Published online Jul 14, 2011. doi: 10.3748/wjg.v17.i26.3109
Revised: December 17, 2010
Accepted: December 24, 2010
Published online: July 14, 2011
AIM: To evaluate the influence of preoperative FOLFOX chemotherapy on CCL20/CCR6 expression in liver metastases of stage IV colorectal cancer (CRC) patients.
METHODS: Using Real Time-PCR, enzyme-linked immunosorbent assay, Western Blots and immunohistochemistry, we have analyzed the expression of CCL20, CCR6 and proliferation marker Ki-67 in colorectal liver metastasis (CRLM) specimens from stage IV CRC patients who received preoperative FOLFOX chemotherapy (n = 53) and in patients who did not receive FOLFOX chemotherapy prior to liver surgery (n = 29).
RESULTS: Of the 53 patients who received FOLFOX, time to liver surgery was ≤ 1 mo in 14 patients, ≤ 1 year in 22 patients and > 1 year in 17 patients, respectively. In addition, we investigated the proliferation rate of CRC cells in liver metastases in the different patient groups. Both CCL20 and CCR6 mRNA and protein expression levels were significantly increased in patients who received preoperative FOLFOX chemotherapy ≤ 12 mo before liver surgery (P < 0.001) in comparison to patients who did not undergo FOLFOX treatment. Further, proliferation of CRLM cells as measured by Ki-67 was increased in patients who underwent FOLFOX treatment. CCL20 and CCR6 expression levels were significantly increased in CRLM patients who had undergone preoperative FOLFOX chemotherapy.
CONCLUSION: This chemokine/receptor up-regulation could lead to increased proliferation/migration through an autocrine mechanism which might be used by surviving metastatic cells to escape cell death caused by FOLFOX.
- Citation: Rubie C, Frick VO, Ghadjar P, Wagner M, Justinger C, Graeber S, Sperling J, Kollmar O, Schilling MK. Effect of preoperative FOLFOX chemotherapy on CCL20/CCR6 expression in colorectal liver metastases. World J Gastroenterol 2011; 17(26): 3109-3116
- URL: https://www.wjgnet.com/1007-9327/full/v17/i26/3109.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i26.3109
Colorectal cancer (CRC) constitutes one of the most common causes of cancer death in the western world. CRC is also a tumor with a high propensity for metastatic spread, mostly to liver and lungs. Liver metastases develop in approximately 50% of CRC patients at some point in the course of their disease and worsen the prognosis for patient survival dramatically[1,2]. To date, long-term survival of patients with colorectal liver metastases (CRLM) can only be achieved by surgical resection. However, in cases where metastases are considered unresectable, chemotherapy is the treatment of choice, although 5-year survival with chemotherapy alone is very poor. Thus, surgical treatment of resectable liver metastases has become the standard treatment[3].
However, surgical resection can only be successful when liver metastases can be thoroughly resected and provided there is no other non-resectable distant metastasis. Moreover, the primary tumor needs to be resectable. Since all these criteria are often not fulfilled, cancer recrudescence occurs in 45% to 75% of CRLM patients within the first five years after primary tumor resection[3,4]. For these reasons, adjuvant chemotherapy has been evaluated in patients with resectable CRLM. To date, the standard approach with regard to adjuvant chemotherapy for CRC patients is a combination of oxaliplatin, fluorouracil and leucovorin[5,6]; termed FOLFOX. Perioperative FOLFOX chemotherapy has been shown to reduce the risk of cancer relapse by a quarter[7]. Thus, the combination of FOLFOX chemotherapy and surgery is a promising tool to improve the prognosis of CRC patients.
It has been shown that both the chemokine CCL20 and its unique receptor CCR6 are expressed in CRC cells[8-11], providing a basis for efficient autocrine and paracrine loops[12]. CCL20 stimulation of CCR6-bearing CRC cells led to increased proliferation and migration in vitro[8,9]. Moreover, our recent data suggest that interactions between CCL20 and the corresponding receptor CCR6 are critical components in the regulation of CRC progression and organ selective CRC metastasis to the liver[10,11].
The purpose of this study was to retrospectively analyze the impact of FOLFOX chemotherapy administered to stage IV CRC patients on CCL20/CCR6 expression in liver metastases. Eighty-two patients underwent radical surgery of the primary CRC and of synchronous and metachronous liver metastases. Twenty-nine patients underwent liver surgery without preoperative FOLFOX and 53 patients received FOLFOX prior to liver surgery. We compared CCL20/CCR6 expression in liver resection specimens from both groups and correlated their expression with proliferation of CRLM cells.
Surgical specimens and corresponding normal tissue from the same samples were collected from patients who underwent surgical resection at our department between 2002 and 2008.
Informed written consent for tissue procurement was obtained from all patients and the study was approved by the local ethics commission of the Ärztekammer des Saarlandes.
