Published online Jan 21, 2011. doi: 10.3748/wjg.v17.i3.354
Revised: June 16, 2010
Accepted: June 23, 2010
Published online: January 21, 2011
AIM: To examine the effect of increasing dietary zinc (Zn) intake and the lack of metallothionein (MT) expression on activity of small intestinal disaccharidases.
METHODS: MT-I and II knockout (MT-/-) and wild-type (MT+/+) female mice at 3.5 wk of age were randomly fed with a diet containing 2 (2 Zn), 15 (15 Zn) or 50 (50 Zn) mg Zn/kg (n = 8/group/genotype) for 5 wk. Small intestinal segments (duodenum, jejunum and ileum) were collected and either fixed in 10% formalin for histological analysis or snap frozen in liquid nitrogen for sucrase, lactase and maltase activity analyses.
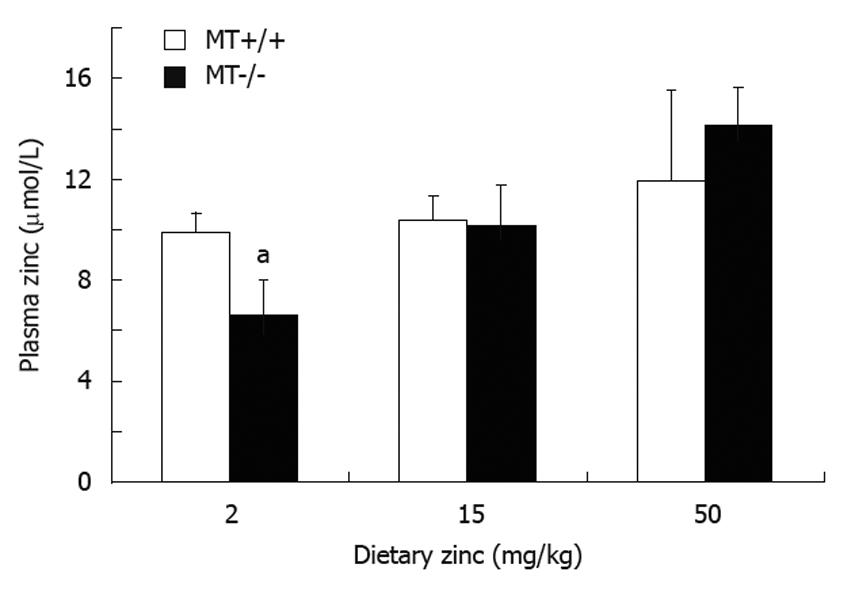
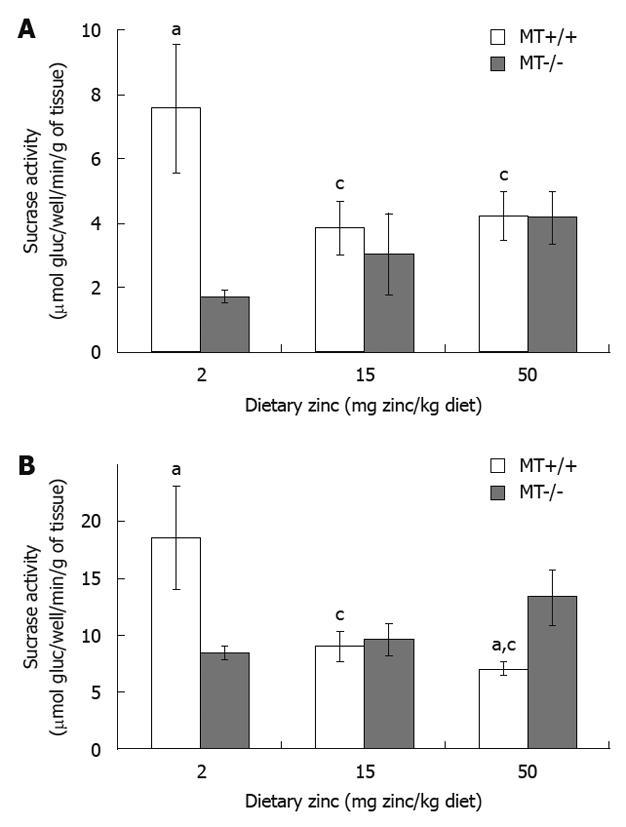
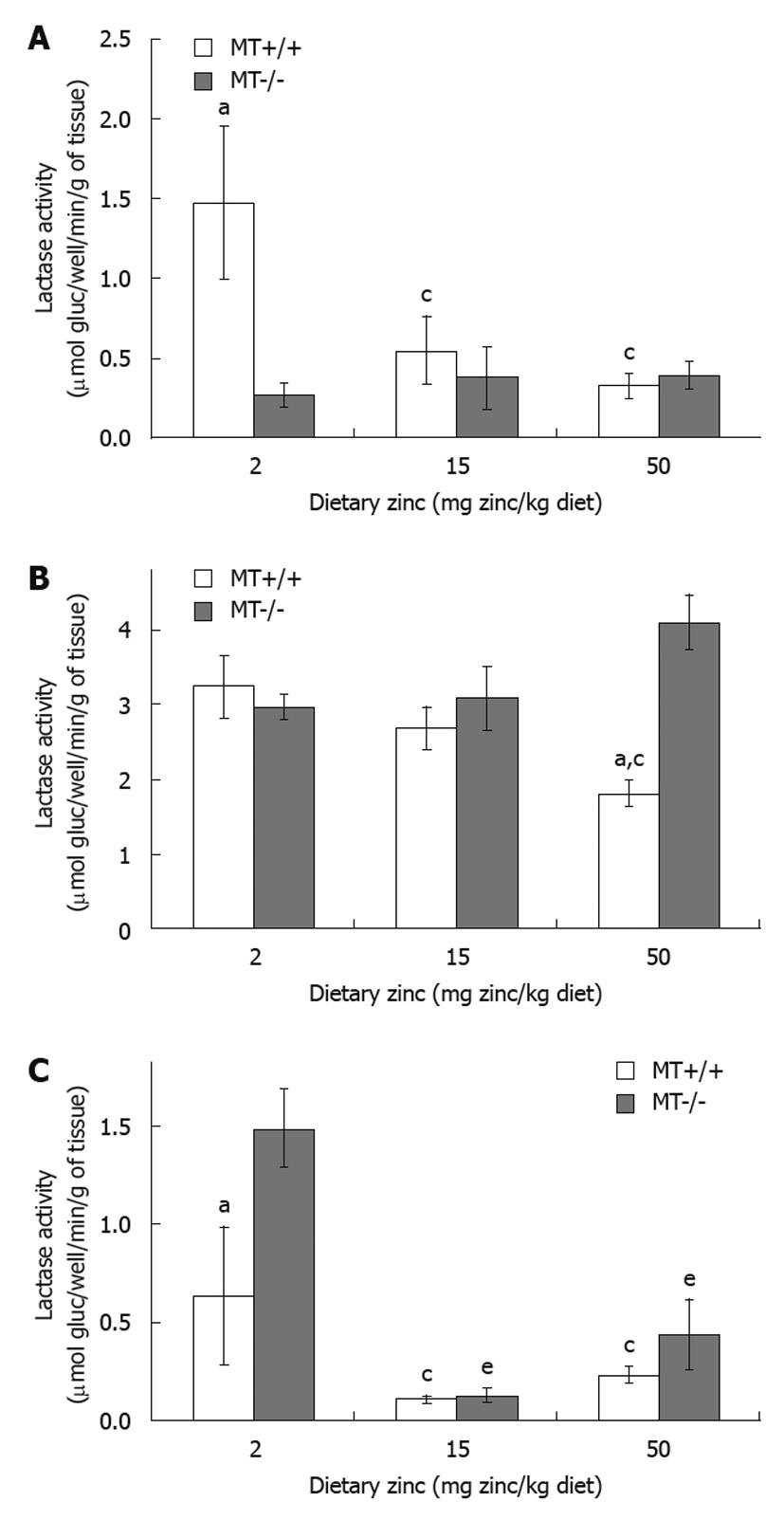
RESULTS: Plasma Zn was significantly (P < 0.05) lower (33%) in MT-/- compared with MT+/+ mice fed the 2 Zn diet. Villus height and crypt depth were increased by approximately 15% in MT+/+ mice compared with MT-/- mice. Duodenal disaccharidase activities were significantly higher in MT+/+ compared with MT-/- mice particularly in those fed the 2 Zn diet. For the 50 Zn diet, jejunal sucrase and lactase activities were significantly higher in MT-/- (13 313 ± 2314; 4107 ± 364 μmol glucose/well/min/g tissue, respectively) compared with MT+/+ mice (7054 ± 608; 1818 ± 174). Similarly, ileal lactase activities were higher in MT-/- (1480 ± 192) compared with MT+/+ (629 ± 353) mice particularly those fed the 2 Zn diet.
CONCLUSION: Increasing dietary Zn has little effect on disaccharidases activity in MT wild-type mice. The presence of MT may enhance morphological and functional development of the gut.
- Citation: Tran CD, Cool J, Xian CJ. Dietary zinc and metallothionein on small intestinal disaccharidases activity in mice. World J Gastroenterol 2011; 17(3): 354-360
- URL: https://www.wjgnet.com/1007-9327/full/v17/i3/354.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i3.354
Gastrointestinal function is characterized by growth, structural and functional changes of the crypts and villi, macromolecular absorption capacity and alteration in the activity of the small intestinal brush-border disaccharidases[1]. It is well documented that weaning is associated with marked changes in the histology and biochemistry of the small intestine, and such changes include increased cell proliferation and differentiation[2] and altered activity of the brush border disaccharidases, lactase and sucrase[1]. These changes are often used as indicators of small intestinal maturity and development following the consumption of a solid diet.
