Published online Jan 21, 2011. doi: 10.3748/wjg.v17.i3.343
Revised: August 27, 2010
Accepted: September 3, 2010
Published online: January 21, 2011
AIM: To assess the absolute number of T-regulatory cells (Tregs; CD4+CD25+Foxp3+) in the peripheral blood of gastric and colorectal cancer patients.
METHODS: We enrolled 70 cancer patients (33 gastric cancer, 37 colorectal cancer) and 17 healthy volunteers. The CD3+CD4+ lymphocytes and CD4+CD25+Foxp3+ Tregs in the peripheral blood were analyzed with flow cytometry. The absolute numbers of Tregs were calculated based on the CD4+CD25+Foxp3+ cells percentage of CD3+CD4+ cells and the absolute numbers of CD3+CD4+ cells per microliter.
RESULTS: The mean number of CD4+CD25+Foxp3+ cells per microliter in colorectal cancer patients was 15.7 (SD: 21.8), for gastric cancer patients 12.2 (SD: 14.3), and for controls 17.5 (SD: 11.4). The absolute number of Tregs was significantly lower in gastric cancer patients than in controls (P = 0.026). There was no statistically significant difference for gastric vs colorectal cancer or colorectal cancer vs controls. The absolute number of Tregs was also significantly depressed in N+vs N- cancer patients [22.0 (27.7) vs 10.1 (9.0), P = 0.013], and in the subgroup of gastric cancer patients [30.3 (27.6) vs 9.6 (8.0), P = 0.003]. No statistical difference was observed in the proportion of Tregs in the CD4+ population between the groups.
CONCLUSION: The absolute number of Tregs in peripheral blood of gastric cancer but not colorectal cancer patients was significantly decreased in comparison with that in healthy controls.
- Citation: Szczepanik AM, Siedlar M, Sierzega M, Goroszeniuk D, Bukowska-Strakova K, Czupryna A, Kulig J. T-regulatory lymphocytes in peripheral blood of gastric and colorectal cancer patients. World J Gastroenterol 2011; 17(3): 343-348
- URL: https://www.wjgnet.com/1007-9327/full/v17/i3/343.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i3.343
There is evidence that regulatory T lymphocytes Tregs might be important for immunotolerance to self- and allo-antigens[1]. Activity of these cells is one of the mechanisms of immune evasion by tumors, which inhibits the antitumor activity of effector cells. They also suppress the antigen-presenting function of dendritic cells and the activity of natural killer cells[2,3]. Tregs are the subset of CD4+ cells that express high levels of the interleukin-2 receptor α chain CD25. Therefore, Tregs are described as CD4+CD25++ cells. However, the value of CD25 as a specific marker is limited, because it is also expressed on activated CD4+ cells. Recent studies have shown that Foxp3, a member of the transcription factor family, represents a specific marker for Tregs[4]. However, Foxp3 is a nuclear protein and it is impossible to use it for isolation of cells. Many previous studies on Tregs in neoplastic diseases that were performed before the discovery of Foxp3 used CD4+CD25++ subpopulation as an equivalent to Tregs. Recently, flow cytometric detection of CD4+CD25+Foxp3+ in human cancer studies has been described[5]. The main outcome from studies in human cancer is the increase in the proportion of Tregs/CD4+ cells among tumor-infiltrating lymphocytes (TILs), metastatic lymph nodes and peripheral blood[6]. The increase in Tregs was in some studies a prognostic factor of poor survival. However, these data are not uniform for all types and locations of tumors[7-9].
The aim of our study was to assess the absolute number of Tregs (CD4+CD25+Foxp3+) in the peripheral blood of gastric and colorectal cancer patients.