Eighty-two patients were included in the study, comprising CRC patients who had FOLFOX chemotherapy before liver surgery (n = 53) and CRC patients who did not have FOLFOX before liver surgery (n = 29). Of the 53 patients who received FOLFOX, time to liver surgery was ≤ 1 mo in 14 patients, ≤ 1 year in 22 patients and > 1 year in 17 patients, respectively. In every patient sample the corresponding non-affected normal liver tissue was also analyzed, thus a total of 164 samples were analyzed. In the 53 patients who received FOLFOX chemotherapy before liver surgery, two cancers were classified as pT1, six as pT2, forty as pT3 and five as pT4, with positive nodal involvement in 40 cases, according to the UICC TNM classification[13]. In the twenty-nine patients who received no FOLFOX chemotherapy before liver surgery, six cancers were classified as pT1, two as pT2, nineteen as pT3 and two as pT4, with positive nodal involvement in 10 cases. The clinical data and patient characteristics were obtained from a prospective database and are summarized in Table 1. In the group who did not receive FOLFOX, 8 patients underwent CRLM resection less than 6 mo and 21 patients underwent CRLM resection 6 mo and longer after resection of primary tumor. In the patient group who received FOLFOX chemotherapy, 7 underwent CRLM resection less than 6 mo after resection of primary tumor and 45 patients underwent CRLM resection 6 mo and longer after resection of primary tumor. One patient underwent resection of primary tumor and CRLM resection at the same time (Table 2).
| Characteristic | CRLM with FOLFOX1(n = 53) | CRLM without FOLFOX2(n = 29) |
| Localization of primary tumor | ||
| Colon | 25 | 13 |
| Rectum | 28 | 16 |
| Gender | ||
| Male | 29 | 17 |
| Female | 24 | 12 |
| Age at surgery (yr) | ||
| Median | 61.4 | 66.7 |
| Range | 35-79 | 43-77 |
| Largest tumor diameter (cm) | ||
| Median | 4.7 | 4.9 |
| Range | 1.3-9.7 | 1.2-10.1 |
| Tumor (T)-category | ||
| of primary tumor | ||
| pT1 | 2 | 6 |
| pT2 | 6 | 2 |
| pT3 | 40 | 19 |
| pT4 | 5 | 2 |
| Lymph node metastasis (N-category)3 | ||
| Positive | 40 | 10 |
| Negative | 13 | 19 |
| Grade | ||
| G1 | 0 | 1 |
| G2 | 32 | 17 |
| G3 | 21 | 11 |
Tissue specimens were collected immediately after surgical resection, snap frozen in liquid nitrogen and then stored at -80°C until they were processed under nucleic acid sterile conditions for protein and RNA extraction. For corresponding normal tissue we used adjacent non-affected tissue to the same resected specimen. All tissues obtained were reviewed by an experienced pathologist and examined for the presence of tumor cells. As minimum criteria for usefulness for our study, we only used tumor tissues in which tumor cells constituted at least > 75% of the tumor biopsy.
Total RNA was isolated using RNeasy columns from Qiagen (Hilden, Germany) according to the manufacturer’s instructions. RNA integrity was confirmed spectrophotometrically and by electrophoresis on 1% agarose gels. For cDNA synthesis, 5 μg of each patient total RNA sample were reverse-transcribed in a final reaction volume of 50 μL containing 1 × TaqMan RT buffer, 2.5 μmol/L random hexamers, 500 μmol/L each dNTP, 5.5 mmol/L MgCl2, 0.4 U/μL RNase inhibitor, and 1.25 U/μL Multiscribe RT. All RT-PCR reagents were purchased from Applied Biosystems (Foster City, CA). The reaction conditions were 10 min at 25°C, 30 min at 48°C, and 5 min at 95°C.
All Q-RT PCR assays containing the primer and probe mix were purchased from Applied Biosystems, (Applied Biosystems, Foster City, CA) and utilized according to the manufacturer’s instructions. PCR reactions were carried out using 10 μL 2 × Taqman PCR Universal Master Mix No AmpErase® UNG and 1 μL gene assay (Applied Biosystems, Foster City, CA), 8 μL Rnase-free water and 1 μL cDNA template (50 mg/L). The theoretical basis of the qRT assays is described in detail elsewhere[14]. All reactions were run in triplicate along with no template controls and an additional reaction in which reverse transcriptase was omitted to assure absence of genomic DNA contamination in each RNA sample. For the signal detection, ABI Prism 7900 sequence detector was programmed to an initial step of 10 min at 95°C, followed by 40 thermal cycles of 15 s at 95°C and 10 min at 60°C and the log-linear phase of amplification was monitored to obtain CT values for each RNA sample.
Gene expression of all target genes was analyzed in relation to the levels of the slope matched housekeeping genes phosphomannomutase (PMM1) and β2-microglobulin (β2M)[15]. Data analysis was performed according to the relative standard curve method. Data are presented in relation to the respective housekeeping genes.
Protein lysates from frozen tissue were extracted with radioimmunoprecipitation (RIPA) buffer containing Complete, a protease inhibitor cocktail (Roche, Penzberg, Germany). Total protein quantification was performed using the Pierce BCA protein assay reagent kit (Pierce, Rockford, IL., USA).
The chemokine protein levels in the different tissue lysates were determined by sandwich-type enzyme-linked immunosorbent assays (ELISA) according to the manufacturer’s instructions. Samples were assayed in duplicate with all values calculated as the mean of the two measurements. CCL20 levels were assayed using a validated commercial ELISA (Duo Set R&D Systems, DY360, Minneapolis, MN, USA). The absorbance was read at 450 nm in a 96-well microtiter plate reader. The chemokine concentration from each tissue lysate was normalized to the total protein content of each sample.
Total protein (25 μg/lane) was separated by SDS-PAGE using a 10% gel and blotted onto nitrocellulose membranes (Hybond ECL, Amersham Biosciences, Piscataway, NJ, USA). Membranes were blocked by incubation in Tris-buffered saline (TBS) containing 5% nonfat dry milk and 0.1% Tween 20 for 2 h at room temperature and then incubated overnight at 4°C with goat anti-human CCR6 antibody (diluted 1:500, C2099-70B, Biomol, Hamburg, Germany). Blots were then washed and incubated at room temperature for 1 h with donkey anti-goat HRP antibody (diluted 1:5000, sc-2056, Santa Cruz Biotechnology, Santa Cruz, CA, USA). Bands were visualized by ECL Western blotting analysis systems (Amersham Biosciences, Piscataway, NJ, USA). The human cell lysate HL-60 (sc-2209, Santa Cruz Biotechnology, Santa Cruz, CA, USA) served as positive control.
