of pancreatic adenocarcinoma cell gemcitabine chemoresistance. Clin Cancer Res. 2005;11:3433-3438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 64] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 17. | Wang Z, Banerjee S, Kong D, Li Y, Sarkar FH. Down-regulation of Forkhead Box M1 transcription factor leads to the inhibition of invasion and angiogenesis of pancreatic cancer cells. Cancer Res. 2007;67:8293-8300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 215] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 18. | Troussard AA, McDonald PC, Wederell ED, Mawji NM, Filipenko NR, Gelmon KA, Kucab JE, Dunn SE, Emerman JT, Bally MB. Preferential dependence of breast cancer cells versus normal cells on integrin-linked kinase for protein kinase B/Akt activation and cell survival. Cancer Res. 2006;66:393-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 95] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 19. | Wong RP, Ng P, Dedhar S, Li G. The role of integrin-linked kinase in melanoma cell migration, invasion, and tumor growth. Mol Cancer Ther. 2007;6:1692-1700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 49] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 20. | Hao JH, Gu QL, Liu BY, Li JF, Chen XH, Ji YB, Zhu ZG, Lin YZ. Inhibition of the proliferation of human gastric cancer cells SGC-7901 in vitro and in vivo using Bcl-2 siRNA. Chin Med J (Engl). 2007;120:2105-2111. [PubMed] |
| 21. | Masson-Gadais B, Salers P, Bongrand P, Lissitzky JC. PKC regulation of microfilament network organization in keratinocytes defined by a pharmacological study with PKC activators and inhibitors. Exp Cell Res. 1997;236:238-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 22. | Tomayko MM, Reynolds CP. Determination of subcutaneous tumor size in athymic (nude) mice. Cancer Chemother Pharmacol. 1989;24:148-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1196] [Cited by in RCA: 1388] [Article Influence: 38.6] [Reference Citation Analysis (0)] |
| 23. | Jackson AL, Linsley PS. Noise amidst the silence: off-target effects of siRNAs? Trends Genet. 2004;20:521-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 253] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 24. | Guo L, Yu W, Li X, Zhao G, Liang J, He P, Wang K, Zhou P, Jiang Y, Zhao M. Targeting of integrin-linked kinase with a small interfering RNA suppresses progression of experimental proliferative vitreoretinopathy. Exp Eye Res. 2008;87:551-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 25. | Brakebusch C, Fässler R. The integrin-actin connection, an eternal love affair. EMBO J. 2003;22:2324-2333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 366] [Cited by in RCA: 350] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 26. | Boulter E, Grall D, Cagnol S, Van Obberghen-Schilling E. Regulation of cell-matrix adhesion dynamics and Rac-1 by integrin linked kinase. FASEB J. 2006;20:1489-1491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 27. | Attwell S, Mills J, Troussard A, Wu C, Dedhar S. Integration of cell attachment, cytoskeletal localization, and signaling by integrin-linked kinase (ILK), CH-ILKBP, and the tumor suppressor PTEN. Mol Biol Cell. 2003;14:4813-4825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 118] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 28. | Kim YB, Lee SY, Ye SK, Lee JW. Epigenetic regulation of integrin-linked kinase expression depending on adhesion of gastric carcinoma cells. Am J Physiol Cell Physiol. 2007;292:C857-C866. [PubMed] |
| 29. | Qian Y, Zhong X, Flynn DC, Zheng JZ, Qiao M, Wu C, Dedhar S, Shi X, Jiang BH. ILK mediates actin filament rearrangements and cell migration and invasion through PI3K/Akt/Rac1 signaling. Oncogene. 2005;24:3154-3165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 125] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 30. | Filipenko NR, Attwell S, Roskelley C, Dedhar S. Integrin-linked kinase activity regulates Rac- and Cdc42-mediated actin cytoskeleton reorganization via alpha-PIX. Oncogene. 2005;24:5837-5849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 95] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 31. | Persad S, Attwell S, Gray V, Delcommenne M, Troussard A, Sanghera J, Dedhar S. Inhibition of integrin-linked kinase (ILK) suppresses activation of protein kinase B/Akt and induces cell cycle arrest and apoptosis of PTEN-mutant prostate cancer cells. Proc Natl Acad Sci U S A. 2000;97:3207-3212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 151] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 32. | D'Amico M, Hulit J, Amanatullah DF, Zafonte BT, Albanese C, Bouzahzah B, Fu M, Augenlicht LH, Donehower LA, Takemaru K. The integrin-linked kinase regulates the cyclin D1 gene through glycogen synthase kinase 3beta and cAMP-responsive element-binding protein-dependent pathways. J Biol Chem. 2000;275:32649-32657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 194] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 33. | Li F, Liu J, Mayne R, Wu C. Identification and characterization of a mouse protein kinase that is highly homologous to human integrin-linked kinase. Biochim Biophys Acta. 1997;1358:215-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 34. | Persad S, Troussard AA, McPhee TR, Mulholland DJ, Dedhar S. Tumor suppressor PTEN inhibits nuclear accumulation of beta-catenin and T cell/lymphoid enhancer factor 1-mediated transcriptional activation. J Cell Biol. 2001;153:1161-1174. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 186] [Cited by in RCA: 185] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 35. | Troussard AA, Tan C, Yoganathan TN, Dedhar S. Cell-extracellular matrix interactions stimulate the AP-1 transcription factor in an integrin-linked kinase- and glycogen synthase kinase 3-dependent manner. Mol Cell Biol. 1999;19:7420-7427. [PubMed] |
| 36. | Troussard AA, Costello P, Yoganathan TN, Kumagai S, Roskelley CD, Dedhar S. The integrin linked kinase (ILK) induces an invasive phenotype via AP-1 transcription factor-dependent upregulation of matrix metalloproteinase 9 (MMP-9). Oncogene. 2000;19:5444-5452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 151] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 37. | Matsumura S, Oue N, Nakayama H, Kitadai Y, Yoshida K, Yamaguchi Y, Imai K, Nakachi K, Matsusaki K, Chayama K. A single nucleotide polymorphism in the MMP-9 promoter affects tumor progression and invasive phenotype of gastric cancer. J Cancer Res Clin Oncol. 2005;131:19-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 82] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 38. | Wu CY, Wu MS, Chiang EP, Chen YJ, Chen CJ, Chi NH, Shih YT, Chen GH, Lin JT. Plasma matrix metalloproteinase-9 level is better than serum matrix metalloproteinase-9 level to predict gastric cancer evolution. Clin Cancer Res. 2007;13:2054-2060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 70] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 39. | Harris AL. Hypoxia--a key regulatory factor in tumour growth. Nat Rev Cancer. 2002;2:38-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3808] [Cited by in RCA: 3950] [Article Influence: 171.7] [Reference Citation Analysis (0)] |
| 40. | Forooghian F, Das B. Anti-angiogenic effects of ribonucleic acid interference targeting vascular endothelial growth factor and hypoxia-inducible factor-1alpha. Am J Ophthalmol. 2007;144:761-768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 2.2] [Reference Citation Analysis (0)] |