The study consisted of 70 patients (33 gastric cancer and 37 colorectal cancer) treated in a single institution between 2006 and 2009. All these patients had histologically confirmed disease. The median age was 68 years (range: 32-82 years). There were 42 male and 28 female patients. All these patients underwent laparotomy. The details of clinicopathological characteristics are summarized in Table 1.
| No. of cases | |
| Sex | |
| Male | 42 |
| Female | 28 |
| Age (yr) | |
| ≤ 65 | 32 |
| > 65 | 38 |
| Tumor grade | |
| 1 | 9 |
| 2 or 3 | 61 |
| Lymph node metastases | |
| No | 24 |
| Yes | 46 |
| Distant metastases | |
| No | 41 |
| Yes | 29 |
| Stage (AJCC 2002) | |
| I | 13 |
| II | 9 |
| III | 13 |
| IV | 35 |
None of the patients received chemotherapy, radiotherapy, immunotherapy or other form of therapy that influenced the immune system. Patients had no history of autoimmune disease or recent infection.
The blood of 17 healthy volunteers was tested as controls. The control group consisted of 10 men and seven women with a mean age of 42 years (range: 25-52 years).
The study was approved by the Ethical Committee of Jagiellonian University.
Blood samples were collected prior to any interventional procedure in sterile EDTA vacutainers. Peripheral blood samples (100 μL) obtained from cancer patients were incubated in TruCount tubes (BD Biosciences, San Jose, CA, USA) with a monoclonal antibody cocktail: FITC-conjugated anti-CD3 and PE-conjugated anti-CD4 (5 μL; BD Biosciences) for 30 min at 4°C. The samples were treated with 400 μL FACS Lysing Solution (BD Biosciences), and after erythrocyte lysis, 10 000 CD3+CD4+ cells along with beads were acquired on a FACSCanto flow cytometer and analyzed with FACSDiva Software (BD Biosciences). The absolute numbers of CD3+CD4+ lymphocytes in samples were calculated on a basis of bead and lymphocyte counts. Tregs (CD4+CD25+Foxp3+) were stained in 200 μL EDTA peripheral blood samples using the Human Regulatory T Cell Staining Kit (eBiosciences, UK), according to manufacturer’s instructions, and acquired on the flow cytometer. The absolute numbers of Tregs were calculated based on the CD4+CD25+Foxp3+ cells percentage of CD3+CD4+ cells and the absolute numbers of CD3+CD4+ cells per microliter.
All quantitative variables were described as mean (SD). The Mann-Whitney U test and the χ2 test were used when appropriate to compare distribution of individual variables between groups. P < 0.05 was considered statistically significant. Statistical analysis was performed using SPSS version 14 software (SPSS Inc., Chicago, IL, USA).
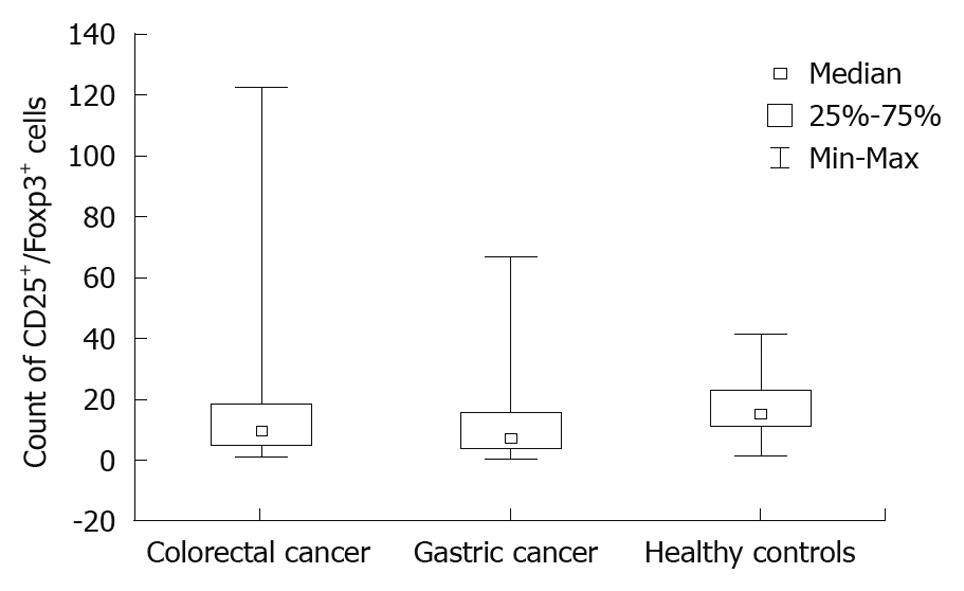
The mean number of CD4+CD25+Foxp3+ cells per microliter in colorectal cancer patients was 15.7 (21.8), for gastric cancer patients 12.2 (14.3) and for controls 17.5 (11.4) (Figure 1).