Resected specimens were routinely fixed with formalin in the immediate postoperative period and paraffin-embedded within the first three hours after procurement. Before staining, 4 μm thick sections were mounted on Superfrost Plus slides, deparaffinized with xylene, and rehydrated in graded ethanol to deionized water. The sections were treated with an antigen retrieval solution (Target Retrieval, Dakocytomation, Carpinteria, CA) and microwaved. CCL20 and CCR6 staining was performed according to the avidin-biotin-peroxidase reaction (Vectastain ABC ELITE Kit, Vector Laboratories Inc., Burlingame, CA) and Ki-67 staining was performed according to the APAAP method (Dako REAL Detection System, Dako, Glostrup, Denmark, K5000). For CCL20 and CCR6 staining, but not for Ki-67 staining, slides were immersed in 3% hydrogen peroxide for 10 min and then treated with avidin and biotin (Avidin/Biotin blocking kit, Vector Laboratories Inc., Burlingame, CA). Sections for CCL20, CCR6 and Ki-67 staining were incubated with serum followed by an overnight incubation with goat anti-human CCR6 polyclonal antibody (1:125, Biomol, Hamburg, Germany, C2099-70B), goat anti-human CCL20 polyclonal antibody (1:150, R&D, Abingdon, UK AF360) or Ki-67 MIB-1 monoclonal antibody (1:75, Dako, Glostrup, Denmark, M7240). Consequently, immunostaining with the avidin-biotin-peroxidase reaction (Vectastain ABC ELITE Kit, Vector Laboratories Inc., Burlingame, CA) was performed on CCL20 and CCR6 slides and a chromogene aminoethyl-carbazide solution (Tissugnost, Darmstadt, Merck) was used. For detection of Ki-67, the alkaline phosphatase-antialkaline phosphatase complex (APAAP) was used with Fast Red Substrate as chromogen (Dako, Glostrup, Denmark, K0597). Consequently, for all sections counterstaining was performed in hematoxylin solution. Negative controls were performed in all cases omitting primary antibody.
All data are presented as mean and SE (standard of the mean). Statistical calculations were done with the MedCalc (MedCalc software, Mariakerke, Belgium) software package[16]. The parametric Student’s t-test was applied, if normal distribution was given; otherwise, the Wilcoxon’s rank sum test was used. Statistical significance was considered on a two-sided significance level (α) of 0.05.