The brush border disaccharidases, lactase and sucrase, are considered accurate markers of enterocyte maturity and functional capacity[3]. It is well documented that dietary modification is correlated with significant changes in the histology and biochemistry of the small intestine[4]. Furthermore, pigs fed an inorganic zinc (Zn) diet and weaned pigs from sows fed an inorganic Zn diet compared with those fed the control diet had improved gut morphology[5,6]. In addition, Zn deficiency has been shown to reduce villous dimensions and increase villous density in the small intestine; however after a short period of Zn supplementation, villous density basal width and the maximum height of individual villi returned to normal[7]. Duff and Ettarh[8] demonstrated that Zn-fed mice produced more crypt cells in the distal part of small intestine compared with controls. Furthermore, it has been shown that weanling rats fed a semi-purified Zn-deficient diet have significantly lower levels of sucrase, maltase, lactase, leucine aminopeptidase, and alkaline phosphatase compared with rats fed a control or high Zn diet[9,10].
Zn is an essential nutrient required for cell growth, differentiation, and survival, and its deficiency causes growth retardation, immunodeficiency, and other health problems[11]. Therefore, Zn homeostasis must be tightly controlled in individual cells. The transcellular uptake of Zn occurs in the distal duodenum and proximal jejunum[12-15] from the brush border membrane. The mechanisms of exogenous zinc uptake have not yet been entirely elucidated, although both saturable and nonsaturable processes are involved[16]. Zn has been shown to be a potent inducer of its endogenous binding protein, metallothionein (MT)[17,18], a low molecular weight, intracellular cysteine-rich, metal-binding protein that consists of 4 isoforms (MT-1 to MT-4), with MT-1 and MT-2 being the most widely expressed isoforms[19]. MT is found mainly in the liver, kidneys, intestine and pancreas[17]. MT synthesis is induced by a number of metals, cytokines and stress hormones as well as by a wide range of chemicals, many of which act indirectly via a stress or inflammatory response[20,21].
Metal regulation of MT genes has been covered in several recent reviews[22,23]. Briefly, the binding of Zn to the metal transcription factor-1 allows the protein to bind to metal response elements in the promoter region which, in turn, initiates MT-gene transcription. Functions of MT include protection from cell apoptosis, promotion of cell proliferation and differentiation, regulation of Zn pools in circulation and in cells, scavenging of free radicals and protection against toxicity of heavy metals[17-19]. We hypothesized that an increase in dietary Zn and a lack of MT-1 and MT-2 expression will alter small intestinal disaccharidases activity. Thus the aims of the present study were to investigate the effects of various concentrations of dietary Zn intake (2, 15, or 50 mg Zn/kg diet) and the lack of MT-1 and MT-2 expression on small intestinal morphology and activity of disaccharidases in mice. This is applicable to the young infant starting complementary feeding whether dietary Zn may aid in nutrient absorption.
Twenty four MT wild-type (MT+/+) C57BL/6 mice were obtained from the University of Adelaide (Adelaide, South Australia) and 24 MT-1 and 2 null (MT-/-) mice were obtained from a breeding colony at the Children, Youth and Women’s Health Service Animal Care Facility (North Adelaide, South Australia). MT-/- (mixed genetic background of OLA129 and C57BL6 strains) mice were F3 derivatives of the interbreeding of normal C57BL6 mice[24].
At 3 wk old, mice were randomly allocated to be fed with either a 2, 15 or 50 mg Zn/kg diet (2 Zn, 15 Zn and 50 Zn) for 5 wk (n = 8/group/genotype) and body weight was recorded weekly. Mice were fed a casein-based diet[25] supplemented with ZnSO4 to 2, 15 or 50 mg Zn/kg. The casein-based diet contained (g/kg): cornflour starch, 514; casein, 180; sucrose, 152; wheat bran, 50; peanut oil, 50; D,L-methionine, 2.5; choline chloride, 1 and codliver oil, 4.4. The mineral profile (g/kg diet) was: KH2PO4, 17.155; CaCO3 14.645; NaCl, 12.530; MgSO4・7H2O, 4.99; FeC6H5O7・5H2O, 0.296; CaPO4, 0.170; MnSO4・4H2O, 0.080; CuSO4, 0.123; KI, 0.00025; (NH4)6Mo7・O24・4H2O, 0.00125; Na2SeO3, 0.00005. The vitamin profile (mg/kg diet) was: thiamine HCl, 70; riboflavin, 30; niacin (nicotinic acid), 50; pantothenic acid, 150; pyridoxal HCl, 15; hydroxycobalamin, 0.02; inositol, 400; ρ-aminobenzoic acid, 50; folic acid, 10; biotin, 0.4 and glucose, 225. For the purpose of this study we chose the 15 mg Zn/kg diet as the normal (control) diet and the 2 and 50 mg Zn/kg diet as the low and high Zn diet respectively. The Zn diets were given to the animals ad libitum and the Zn content of the diets was validated by atomic absorption spectrophotometry using a Perkin-Elmer 3030 (Überlingen, Germany).