The difference between colorectal cancer patients and the control group was not significant (P = 0.079). There was a significant difference between the gastric cancer patients and the control group (P = 0.026). The difference between the gastric cancer and colorectal cancer patients was not significant.
The absolute number of CD4+CD25+Foxp3+ cells did not differ according to sex in either the colorectal or gastric cancer patients. Patients aged ≤ 65 years and > 65 years had similar results for both cancer types (Table 2). The analysis of TNM stage revealed that, for more advanced-stage cancer, Treg count was lower but the difference was not significant.
| Colorectal cancer | P1 | Gastric cancer | P1 | Overall | P1 | |
| Overall | 15.7 (21.8) | 12.2 (14.3) | 0.449 | |||
| Sex | 0.779 | 0.912 | 0.801 | |||
| Male | 16.9 (25.2) | 11.3 (11.9) | 14.4 (20.3) | |||
| Female | 13.5 (14.6) | 13.4 (17.5) | 13.5 (15.9) | |||
| Age (yr) | 0.849 | 0.080 | 0.309 | |||
| ≤ 65 | 18.7 (28.5) | 10.3 (16.9) | 15.0 (24.1) | |||
| > 65 | 12.7 (12.2) | 13.7 (12.4) | 13.2 (12.2) | |||
| Tumor grade | 0.501 | NA | 1.000 | 0.865 | ||
| 1 | 16.8 (15.5) | 14.9 (15.5) | ||||
| 2 or 3 | 15.9 (24.8) | 15.2 (21.9) | ||||
| Lymph node metastases | 0.210 | 0.003 | 0.013 | |||
| No | 19.9 (28.1) | 30.3 (27.6) | 22.0 (27.7) | |||
| Yes | 11.0 (10.4) | 9.6 (8.0) | 10.1 (9.0) | |||
| Distant metastases | 0.596 | 0.882 | 0.447 | |||
| No | 17.6 (24.6) | 15.8 (20.4) | 17.0 (23.1) | |||
| Yes | 10.0 (7.8) | 9.9 (8.3) | 9.9 (8.0) | |||
| Stage | 0.267 | 0.195 | 0.088 | |||
| I or II | 19.9 (28.9) | 33.4 (30.9) | 22.4 (29.0) | |||
| III or IV | 10.9 (10.4) | 9.4 (7.8) | 9.9 (8.9) |
In colorectal cancer patients, Treg absolute count was not related to tumor grade, lymph node status or distant metastases. There was also no difference between gastric cancer subgroups according to Lauren’s histological classification. In gastric cancer patients, the number of CD4+CD25+Foxp3+ cells was significantly lower in peripheral blood of N+ patients (P = 0.003). For the pooled group of patients (gastric and colorectal cancer), this difference was also significant (P = 0.013). There was no difference between M- an M+ gastric cancer patients for absolute number of Tregs, or in the entire group of cancer patients.
The CD4+CD25+Foxp3+/CD4+ lymphocyte ratio did not show any differences between N+ and N- and M+ and M- patients for both types of cancer. This proportion was not related to sex or age (Table 3). There was no difference between stages I/II and III/IV for either type of cancer, nor in the pooled group of cancer patients.