Prior to CRLM resection, 82 patients were enrolled in our study over a 6 year period. The median age of patients at surgery was 61.4 years (35-79) in the FOLFOX group and 66.7 years (43-77) in the patient group without FOLFOX. There were 29 males and 24 females in the FOLFOX group and 17 male and 12 female patients in the non-FOLFOX patient group. The demographic and clinical characteristics of patients are shown in Tables 1 and 2. FOLFOX and non-FOLFOX patients showed no statistically relevant differences with respect to T-stage, grading and timing between primary tumor resection and CRLM resection. However, with respect to N-stage our data revealed a significant difference between FOLFOX and non-FOLFOX patients (P < 0.05), as shown in Table 1. Thus, the non-FOLFOX group included a higher percentage of patients without lymph node metastasis (65.5%) compared to the FOLFOX group under investigation (24.5%).
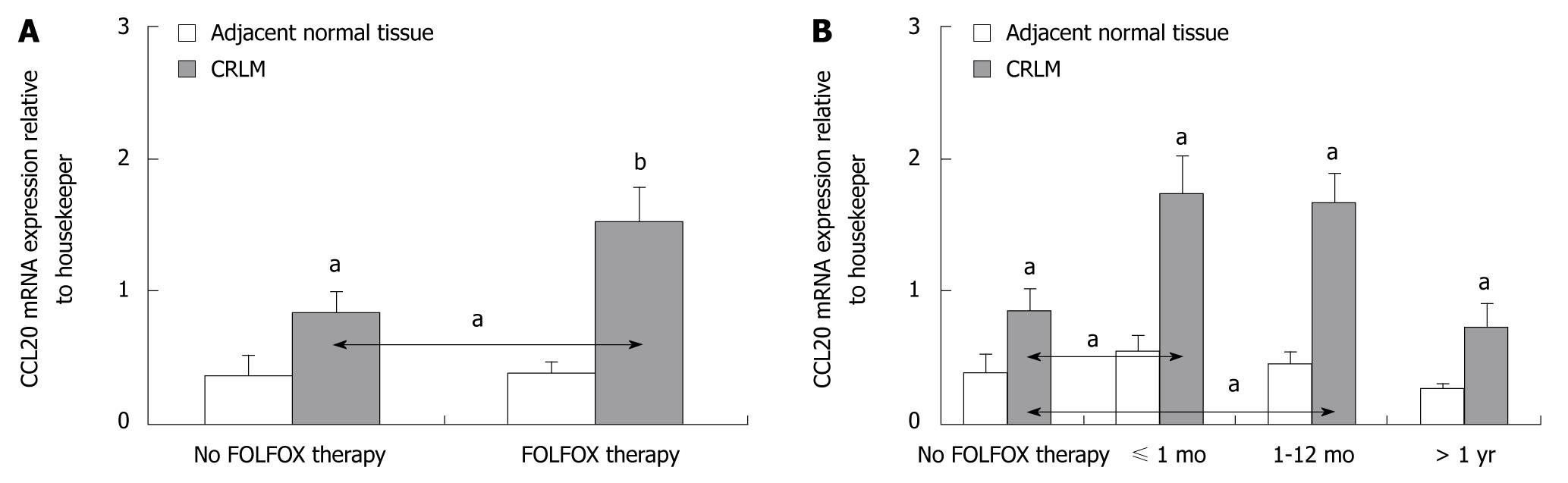
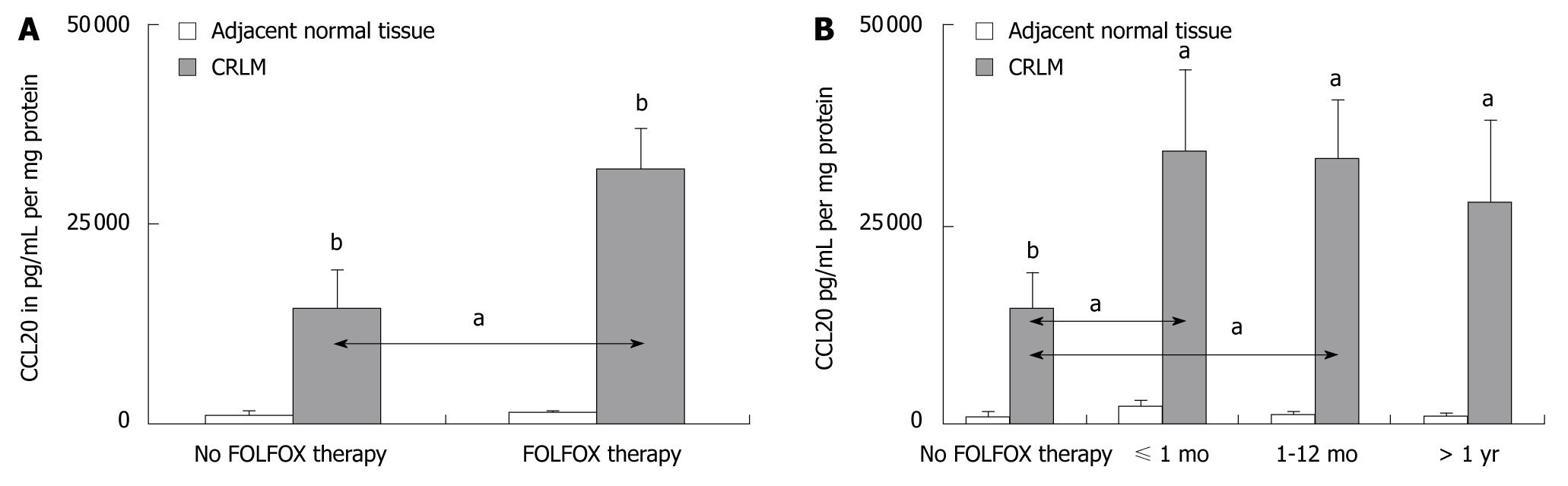
Significantly greater levels of CCL20 mRNA and CCL20 protein expression were observed in CRLM tissues compared to corresponding normal tissue in all patients (P < 0.05 and P < 0.001, respectively) (Figures 1 and 2). However, patients who were preoperatively treated with FOLFOX chemotherapy showed significantly higher levels of CCL20 mRNA and CCL20 protein expression as compared to patients without FOLFOX treatment (P < 0.05) (Figures 1A and 2A). When the interval between FOLFOX treatment and surgery was considered, only patients who received FOLFOX chemotherapy ≤ 12 mo before liver surgery expressed significantly higher amounts of CCL20 mRNA and CCL20 protein, as compared to patients without FOLFOX treatment, respectively (P < 0.05) (Figures 1B and 2B).
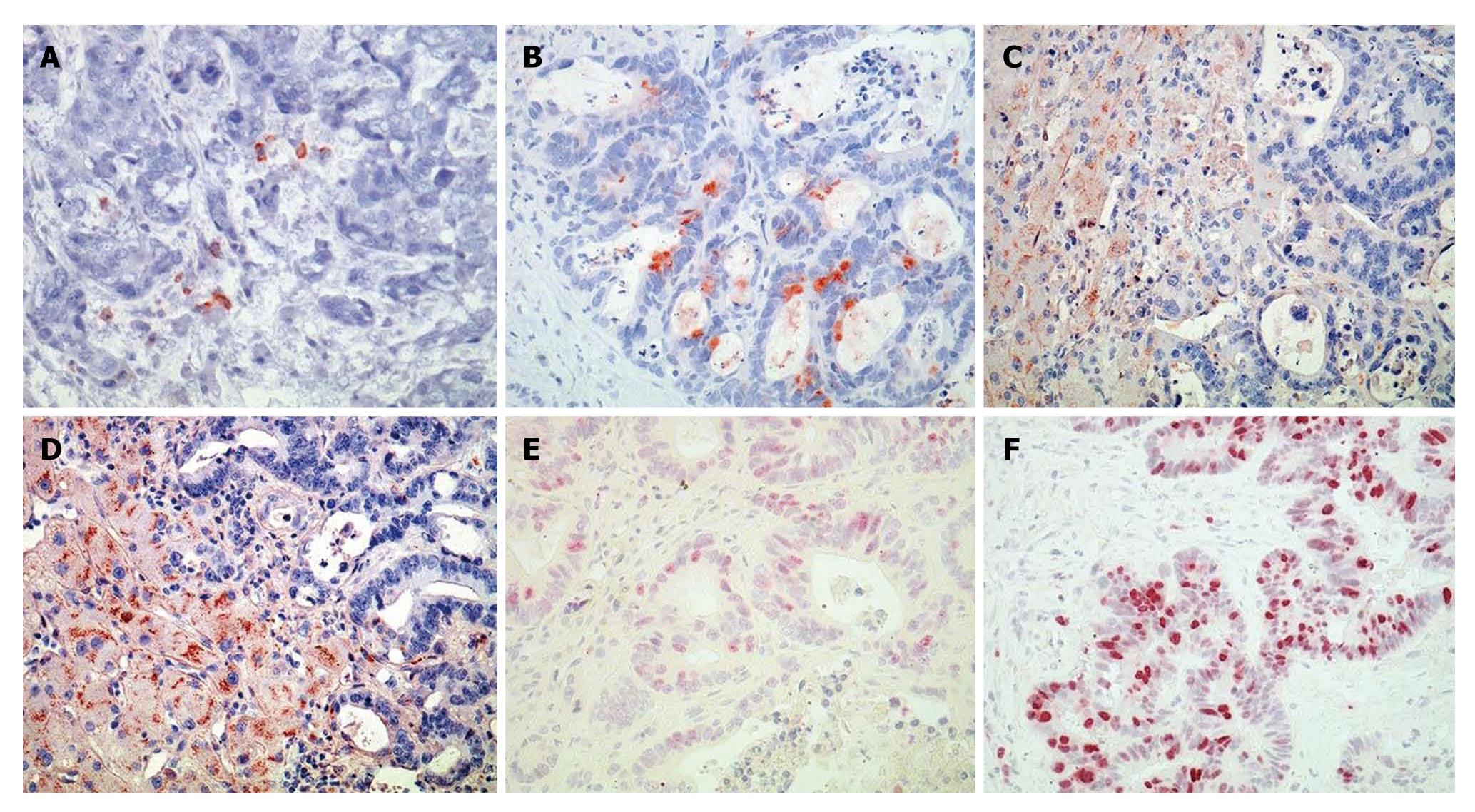
Immunostaining of the CRLM tissue revealed positive staining for CCL20 in 56% (16/29) of patients without and in 87% (46/53) of patients with preoperative FOLFOX chemotherapy (Figure 3A and B), respectively. CCL20 was immunolocalized with lesser intensity in the tumor tissue sections of CRLM patients without FOLFOX, as shown for a representative patient in Figure 3A, compared to patients who underwent preoperative FOLFOX chemotherapy, as shown in Figure 3B for a representative patient.
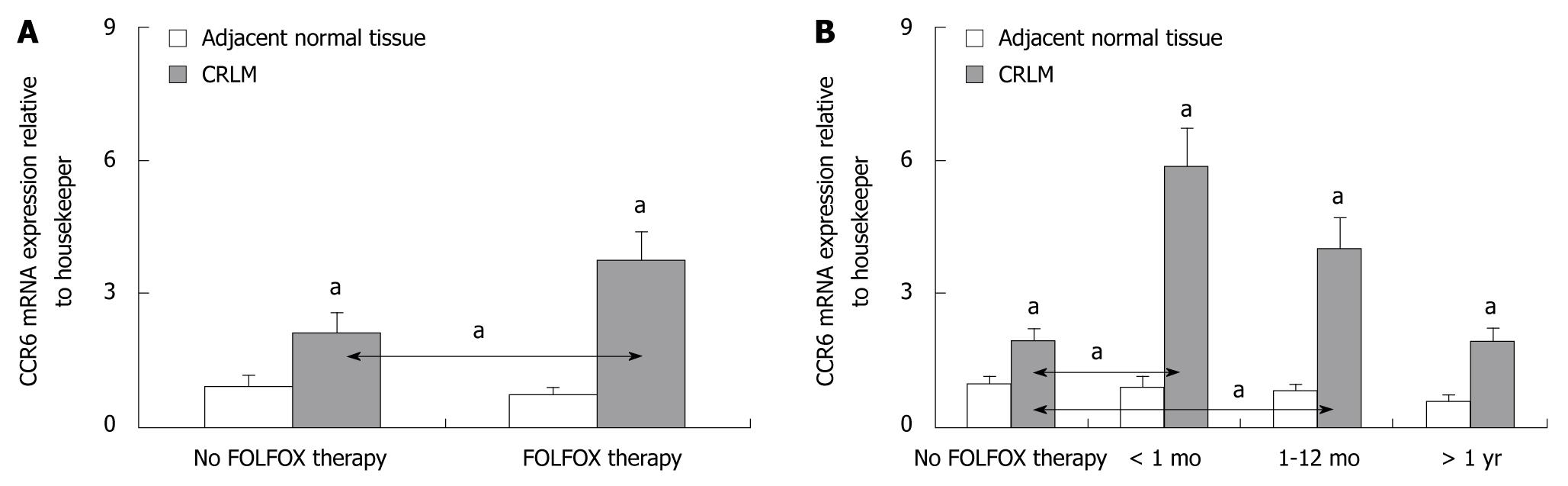
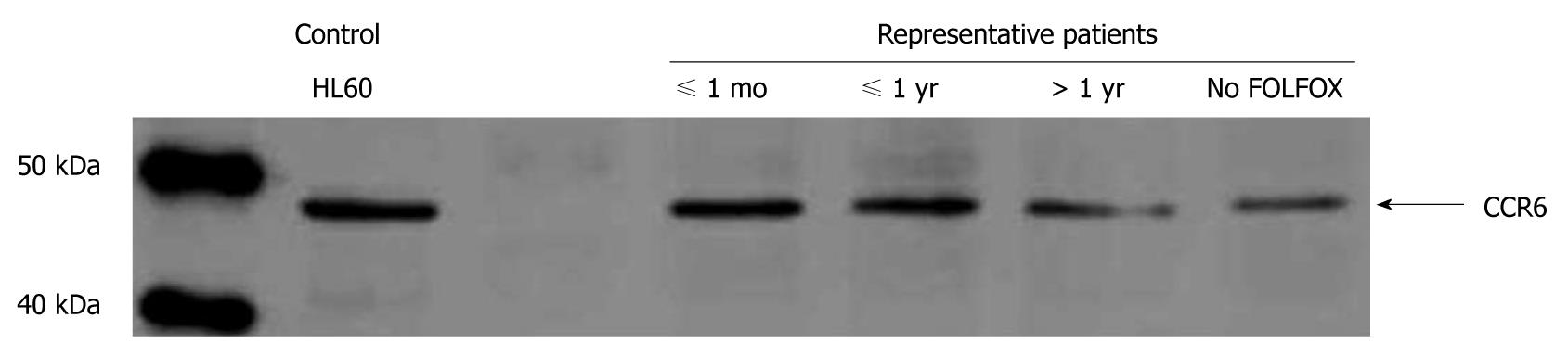