At the end of the experimental period, all mice were CO2 asphyxiated and blood was withdrawn by cardiac puncture. The mice were then killed by cervical dislocation. The liver and gut were excised and the pancreas and mesentery removed. The gut was separated into stomach, small intestine, cecum and colon. The contents of the small intestine were flushed thoroughly with saline. The small intestine was then divided into the duodenum, from the gastro-duodenal junction to the ligament of Treitz, and into two segments of equal length comprising the jejunum, and ileum. A 4 cm segment of the duodenum, jejunum and ileum were excised for histological assessment and disaccharidase activity analysis, respectively. The protocol adhered to the Australian Code of Practice for the Care and Use of Animals for Scientific Purposes and approval was obtained from the Animal Care and Ethics Committee of the Women’s and Children’s Hospital (South Australia).
Blood samples were placed in lithium heparin tubes to obtain plasma, which was stored at -20°C until analysis. Plasma samples were diluted (1:2) in 10% trichloroacetic acid (Sigma-Aldrich, Sydney Australia) and were analyzed for Zn concentration (μmol/L) by atomic absorption spectrophotometry using a Perkin-Elmer 3030 (Perkin-Elmer Pty Ltd, Uberlingen, Germany).
Small intestinal segments of 2 cm were removed and fixed in 10% formalin overnight, processed and embedded in paraffin wax (cross section), from which 4 μm-thick small intestinal sections were cut using the RM2235 microtome (Leica, Germany) and mounted onto glass slides. Sections were dewaxed and stained with Lilie-Mayer’s hematoxylin and eosin and coverslipped. On each section, approximately 40 crypt depths and villi heights (expressed as μm) were measured using the Eclipse50i light microscope (Nikon, Japan) and Image ProPlus 5.0 package (Media Cybernetics, USA).
Disaccharidase activities in intestinal segments were assessed using a microplate modification of the Dahlqvist[26] assay in duplicates. Gut segments were homogenized in 10 mmol/L phosphate-buffered saline by Ultra-TurraxT25 homogenizer (Janke and Kunskel, Germany). Homogenates were centrifuged at 3500 rpm at 4°C for 10 min. The supernatant was then aliquoted and snap-frozen at -80°C for subsequent sucrase, maltase and lactase activity assays. Glucose standards (0, 1.25, 2.5, 5, 10, 20, 30 and 40 nmol/L) were prepared. Samples were diluted 1:20, 1:50 and 1:100 with 50 mmol/L phosphate buffer and then pipetted onto a 96-well plate. A solution of 0.2 mol/L sucrose, lactose or 0.004 mol/L maltose was added (50 μL/well) and the plate incubated at 37°C for 30 min. Tris-glucose oxidase was added to all wells and incubated at 37°C for another 30 min. Absorbance was determined using a Tecan spectrophotometer (Sunrise, Austria) set at 490 nm wavelength. Disaccharidase activities were analyzed using TableCurve (Systat Software, USA) and results were expressed as μmol glucose/well/min/g of tissue.
Histological analysis data were not normally distributed therefore data were log transformed and are presented as geometric mean ± SE of the mean. Disaccharidase activity data are expressed as mean ± SE of the mean. All data were analyzed using two-way analysis of variance followed by Tukey’s post-hoc test (SigmaStats3.0). The significance level was determined as P < 0.05.
There were no differences in body weights of both MT+/+ and MT-/- mice between the dietary Zn groups (data not shown). There were no differences in plasma Zn levels between MT+/+ and MT-/- mice when fed the 15 Zn or 50 Zn diet. However, there was a significant (P < 0.05) decrease (33%) in plasma Zn in MT-/- mice fed the 2 Zn diet compared with MT+/+ counterparts (Figure 1). There was an apparent trend of an increase in plasma Zn levels as the dietary Zn levels increased in MT-/- mice. Whereas in MT+/+ mice this was more tightly controlled with no difference in plasma Zn with increasing dietary Zn.
An increase in dietary Zn did not change small intestinal weight between MT+/+ and MT-/- mice. However, there was a significant (P < 0.05) difference in small intestinal weight in MT-/- mice fed 2 Zn (0.74 ± 0.04 g) and 50 Zn (0.70 ± 0.03 g) compared with those fed the 15 Zn (0.59 ± 0.02 g). Interestingly, MT-/- mice had a significantly (P < 0.05) longer small intestine compared with MT+/+ mice, in particular in mice with the 2 Zn (29.8 ± 0.8 cm vs 25.7 ± 0.9 cm, respectively) and 50 Zn diets (29.1 ± 0.6 cm vs 26.8 ± 0.6 cm, respectively).