| Colorectal cancer | P1 | Gastric cancer | P1 | Overall | P1 | |
| Overall | 2.18 (1.79) | 2.01 (1.56) | 0.760 | |||
| Sex | 0.332 | 0.455 | 0.820 | |||
| Male | 2.31 (1.68) | 1.89 (1.62) | 2.12 (1.65) | |||
| Female | 1.98 (2.01) | 2.18 (1.51) | 2.08 (1.75) | |||
| Age (yr) | 0.456 | 0.610 | 0.777 | |||
| ≤ 65 | 2.32 (1.58) | 1.88 (1.63) | 2.13 (1.59) | |||
| > 65 | 2.05 (2.00) | 2.11 (1.54) | 2.08 (1.76) | |||
| Tumor grade | 0.477 | 1.000 | 0.758 | |||
| 1 | 2.46 (1.72) | 0 | 2.19 (1.80) | |||
| 2 or 3 | 2.11 (1.90) | 2.10 (1.36) | 2.09 (1.80) | |||
| Lymph node metastases | 0.316 | 0.388 | 0.172 | |||
| No | 2.60 (2.13) | 2.73 (2.04) | 2.63 (2.07) | |||
| Yes | 1.73 (1.25) | 1.94 (1.53) | 1.85 (1.41) | |||
| Distant metastases | 0.818 | 0.854 | 0.798 | |||
| No | 2.23 (1.98) | 1.95 (1.72) | 2.14 (1.88) | |||
| Yes | 2.03 (1.09) | 2.05 (1.49) | 2.04 (1.36) | |||
| Stage | 0.254 | 0.423 | 0.138 | |||
| I or II | 2.56 (2.22) | 2.75 (2.35) | 2.59 (2.18) | |||
| III or IV | 1.66 (1.33) | 1.66 (1.32) | 1.66 (1.31) |
The prevalence of Tregs in various compartments in patients with tumors has been described as a potential prognostic factor. Tregs have been found in TILs (primary and metastatic tumors), in metastatic lymph nodes, malignant ascites, pleural effusion, and peripheral blood[6,10]. The prognostic significance of these findings is not uniform. Moreover, the impact of Tregs has been reported as their density in tumor stroma, proportion of TILs, Tregs/CD4 ratio, Tregs/CD3 ratio, or Tregs/CD8 ratio[11-14]. Mostly, the proportion of CD25+Foxp3+ cells among CD4+ TILs has been analyzed.
Most of these studies were retrospective and based on the immunohistochemical examination of paraffin-embedded, previously collected specimens. On the other hand, peripheral blood is easily accessible and enables repeated measurements prior to surgery or in the follow-up period. Therefore, it is important to establish the pattern of Tregs in peripheral blood of patients with various tumors. However, the number of Tregs in peripheral blood changes less markedly than in TILs[11].
The present study was a pilot study, therefore, assessment of the number of cells was performed preoperatively only. The results of the assessment in the early postoperative period would probably be influenced by the pro-inflammatory and anti-inflammatory post-injury reactions. This might change the lymphocyte subpopulations. Our present results can form a background for subsequent studies on Tregs in the preoperative period and follow-up of cancer patients.
In our study, the absolute number of CD25+Foxp3+ cells in peripheral blood did not differ between gastric and colorectal cancer patients. There was no difference between sex and age groups. Some authors have described increased prevalence of Tregs among T-lymphocyte populations in elderly patients[15]. Others have not observed this tendency[16]. There is also no clear evidence in the literature that the Tregs population is sex-related.
The absolute number of Tregs was significantly lower in gastric cancer patients than in controls. This is probably in contrast with other studies in gastric cancer patients, however, it is impossible to compare these results directly. The main problem is the difference in description of Tregs. Some early studies have analyzed the population of CD4+CD25+ cells, others CD4+CD25high cells, and more recently, CD4+CD25+Foxp3+ lymphocytes[12,13,17-20]. Most probably this is not the last word in the identification of functionally active Tregs. Even if these populations have a common core, they are not identical. Moreover, the technical process of identification can vary and bias the final result.
In colorectal cancer patients, the absolute number of Tregs in peripheral blood did not differ from that in healthy controls. There are not sufficient data to compare this result with other studies, because the main colorectal cancer studies have concentrated on prevalence of Tregs among TILs[12,13,21]. The pattern of Tregs localization and its correlation with tumor stage and prognosis also differs between gastric and colorectal cancer. The high prevalence of Tregs in colorectal cancer has been reported as a positive factor, contrary to gastric cancer for which it has been reported as a negative factor[7,8,11,13,22]. The reason for these differences might also be related to the different results observed in gastric and colorectal cancer patients in our study. However, the regulatory mechanisms of Treg maturation, activation and distribution are not fully understood. Therefore, we could not clarify the observed differences between the results in gastric and colorectal cancer.
We found that N+ gastric cancer patients had significantly lower absolute counts of Tregs in peripheral blood than had N- patients.