Both CCR6 mRNA and CCR6 protein expression levels were significantly increased in CRLM tissues of all patients compared to the corresponding normal liver tissue (P < 0.05) (Figure 4). Further, CCR6 mRNA and CCR6 protein expression levels were significantly higher in CRLM tissues of patients who underwent preoperative FOLFOX chemotherapy compared to patients without FOLFOX (P < 0.05) (Figure 4A). CCR6 up-regulation was limited to those patients who received preoperative FOLFOX chemotherapy ≤ 12 mo before liver surgery (P < 0.05) (Figures 4B and 5).
Immunostaining revealed positive staining for CCR6 in 59% (17/29) of patients without and in 87% (46/53) of patients with preoperative FOLFOX chemotherapy (Figure 3C and D), respectively. CCR6 staining intensities were stronger in the FOLFOX group. However, intense laminar CCR6 immunostaining was found mainly in the benign-appearing tissue sections of CRLM patients. Thus, CCR6 staining localized to a streak of hepatocytes along the tumor invasion front. These hepatocytes appeared clearly distinct from normal hepatocytes (Figure 3C and D).
Proliferation of CRLM cells as demonstrated by Ki-67 staining revealed a more frequent immunostaining pattern in CRLM tissues of patients who underwent preoperative FOLFOX (Figure 3F) compared to patients without FOLFOX (Figure 3E). In CRLM patients without FOLFOX treatment (n = 29) (Figure 3E) we observed weak or no Ki-67 immunostaining in 11 patients (38%), moderate immunostaining intensities in 13 patients (45%) and strong immunostaining intensities in 5 patients (17%). In CRLM patients with preoperative FOLFOX treatment (n = 53) (Figure 3F) we observed weak or no Ki-67 immunostaining in 16 patients (30%), moderate immunostaining intensities in 11 patients (21%) and strong immunostaining intensities in 26 patients (49%).
At present, standard treatment of CRC patients without distant metastasis consists of surgical resection of the primary tumor followed by adjuvant FOLFOX chemotherapy in patients with lymph node metastasis[17]. For patients with synchronous liver metastasis there are three treatment options: colectomy with synchronous or heterochronous CRLM surgery; perioperative chemotherapy with FOLFOX, FOLFIRI or CapeOX followed by colectomy and CRLM resection; and colectomy followed by chemotherapy and staged CRLM resection[5,6]. As liver resection offers the chance of long-term survival only for patients with resectable CRLM, chemotherapy is often applied to render formerly unresectable CRLM patients resectable. Moreover, superior survival for patients who have undergone resection has been demonstrated by several studies[18,19]. This improved survival may be due to the lower tumor burden.