Histological analysis (Tables 1 and 2) of the small intestine in MT+/+ and MT-/- mice showed that increasing dietary Zn concentrations did not alter villus height and crypt depth (P > 0.05). Differences in ileal villus height were insignificant irrespective of genotypes and dietary groups (Table 1). However, shorter villi were observed in the duodenum and jejunum of MT-/- mice fed with 15 Zn and/or 50 Zn diet(s) compared with MT+/+ mice (Table 1). Crypt depth was also significantly shorter in the duodenum (all dietary groups), jejunum (15 Zn and 50 Zn) and ileum (2 Zn) in MT-/- mice (P < 0.05) (Table 2).
| Intestinal segments | Dietary Zn intake (mg/kg) | Genotype | |
| MT+/+ | MT-/- | ||
| Duodenum | 2 | 0.099 ± 0.006a | 0.069 ± 0.004 |
| 15 | 0.182 ± 0.014a | 0.069 ± 0.005 | |
| 50 | 0.104 ± 0.004a | 0.085 ± 0.009 | |
| Jejunum | 2 | 0.086 ± 0.004 | 0.080 ± 0.003 |
| 15 | 0.084 ± 0.003a | 0.066 ± 0.004 | |
| 50 | 0.093 ± 0.003a | 0.077 ± 0.003 | |
| Ileum | 2 | 0.091 ± 0.004a | 0.077 ± 0.005 |
| 15 | 0.087 ± 0.006 | 0.075 ± 0.004 | |
| 50 | 0.085 ± 0.004 | 0.075 ± 0.004 | |
MT-/- mice fed the 2 Zn diet had a significantly reduced duodenal and jejunal sucrase activity compared with MT+/+ mice (Figure 2). Interestingly, sucrase levels were significantly lowered in the jejunum of MT+/+ mice receiving 15 Zn and 50 Zn (by 12%) compared with 2 Zn (Figure 2B). MT+/+ mice fed with 50 Zn also showed a lower activity in the jejunum compared with MT-/- mice. Results in both figures showed a trend of a decrease in sucrase activity in MT+/+ mice and an increase in MT-/- mice as dietary Zn concentration increased (P < 0.05). No significant differences were obtained for the ileum regardless of dietary Zn and genotypes (P > 0.05).
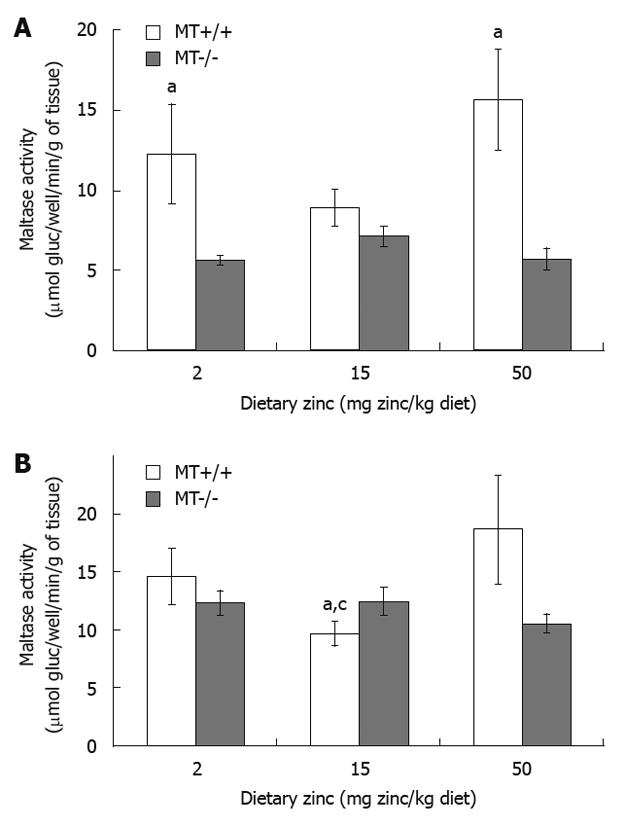
Duodenal maltase activity of MT+/+ mice fed with 2 Zn and 50 Zn was 37% and 47% higher, respectively, than that of MT-/- mice receiving same levels of dietary Zn (Figure 3A). When dietary Zn intake was increased from 15 Zn to 50 Zn, MT+/+ mice exhibited a marked increase in jejunal maltase activity (Figure 3B). Significant differences were also observed between MT+/+ and MT-/- mice receiving 15 Zn (P < 0.05). No differences were seen in ileal maltase activity regardless of dietary Zn and genotypes (P > 0.05).
In MT+/+ mice, there was a trend of a decrease in lactase activity when the dietary Zn levels increased in different regions of the small intestine (Figure 4A-C). For example, MT+/+ mice receiving 50 Zn showed 28% reduction (P < 0.05) in jejunal lactase activity compared with the 2 Zn group (Figure 4B). Interestingly, in MT-/- mice, while the increase in dietary Zn levels did not appear to alter the lactase activity in the duodenum (Figure 4A) and in the jejunum (Figure 4B), the ileal activity was significantly lower in the 15 Zn and 50 Zn groups by 84% and 55%, respectively, compared with MT-/- mice fed with 2 Zn (Figure 4C). Genotype comparisons showed that, in MT-/- mice, the lactase activity in the duodenum was significantly lower when fed the low Zn diet (2 Zn, Figure 4A), but was significantly higher (P < 0.05) in the jejunum when fed the high Zn diet (50 Zn, Figure 4B) and in the ileum when fed the low Zn diet (2 Zn, Figure 4C), compared with their MT+/+ counterparts.