Our study included 25 node-positive patients and this probably influenced the mean Treg count in the whole group. Several studies have revealed an increase in Tregs in metastatic lymph nodes in gastric and esophageal cancer[23]. Our study might have demonstrated Treg migration to the metastatic lymph nodes and accumulation in the peritumoral infiltrate in more advanced tumors. There is some evidence that, in gastric cancer, Tregs might migrate to the tumor microenvironment via a chemokine-mediated mechanism[24].
The increase in Tregs among TILs has also been observed in colorectal cancer patients[25], but we did not observe a significant drop in peripheral blood Tregs in node-positive colorectal cancer patients. However, for the pooled N+vs N- group, the difference was significant. The lack of difference between M- and M+ patients supports the hypothesis that the peripheral Tregs population does not change significantly during metastasis formation. The increase in CD4+CD25high to CD4+ cell ratio in comparison to that in healthy donors has been described in metastatic cancer[26]. However, the absolute numbers of Tregs in these populations have not been reported.
In our study, the number of patients was < 40 for each cancer type. These relatively small numbers preclude statistical analysis of Tregs counts at single TNM stages. Therefore, we performed our analysis on I/II vs III/IV stages. The absolute number of Tregs was lower in the more advanced group, but the difference did not reach statistical significance.
The proportion of Tregs to CD4+ cells was 2.18% for colorectal cancer and 2.01% for gastric cancer patients. This value is located between that reported in the literature of 4%-6% for gastrointestinal cancer and 1%-3% for the healthy population[27]. However, the calculated percentages of Tregs (of CD4 or of CD3) or proportions of Tregs/CD8 is influenced by at least two variables. It should be considered that subpopulations of lymphocytes can change during tumor progression[28]. Therefore, the ratio and absolute numbers should be included.
In conclusion, our study was focused on the peripheral blood Tregs as a potential marker of disease, which was relatively easy to measure during the pretreatment and follow-up periods. The absolute number of Tregs in the peripheral blood of gastric cancer patients was significantly decreased in comparison to that in the healthy controls. This phenomenon was even strongly expressed in patients with lymph node metastasis. This was not observed in colorectal cancer patients. Our findings suggest that the population of Tregs in peripheral blood does not simply mimic stromal Tregs. Further studies on larger groups of patients are necessary to evaluate the Treg population in cancer patients.
The prevalence of T regulatory lymphocytes (Tregs) in various cancers has been described as a prognostic factor. The assessment of the absolute number of Tregs (CD4+CD25+Foxp3+) in the peripheral blood of gastric and colorectal cancer patients can be used to monitor disease during treatment.
Our study was focused on peripheral blood Tregs as a potential disease marker, which was relatively easy to measure during pretreatment and follow-up periods. The prevalence of Tregs in various compartments in patients with tumors has been described as a potential prognostic factor. Tregs have been found in tumor-infiltrated tissues and fluids, but the prognostic significance of these findings is not uniform. The impact of Tregs has been reported as their density in tumor stroma or the proportion of Tregs among tumor-inflitrating lymphocytes (TILs), Tregs/CD4 ratio, Tregs/CD3 ratio, or Tregs/CD8 ratio. However, overall, the proportion of CD25+Foxp3+ cells amongst CD4+ TILs is analyzed.
Many studies of Tregs as a prognostic factor have been retrospective and based on immunohistochemical examination of paraffin-embedded, previously collected specimens. On the other hand, peripheral blood is easily accessible and enables repeated measurements prior to surgery or in the follow-up period. Therefore, it is important to establish the pattern of Tregs in peripheral blood of patients with various tumors.
In utilizing peripheral blood Tregs as a potential disease marker, this study demonstrates a way of improving the assessment and management of patients with gastric cancer.
The authors assessed the absolute number of Tregs (CD4+CD25+Foxp3+) in the peripheral blood of gastric and colorectal cancer patients. The absolute numbers of Tregs were calculated based on the CD4+CD25+Foxp3+ cells percentage of CD3+CD4+ cells and the absolute numbers of CD3+CD4+ cells per microliter. The absolute number of Tregs in the peripheral blood of gastric cancer patients was significantly decreased in comparison to the healthy controls. This phenomenon was even strongly expressed in patients with lymph node metastasis, but not observed in colorectal cancer patients. The findings suggest that the population of Tregs in peripheral blood does not simply mimic stromal Tregs. Further studies on larger groups of patients are necessary to evaluate the Treg population in the blood of cancer patients.