The impact of chemotherapy on metastatic lymph node lesions was addressed in another study, where patients with 4 or more lymph node metastases around the primary cancer were considered to benefit from perioperative chemotherapy[20]. Chemotherapy options for metastatic CRC have significantly changed in recent years. While the optimal adjuvant systemic chemotherapy has yet to be determined, FOLFOX treatment seems to be one of the most effective CRLM treatment options[21] and perioperative FOLFOX chemotherapy is most commonly used to reduce the risk of cancer relapse in CRC patients[7].
Although pathological effects of chemotherapy for CRLM have been discussed[21-24], the pathological response to chemokine expression in CRLM after chemotherapy has not been reported. Since CCL20 and CCR6 have both been shown to be expressed on CRC cells[10,11] and CCL20 stimulation of CCR6-bearing CRC cells led to increased proliferation and migration in vitro[8,9], we investigated the impact of FOLFOX chemotherapy in stage IV CRC patients on CCL20/CCR6 expression in liver metastatic tissue.
Our results have shown that both CCL20 and CCR6 expression were significantly increased in patients who had received preoperative FOLFOX chemotherapy ≤ 12 mo before liver surgery as compared to patients who had not received FOLFOX chemotherapy prior to liver surgery. While CCL20 expression was significantly elevated in CRLM tissues, we detected CCR6 signals only sporadically in the tumor cells. Yet, CCR6 expression appeared rather cumulative in deformed hepatic cells along the tumor invasion front, which may represent a stimulative signal for the tumor to further expand into the neighboring hepatic tissue. Further, we demonstrated that CRLM cells of patients who had preoperative FOLFOX chemotherapy are characterized by an increased proliferation rate. This was measured by the expression of the human Ki-67 protein which is strictly associated with cell proliferation. During interphase, the antigen can be exclusively detected within the nucleus, whereas in mitosis most of the protein is relocated to the surface of the chromosomes[25].