The development profile of disaccharidase activity in rodents seems to be well correlated with the transition from milk to solid food consumption[1]. Although it has been reported that dietary modification is associated with altered activity of the brush border disaccharidases[6,8,27], the influence of dietary Zn levels and the interaction of dietary Zn with its endogenous binding protein MT on small intestinal maturation has been unclear. Thus, in the present study, we examined the effect of increasing dietary Zn concentration and the role of MT-1 and 2 gene knockout on morphological changes and disaccharidase levels in weanling mice. To our knowledge, this is the first study to assess Zn supplementation on small intestinal morphology in wild-type and MT-1 and 2 null mice.
The measurements of villus height and crypt depth provide an indication of the maturity and functional capacity of small intestinal enterocytes. In the present study, although we have shown that increasing dietary Zn intake did not appear to significantly increase growth of the small intestine morphologically, as demonstrated in the histological analysis of villus height and crypt depth, the MT+/+ mice fed the high Zn diet had a higher villus height in most of the regions of the small bowel. This is consistent with a previous study[6], showing villus height in the jejunum of sows fed Zn was greater than those fed the control diet. Similar trends were observed in other studies showing that, although not significant, pigs supplemented with Zn tend to have an increased villus height:crypt depth ratio in the jejunum and higher goblet cell counts in the ileum[5,28]. It has been speculated that the differences in the intestinal morphology of pigs are being masked because of deterioration in the mucosa immediately after weaning, a common phenomenon in weaning pigs. However, growth performance was improved by the addition of ZnO which responded linearly to incremental doses of Zn[28]. In our current study, we further showed that the presence of MT markedly increases villus height and crypt depth in the small intestine, in particular in mice fed a high dietary Zn, and that crypt depth is generally greater in the wild-type mice than the null mice, suggesting the importance of MT-I and II in supporting optimal intestinal growth in response to dietary Zn supply.
Considering the role of Zn as a coenzyme for more than 300 enzymes particularly in RNA-DNA synthesis and cell proliferation[11], these improvements in intestinal morphology may be explained by the beneficial effects of Zn on cell proliferation, differentiation, and protein synthesis[29]. This is supported by the trend of increase in plasma Zn with increasing dietary Zn in the present study and is consistent with our previous study[30]. In contrast, Zn deficiency has been shown to cause villus atrophy, elevated levels of mucosal cell apoptosis, ulceration, inflammation as well as reduction in crypts proliferation[8]. Furthermore, it has been reported that Zn deficiency may impair carbohydrate digestion, as reflected in decreased disaccharidase activities, and may contribute to poor nutrition[10]. The results of these findings suggest that dietary addition of Zn in the normal diet of mice is vital for small intestinal mucosal integrity as well as improved small intestinal morphology.
In general, intestinal disaccharidase activities are lower in MT-/- compared with MT+/+ mice particularly at the low dietary Zn concentration (except for jejunal sucrase and lactase activity in the ileum), indicating that the combination of low dietary Zn and the lack of MT expression have a negative impact on the activity of disaccharidases in the small intestine. MT is a binding protein that regulates the quantity of Zn absorbed by binding Zn within the mucosal cells, thereby regulating its transfer across the basolateral membrane into the circulation and deposition in the liver[17,31]. We have shown that MT-/- mice accumulate less Zn[25], whereas transgenic mice accumulate more[19]. This is consistent with our data where MT-/- mice have lowered plasma Zn compared with MT+/+ mice, in particular with low dietary Zn intake, suggesting that the presence of MT-I and II plays a role in maintaining Zn homeostasis at low dietary Zn intake. It is possible that the low Zn status may be a contributing factor to decreased levels of disaccharidase activities in MT-/- mice. This is consistent with other studies[7,9,10,32] which reported that Zn deficiency causes marked reductions in intestinal mucosal protein content and disaccharidase activity. Furthermore, MT has been shown to have metallo-regulatory functions in cellular growth and differentiation[33], and the lack of MT-I and MT-II expression may also be another contributing factor to the lower levels of disaccharidase activities in MT-/- mice.
Interestingly, increasing dietary Zn in the diet did not have a significant increase on intestinal disaccharidase activity in the wild-type mice. This is consistent with previous studies[7,34] where Zn supplementation has little or no effect on brush border disaccharidases. We have shown that increasing the dietary Zn concentration to 50 mg Zn/kg diet increases maltase activity only. It is possible that the highest dietary Zn concentration in the present study may be too low to induce disaccharidase activity.