Peer reviewer: Ki-Baik Hahm, MD, PhD, Professor, Gachon Graduate School of Medicine, Department of Gastroenterology, Lee Gil Ya Cancer and Diabetes Institute, Lab of Translational Medicine, 7-45 Songdo-dong, Yeonsu-gu, Incheon, 406-840, South Korea
S- Editor Sun H L- Editor Kerr C E- Editor Zheng XM
| 1. | Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151-1164. |
| 2. | Ralainirina N, Poli A, Michel T, Poos L, Andrès E, Hentges F, Zimmer J. Control of NK cell functions by CD4+CD25+ regulatory T cells. J Leukoc Biol. 2007;81:144-153. |
| 3. | André S, Tough DF, Lacroix-Desmazes S, Kaveri SV, Bayry J. Surveillance of antigen-presenting cells by CD4+ CD25+ regulatory T cells in autoimmunity: immunopathogenesis and therapeutic implications. Am J Pathol. 2009;174:1575-1587. |
| 5. | Crellin NK, Garcia RV, Levings MK. Flow cytometry-based methods for studying signaling in human CD4+CD25+FOXP3+ T regulatory cells. J Immunol Methods. 2007;324:92-104. |
| 6. | Ha TY. The role of regulatory T cells in cancer. Immune Netw. 2009;9:209-235. |
| 7. | Ichihara F, Kono K, Takahashi A, Kawaida H, Sugai H, Fujii H. Increased populations of regulatory T cells in peripheral blood and tumor-infiltrating lymphocytes in patients with gastric and esophageal cancers. Clin Cancer Res. 2003;9:4404-4408. |
| 8. | Correale P, Rotundo MS, Del Vecchio MT, Remondo C, Migali C, Ginanneschi C, Tsang KY, Licchetta A, Mannucci S, Loiacono L. Regulatory (FoxP3+) T-cell tumor infiltration is a favorable prognostic factor in advanced colon cancer patients undergoing chemo or chemoimmunotherapy. J Immunother. 2010;33:435-441. |
| 9. | Heimberger AB, Abou-Ghazal M, Reina-Ortiz C, Yang DS, Sun W, Qiao W, Hiraoka N, Fuller GN. Incidence and prognostic impact of FoxP3+ regulatory T cells in human gliomas. Clin Cancer Res. 2008;14:5166-5172. |
| 10. | Haas M, Dimmler A, Hohenberger W, Grabenbauer GG, Niedobitek G, Distel LV. Stromal regulatory T-cells are associated with a favourable prognosis in gastric cancer of the cardia. BMC Gastroenterol. 2009;9:65. |
| 11. | Yuan XL, Chen L, Li MX, Dong P, Xue J, Wang J, Zhang TT, Wang XA, Zhang FM, Ge HL. Elevated expression of Foxp3 in tumor-infiltrating Treg cells suppresses T-cell proliferation and contributes to gastric cancer progression in a COX-2-dependent manner. Clin Immunol. 2010;134:277-288. |
| 12. | Suzuki H, Chikazawa N, Tasaka T, Wada J, Yamasaki A, Kitaura Y, Sozaki M, Tanaka M, Onishi H, Morisaki T. Intratumoral CD8(+) T/FOXP3 (+) cell ratio is a predictive marker for survival in patients with colorectal cancer. Cancer Immunol Immunother. 2010;59:653-661. |
| 13. | Frey DM, Droeser RA, Viehl CT, Zlobec I, Lugli A, Zingg U, Oertli D, Kettelhack C, Terracciano L, Tornillo L. High frequency of tumor-infiltrating FOXP3(+) regulatory T cells predicts improved survival in mismatch repair-proficient colorectal cancer patients. Int J Cancer. 2010;126:2635-2643. |
| 14. | Sinicrope FA, Rego RL, Ansell SM, Knutson KL, Foster NR, Sargent DJ. Intraepithelial effector (CD3+)/regulatory (FoxP3+) T-cell ratio predicts a clinical outcome of human colon carcinoma. Gastroenterology. 2009;137:1270-1279. |