Investigating the proliferation of CRLM cells in CRLM resection specimens, we measured proliferation after the event of FOLFOX exposure. FOLFOX treatment promotes apoptosis of CRC cells, thus inhibiting their proliferation. However, after FOLFOX chemotherapy, not only cell proliferation as measured by KI-67 staining, but also CCL20 and CCR6 expression levels, were increased as compared to patients without FOLFOX chemotherapy. In vitro data have shown an increased proliferation and migration of CCR6-bearing tumor cells after CCL20 stimulation[8,9]. Thus, the observed up-regulation of CCL20 expression by surviving metastatic CRC cells after FOLFOX treatment might represent a CCL20/CCR6 dependent autocrine mechanism, potentially leading to increased proliferation and migration. Since FOLFOX chemotherapy induces non-specific cell death, this mechanism might be used by surviving metastatic cells within the liver to escape FOLFOX-induced cell death. Interestingly, if the interval between preoperative FOLFOX chemotherapy and liver surgery was > 1 year, the up-regulation of neither CCL20 nor CCR6 remained statistically significant. Generally, this suggests that the up-regulation of CCL20 and CCR6 by FOLFOX is a temporary event.
The disruption of chemokine/chemokine receptor interactions is a promising strategy in the treatment of cancer. A CCR5 inhibitor is already in the clinic for the treatment of human immunodeficiency virus (HIV)-infected patients. Other different chemokine antagonists are currently under investigation in phase I-III trials for infectious diseases, autoimmune diseases and cancer. Since CCR6 and CCL20 may play a role in CRC, leading to proliferation and migration via autocrine or paracrine mechanisms, progression of CRC might be advantaged by CCR6/CCL20 interactions. Thus, the effect of chemotherapy on the expression of cancer-related chemokines and their receptors might explain in part the frequent recurrence of metastasis in patients after this treatment. Therefore, CCL20 and CCR6 interactions may constitute a potential new target for specific treatment interventions in the treatment of CRC. Such a novel approach might be effective in combination with FOLFOX as preoperative treatment prior to resection of CRLM.
Although long-term survival of patients with colorectal liver metastases (CRLM) can only be achieved by surgical resection, cancer recrudescence can only be avoided if CRLM are thoroughly resected and if no other non-resectable distant metastases are present. As liver resection offers the chance of long-term survival only for patients with resectable CRLM, chemotherapy is often applied to render formerly unresectable CRLM patients resectable. Thus, a combination of perioperative chemotherapy and surgery is frequently applied to improve prognosis in colorectal cancer (CRC) patients.
As a standard approach of adjuvant chemotherapy, a combination of oxaliplatin, fluorouracil and leucovorin (termed FOLFOX) is most commonly used to reduce the risk of cancer relapse in CRC patients. Although pathological effects of chemotherapy for CRLM have been discussed, the pathological response regarding chemokine expression in CRLM after chemotherapy has not been reported. Thus, application of FOLFOX may enhance the expression of chemokines which are known to be up-regulated with CRC.
Recently, interactions of the chemokine/chemokine receptor pair CCL20/CCR6 became known as critical components in the regulation of CRC progression and organ selective CRC metastasis to the liver. Thus, the authors retrospectively analyzed the impact of FOLFOX chemotherapy in stage IV CRC patients on CCL20/CCR6 expression in liver metastases. The results have shown that both CCL20 and CCR6 expression levels were significantly increased in patients who had received preoperative FOLFOX chemotherapy ≤ 12 mo before liver surgery as compared to patients who had not received FOLFOX chemotherapy prior to liver surgery.
As the disruption of chemokine/chemokine receptor interactions is a promising strategy in the treatment of cancer, various chemokine antagonists are currently under investigation in phase I-III trials for infectious diseases, autoimmune diseases and cancer. Since CCL20/CCR6 interactions may play a role in the progression of CRC, the effect of chemotherapy on the expression of cancer-related chemokines and their receptors might explain in part the frequent recurrence of metastasis in patients after this treatment. Thus, CCL20/CCR6 interactions may constitute a potential target for specific CRC treatment interventions, especially in combination with FOLFOX as preoperative treatment prior to CRLM resection.
The authors have investigated the expression of CCL20/CCR6 in resected liver metastases of colorectal cancer with regard to preoperative FOLFOX chemotherapy. In their retrospective data analysis, they found a correlation of chemotherapy with an upregulation of CCL20/CCR6 after FOLFOX treatment within 12 mo before surgery. This result is a descriptive observation without further elucidation of the underlying mechanisms. However, it may be a basis for further research in this field to clarify tumor cell resistance against specific chemotherapeutic agents.