MT has long been implicated in the regulation of absorption and excretion of Zn by the intestine[31,35] and the presence of MT has been shown to restrict Zn absorption at high Zn concentrations[12]. It has been argued that MT limits Zn absorption by sequestering it in the intestinal wall, thereby transiently reducing its absorption and favoring Zn transfer back into the gut lumen[12,14]. Furthermore, it has been shown that at high Zn concentration, MT-/- mice absorb Zn more readily and retain more Zn in the intestinal wall compared to MT-transgenic mice[36]. The high Zn intakes may explain why MT-/- mice have significantly greater jejunal sucrase and lactase activities than MT+/+ mice by inducing small intestinal disaccharidase activities. These adaptive mechanisms play a central role in nutrient processing and absorption which maintain normal homeostasis in these mice. This is also consistent with MT-/- mice having a heavier and longer small intestine, an adaptive mechanism to absorb more Zn to maintain Zn homeostasis when dietary Zn is high. These changes in intestinal disaccharidase activity in response to the interaction of dietary Zn and endogenous MT remain to be investigated. It is possible that intestinal disaccharidase activity and MT expression may not be related. To our knowledge this is the first study to report intestinal brush border enzyme activities in MT-/- mice. Thus, the role of MT in the expression of disaccharidase activities, particularly in MT-/- mice, warrants further investigation.
In conclusion, limiting the level of dietary Zn in the diet does not affect the activity of small intestinal brush border disaccharidases in wild-type mice. However, the presence of MT enhances the morphological and functional development of the gastrointestinal tract. Thus, simple Zn supplementation may be insufficient to drive growth and development of the small intestine. In light of the present findings, future studies investigating the close interaction between Zn and MT on gut maturation and development are warranted.
The development of brush border enzymes and gut maturation is associated with complementary feeding. However, it is unclear whether the activity of the brush border disaccharidases is influenced by dietary zinc levels and the presence of the endogenous binding protein metallothionein.
The role of metallothionein on gut maturation and development, in particular, in small intestinal morphology and brush border enzymes is an important area of research in the future.
The present study demonstrated that the presence of metallothionien, a zinc binding protein, may have a positive impact on villous morphology and digestive and absorptive function of the small intestine.
Future studies investigating the role of different levels of metallothionein in the gut on small intestinal morphology and the activities of brush border enzymes which may elucidate the underlying mechanism of metallothionein in altering intestinal physiology, villous architecture, and enzyme activities.
Disaccharidases - enzymes located on the brush border and which are essential for digestion and absorption.
Tran et al report on a basic research study in metallothionein wild-type and knockout mice testing the influence of different amounts of alimentary zinc on small intestinal disaccharidases. The paper is well written and the point is clearly made.
Peer reviewers: Dr. Stefan Wirth, Professor, Children’s Hospital, Heusnerstt, 40, Wuppertal 42349, Germany; Dr. Devinder Kumar Dhawan, Professor, Department of Biophysics and Coordinator, Nuclear Medicine, Panjab University, Chandigarh 160014, India
S- Editor Tian L L- Editor Cant MR E- Editor Lin YP
| 1. | Sabat P, Veloso C. Ontogenic development of intestinal disaccharidases in the precocial rodent Octodon degus (Octodontidae). Comp Biochem Physiol A Mol Integr Physiol. 2003;134:393-397. |
| 2. | Lin CH, Correia L, Tolia K, Gesell MS, Tolia V, Lee PC, Luk GD. Early weaning induces jejunal ornithine decarboxylase and cell proliferation in neonatal rats. J Nutr. 1998;128:1636-1642. |
| 3. | Pluske JR, Thompson MJ, Atwood CS, Bird PH, Williams IH, Hartmann PE. Maintenance of villus height and crypt depth, and enhancement of disaccharide digestion and monosaccharide absorption, in piglets fed on cows' whole milk after weaning. Br J Nutr. 1996;76:409-422. |
| 4. | Henning SJ. Biochemistry of intestinal development. Environ Health Perspect. 1979;33:9-16. |
| 5. | Caine WR, Metzler-Zebeli BU, McFall M, Miller B, Ward TL, Kirkwood RN, Mosenthin R. Supplementation of diets for gestating sows with zinc amino acid complex and gastric intubation of suckling pigs with zinc-methionine on mineral status, intestinal morphology and bacterial translocation in lipopolysaccharide-challenged early-weaned pigs. Res Vet Sci. 2009;86:453-462. |