| 15. | Rosenkranz D, Weyer S, Tolosa E, Gaenslen A, Berg D, Leyhe T, Gasser T, Stoltze L. Higher frequency of regulatory T cells in the elderly and increased suppressive activity in neurodegeneration. J Neuroimmunol. 2007;188:117-127. |
| 16. | Hwang KA, Kim HR, Kang I. Aging and human CD4(+) regulatory T cells. Mech Ageing Dev. 2009;130:509-517. |
| 17. | Ichihara F, Kono K, Takahashi A, Kawaida H, Sugai H, Fujii H. Increased populations of regulatory T cells in peripheral blood and tumor-infiltrating lymphocytes in patients with gastric and esophageal cancers. Clin Cancer Res. 2003;9:4404-4408. |
| 18. | Kono K, Kawaida H, Takahashi A, Sugai H, Mimura K, Miyagawa N, Omata H, Fujii H. CD4(+)CD25high regulatory T cells increase with tumor stage in patients with gastric and esophageal cancers. Cancer Immunol Immunother. 2006;55:1064-1071. |
| 19. | Wenger FA, Jacobi CA, Zieren J, Döcke W, Volk HD, Müller JM. Tumor size and lymph-node status in pancreatic carcinoma - is there a correlation to the preoperative immune function? Langenbecks Arch Surg. 1999;384:473-478. |
| 20. | Shen X, Li N, Li H, Zhang T, Wang F, Li Q. Increased prevalence of regulatory T cells in the tumor microenvironment and its correlation with TNM stage of hepatocellular carcinoma. J Cancer Res Clin Oncol. 2010;136:1745-1754. |
| 21. | Michel S, Benner A, Tariverdian M, Wentzensen N, Hoefler P, Pommerencke T, Grabe N, von Knebel Doeberitz M, Kloor M. High density of FOXP3-positive T cells infiltrating colorectal cancers with microsatellite instability. Br J Cancer. 2008;99:1867-1873. |
| 22. | Shen Z, Zhou S, Wang Y, Li RL, Zhong C, Liang C, Sun Y. Higher intratumoral infiltrated Foxp3+ Treg numbers and Foxp3+/CD8+ ratio are associated with adverse prognosis in resectable gastric cancer. J Cancer Res Clin Oncol. 2010;136:1585-1595. |
| 23. | Kawaida H, Kono K, Takahashi A, Sugai H, Mimura K, Miyagawa N, Omata H, Ooi A, Fujii H. Distribution of CD4+CD25high regulatory T-cells in tumor-draining lymph nodes in patients with gastric cancer. J Surg Res. 2005;124:151-157. |
| 24. | Mizukami Y, Kono K, Kawaguchi Y, Akaike H, Kamimura K, Sugai H, Fujii H. CCL17 and CCL22 chemokines within tumor microenvironment are related to accumulation of Foxp3+ regulatory T cells in gastric cancer. Int J Cancer. 2008;122:2286-2293. |
| 25. | Yaqub S, Henjum K, Mahic M, Jahnsen FL, Aandahl EM, Bjørnbeth BA, Taskén K. Regulatory T cells in colorectal cancer patients suppress anti-tumor immune activity in a COX-2 dependent manner. Cancer Immunol Immunother. 2008;57:813-821. |
| 26. | Audia S, Nicolas A, Cathelin D, Larmonier N, Ferrand C, Foucher P, Fanton A, Bergoin E, Maynadie M, Arnould L. Increase of CD4+ CD25+ regulatory T cells in the peripheral blood of patients with metastatic carcinoma: a Phase I clinical trial using cyclophosphamide and immunotherapy to eliminate CD4+ CD25+ T lymphocytes. Clin Exp Immunol. 2007;150:523-530. |
| 27. | Baecher-Allan C, Wolf E, Hafler DA. Functional analysis of highly defined, FACS-isolated populations of human regulatory CD4+ CD25+ T cells. Clin Immunol. 2005;115:10-18. |