Peer reviewer: Thilo Hackert, MD, Department of Surgery, University of Heidelberg, Im Neuenheimer Feld 110, 69120 Heidelberg, Germany
S- Editor Tian L L- Editor Logan S E- Editor Ma WH
| 1. | Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71-96. |
| 2. | Leonard GD, Brenner B, Kemeny NE. Neoadjuvant chemotherapy before liver resection for patients with unresectable liver metastases from colorectal carcinoma. J Clin Oncol. 2005;23:2038-2048. |
| 3. | Fong Y, Fortner J, Sun RL, Brennan MF, Blumgart LH. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg. 1999;230:309-318; discussion 318-321. |
| 4. | Scheele J, Stang R, Altendorf-Hofmann A, Paul M. Resection of colorectal liver metastases. World J Surg. 1995;19:59-71. |
| 5. | André T, Boni C, Mounedji-Boudiaf L, Navarro M, Tabernero J, Hickish T, Topham C, Zaninelli M, Clingan P, Bridgewater J. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med. 2004;350:2343-2351. |
| 6. | André T, Boni C, Navarro M, Tabernero J, Hickish T, Topham C, Bonetti A, Clingan P, Bridgewater J, Rivera F. Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. J Clin Oncol. 2009;27:3109-3116. |
| 7. | Benoist S, Nordlinger B. The role of preoperative chemotherapy in patients with resectable colorectal liver metastases. Ann Surg Oncol. 2009;16:2385-2390. |
| 8. | Yang CC, Ogawa H, Dwinell MB, McCole DF, Eckmann L, Kagnoff MF. Chemokine receptor CCR6 transduces signals that activate p130Cas and alter cAMP-stimulated ion transport in human intestinal epithelial cells. Am J Physiol Cell Physiol. 2005;288:C321-C328. |
| 9. | Brand S, Olszak T, Beigel F, Diebold J, Otte JM, Eichhorst ST, Göke B, Dambacher J. Cell differentiation dependent expressed CCR6 mediates ERK-1/2, SAPK/JNK, and Akt signaling resulting in proliferation and migration of colorectal cancer cells. J Cell Biochem. 2006;97:709-723. |
| 10. | Rubie C, Oliveira V, Kempf K, Wagner M, Tilton B, Rau B, Kruse B, Konig J, Schilling M. Involvement of chemokine receptor CCR6 in colorectal cancer metastasis. Tumour Biol. 2006;27:166-174. |
| 11. | Ghadjar P, Coupland SE, Na IK, Noutsias M, Letsch A, Stroux A, Bauer S, Buhr HJ, Thiel E, Scheibenbogen C. Chemokine receptor CCR6 expression level and liver metastases in colorectal cancer. J Clin Oncol. 2006;24:1910-1916. |
| 12. | Ghadjar P, Rubie C, Aebersold DM, Keilholz U. The chemokine CCL20 and its receptor CCR6 in human malignancy with focus on colorectal cancer. Int J Cancer. 2009;125:741-745. |
| 13. | Wittekind CH, Meyer HJ, Bootz F. UICC TNM classification of malignant tumors. 6th ed. Berlin, Heidelberg, New York: Springer 2002; . |
| 14. | Bustin SA. Absolute quantification of mRNA using real-time reverse transcription polymerase chain reaction assays. J Mol Endocrinol. 2000;25:169-193. |
| 15. | Rubie C, Kempf K, Hans J, Su T, Tilton B, Georg T, Brittner B, Ludwig B, Schilling M. Housekeeping gene variability in normal and cancerous colorectal, pancreatic, esophageal, gastric and hepatic tissues. Mol Cell Probes. 2005;19:101-109. |
| 16. | Schoonjans F, Zalata A, Depuydt CE, Comhaire FH. MedCalc: a new computer program for medical statistics. Comput Methods Programs Biomed. 1995;48:257-262. |
| 17. | Schmiegel W, Reinacher-Schick A, Arnold D, Graeven U, Heinemann V, Porschen R, Riemann J, Rödel C, Sauer R, Wieser M. [Update S3-guideline „colorectal cancer“ 2008]. Z Gastroenterol. 2008;46:799-840. |
| 18. | Scheele J, Altendorf-Hofmann A, Grube T, Hohenberger W, Stangl R, Schmidt K. Resection of colorectal liver metastases. What prognostic factors determine patient selection? Chirurg. 2001;72:547-560. |
| 19. | Kato T, Yasui K, Hirai T, Kanemitsu Y, Mori T, Sugihara K, Mochizuki H, Yamamoto J. Therapeutic results for hepatic metastasis of colorectal cancer with special reference to effectiveness of hepatectomy: analysis of prognostic factors for 763 cases recorded at 18 institutions. Dis Colon Rectum. 2003;46:S22-S31. |
| 20. | Minagawa M, Yamamoto J, Miwa S, Sakamoto Y, Kokudo N, Kosuge T, Miyagawa S, Makuuchi M. Selection criteria for simultaneous resection in patients with synchronous liver metastasis. Arch Surg. 2006;141:1006-1112; discussion 1013. |
| 21. | Sawayama H, Hayashi N, Honda S, Baba Y, Toyama E, Watanabe M, Takamori H, Beppu T, Baba H. Treatment results of FOLFOX chemotherapy before surgery for lymph node metastasis of advanced colorectal cancer with synchronous liver metastasis: the status of LN metastasis and vessel invasions at the primary site in patients who responded to FOLFOX. Int J Clin Oncol. 2010;15:70-76. |
| 22. | Cleary JM, Tanabe KT, Lauwers GY, Zhu AX. Hepatic toxicities associated with the use of preoperative systemic therapy in patients with metastatic colorectal adenocarcinoma to the liver. Oncologist. 2009;14:1095-1105. |
| 23. | Fernandez FG, Ritter J, Goodwin JW, Linehan DC, Hawkins WG, Strasberg SM. Effect of steatohepatitis associated with irinotecan or oxaliplatin pretreatment on resectability of hepatic colorectal metastases. J Am Coll Surg. 2005;200:845-853. |
| 24. | Zorzi D, Laurent A, Pawlik TM, Lauwers GY, Vauthey JN, Abdalla EK. Chemotherapy-associated hepatotoxicity and surgery for colorectal liver metastases. Br J Surg. 2007;94:274-286. |
| 25. | Scholzen T, Gerdes J. The Ki-67 protein: from the known and the unknown. J Cell Physiol. 2000;182:311-322. |