| 6. | Payne RL, Bidner TD, Fakler TM, Southern LL. Growth and intestinal morphology of pigs from sows fed two zinc sources during gestation and lactation. J Anim Sci. 2006;84:2141-2149. |
| 7. | Southon S, Gee JM, Bayliss CE, Wyatt GM, Horn N, Johnson IT. Intestinal microflora, morphology and enzyme activity in zinc-deficient and Zn-supplemented rats. Br J Nutr. 1986;55:603-611. |
| 8. | Duff M, Ettarh RR. Crypt cell production rate in the small intestine of the zinc-supplemented mouse. Cells Tissues Organs. 2002;172:21-28. |
| 9. | Park JH, Grandjean CJ, Antonson DL, Vanderhoof JA. Effects of short-term isolated zinc deficiency on intestinal growth and activities of several brush border enzymes in weaning rats. Pediatr Res. 1985;19:1333-1336. |
| 10. | Gebhard RL, Karouani R, Prigge WF, McClain CJ. The effect of severe zinc deficiency on activity of intestinal disaccharidases and 3-hydroxy-3-methylglutaryl coenzyme A reductase in the rat. J Nutr. 1983;113:855-859. |
| 11. | Frassinetti S, Bronzetti G, Caltavuturo L, Cini M, Croce CD. The role of zinc in life: a review. J Environ Pathol Toxicol Oncol. 2006;25:597-610. |
| 12. | Cousins RJ. Absorption, transport, and hepatic metabolism of copper and zinc: special reference to metallothionein and ceruloplasmin. Physiol Rev. 1985;65:238-309. |
| 13. | Weigand E. Absorption of trace elements: zinc. Int J Vitam Nutr Res Suppl. 1983;25:67-81. |
| 14. | Lonnerdal B. Intestinal absorption of Zn. Zn in human biology. Berlin: Springer-Verlag 1989; 33-55. |
| 16. | Krebs NF. Overview of zinc absorption and excretion in the human gastrointestinal tract. J Nutr. 2000;130:1374S-1377S. |
| 17. | Coyle P, Philcox JC, Carey LC, Rofe AM. Metallothionein: the multipurpose protein. Cell Mol Life Sci. 2002;59:627-647. |
| 18. | Kelly EJ, Quaife CJ, Froelick GJ, Palmiter RD. Metallothionein I and II protect against zinc deficiency and zinc toxicity in mice. J Nutr. 1996;126:1782-1790. |
| 19. | Davis SR, Cousins RJ. Metallothionein expression in animals: a physiological perspective on function. J Nutr. 2000;130:1085-1088. |
| 20. | Hamer DH. Metallothionein. Annu Rev Biochem. 1986;55:913-951. |
| 21. | Bremner I. Nutritional and physiological significance of metallothionein. Experientia Suppl. 1987;52:81-107. |
| 22. | Miles AT, Hawksworth GM, Beattie JH, Rodilla V. Induction, regulation, degradation, and biological significance of mammalian metallothioneins. Crit Rev Biochem Mol Biol. 2000;35:35-70. |
| 23. | Andrews GK. Regulation of metallothionein gene expression by oxidative stress and metal ions. Biochem Pharmacol. 2000;59:95-104. |
| 24. | Michalska AE, Choo KH. Targeting and germ-line transmission of a null mutation at the metallothionein I and II loci in mouse. Proc Natl Acad Sci USA. 1993;90:8088-8092. |
| 25. | Tran CD, Butler RN, Philcox JC, Rofe AM, Howarth GS, Coyle P. Regional distribution of metallothionein and zinc in the mouse gut: comparison with metallothionien-null mice. Biol Trace Elem Res. 1998;63:239-251. |
| 26. | Dahlqvist A. Method for assay of intestinal disaccharidases. Anal Biochem. 1964;7:18-25. |
| 27. | Hedemann MS, Jensen BB, Poulsen HD. Influence of dietary zinc and copper on digestive enzyme activity and intestinal morphology in weaned pigs. J Anim Sci. 2006;84:3310-3320. |
| 28. | Mavromichalis I, Peter CM, Parr TM, Ganessunker D, Baker DH. Growth-promoting efficacy in young pigs of two sources of zinc oxide having either a high or a low bioavailability of zinc. J Anim Sci. 2000;78:2896-2902. |
| 29. | Tran CD. Advances of Zinc in Health Research. Micronutrient and Health Research. New York: Nova Science Publishers, Inc 2008; 23-70. |
| 30. | Fong L, Tan K, Tran C, Cool J, Scherer MA, Elovaris R, Coyle P, Foster BK, Rofe AM, Xian CJ. Interaction of dietary zinc and intracellular binding protein metallothionein in postnatal bone growth. Bone. 2009;44:1151-1162. |
| 31. | Richards MP, Cousins RJ. Mammalian zinc homeostasis: requirement for RNA and metallothionein synthesis. Biochem Biophys Res Commun. 1975;64:1215-1223. |
| 32. | Zarling EJ, Mobarhan S, Donahue PE. Does zinc deficiency affect intestinal protein content or disaccharidase activity? J Lab Clin Med. 1985;106:708-711. |
| 33. | Thirumoorthy N, Manisenthil Kumar KT, Shyam Sundar A, Panayappan L, Chatterjee M. Metallothionein: an overview. World J Gastroenterol. 2007;13:993-996. |
| 34. | Jones PE, Peters TJ. Oral zinc supplements in non-responsive coeliac syndrome: effect on jejunal morphology, enterocyte production, and brush border disaccharidase activities. Gut. 1981;22:194-198. |