INTRODUCTION
Chronic inflammation is thought to be the leading cause of many human cancers including colorectal cancer (CRC). Ulcerative colitis (UC) and Crohn’s disease (CD), the two major forms of inflammatory bowel disease (IBD), are associated with an increased risk of developing colitis-associated colorectal cancer (CAC). The risk of CRC in UC patients is 2% after 10 years, 8% after 20 years and 18% after 30 years of active disease[1]. Although more recently other studies have estimated a lower risk in this class of patients, the overall incidence rate ratio for developing CRC calculated in UC patients was found by Bernstein et al[2] to be 2.75 [95% confidence interval (95% CI), 1.91-3.97] compared to the general population.
While the relationship between UC and CRC is well established, the risk associated with CD has been unclear until recently. Indeed, the heterogeneous nature of CD which can involve any part of the gut in a non-continuous way, with many patients having no colonic involvement, makes it difficult to estimate the actual risk of developing CRC in these patients. A milestone Swedish study demonstrated a relative risk of CRC of 5.6 for CD patients with exclusive localization in the colon and 3.2 for patients with ileo-colitis[3]. In contrast, patients with exclusive ileal localization of the disease had no increased risk. A meta-analysis of CRC risk in CD revealed an overall relative risk of 2.5 (95% CI, 1.3-4.7)[4]. In the subset of patients with exclusive colonic localization, the risk was 4.5 (95% CI, 1.3-14.9) while the risk in patients with ileal disease was not significantly increased. Interestingly, when comparing Crohn’s colitis with UC of similar extent, the relative risk of developing CRC is similar between the two groups.
Several risk factors concur to determine the probability of developing CRC in single patients. The observation that the cumulative risk increases over the years indicates that disease duration does play a role[1,3]. In addition, the extension of the disease has been shown to increase the risk of CAC; this being 1.7 in patients with ulcerative proctitis, 2.8 in those with left-sided colitis and 14.8 in patients with extensive colitis[3]. Also, the severity of inflammation independently correlates with the risk of developing CAC[5]. The same independent risk factors have been linked to the risk of developing CRC in CD. In addition, in CD patients, perianal disease, bypasses and strictures might be sites of increased risk of neoplastic transformation[6-8].
Overall, these data indicate that chronic inflammation of the colon such as that observed during either UC or CD increases the risk of developing CRC. However, the mechanisms involved in this process are still poorly understood. The current opinion regarding the pathogenesis of IBD is that, in genetically susceptible individuals, there is an overreaction of the immune system toward antigens of the gut microbiota leading to chronic inflammation[9]. UC and CD are characterized by different immune responses. While UC is caused by an atypical T helper (Th)2-mediated immune response characterized by high levels of IL-5 (but not IL-4) and IL-13, in CD there is a prevalent activation of Th1 cells with high expression of TNF-α and IFN-γ[10-13]. More recently, a new subset of IL-17-producing T helper cells, the Th17 cells, has been shown to play a role in the pathogenesis of CD[14,15]. Finally, in addition to CD4+ T cells, CD8+ T cells, natural killer, natural killer T cells and regulatory T cells have also been implicated in the pathogenesis of IBD[16-18].
Given the role played by these cell subsets and by the cytokines they express in the induction and maintenance of gut inflammation, their role has also been investigated in the pathogenesis of CAC. Here we review some of the recent data that implicate immune cells and inflammatory cytokines in the pathogenesis of CAC.
INFLAMMATION AND TUMOR INITIATION: A DOUBLE-EDGED SWORD
Chronic inflammation is thought to induce dysplasia by inducing DNA modifications in intestinal epithelial cells. Indeed, chronic accumulation of activated immune cells such as neutrophils, macrophages and dendritic cells is accompanied by the release of oxygen and nitrogen reactive species, which are known to induce genomic mutations[19,20]. Moreover, chronic inflammation is associated with DNA methylation and histone modification[21-23]. All these processes have been associated with the altered expression of genes involved in carcinogenesis such as p53, APC, K-ras and Bcl-2[24]. Once initiated, dysplastic cells are subjected to the effect of cell-derived growth factors and cytokines which contribute to tumor growth. However, lines of evidence have also indicated that, under certain conditions, immune cell subsets and cytokines fight to maintain dysplastic cells in check thus preventing tumor progression. A change in the immune response and/or an adaptation to the selective pressure of the immune system, referred to as immune-editing, at a certain point will select dysplastic cell clones able to grow sustained by the presence of growth factors and proinflammatory cytokines released in the surrounding microenvironment, thus changing the role of the immune system from a negative regulator of tumor growth to cancer promoter[25]. Although most of the data sustaining this mechanism derive from models of sporadic cancer, it is possible that a similar alteration of the balance between immune system and dysplastic cells might also occur during long-standing intestinal inflammation.
CD4+ T cells and colitis-associated carcinogenesis
Whether T cells are required for the development of colitis-associated CRC is an open question. In the azoxymethane/dextran sulphate sodium (AOM/DSS) experimental model of CAC, RAG1-deficient mice that do not have B and T cells did not develop tumors even in the presence of colitis[26]. These results indicate that lymphocytes are required to promote tumor growth in the context of colitis. However, it is worth considering that an enhanced activity of natural killer (NK) cells, which are still present in RAG1-/- mice, might be responsible for tumor protection in these mice. Indeed, depletion of suppressive subsets of T cells (i.e. regulatory T cells) has been shown to increase NK cell activity and tumor rejection[27-29]. Experiments with RAG1-/-//γ-chain-/- double knockout mice which lack B, T and NK cells would help to address this issue.
With regard to T helper cell subsets, the role of Th1 and Th2 cells in CAC has been shown by Osawa et al[30]. The authors compared CAC development in IL4-/- and IFN-γ-/- deficient mice which have a biased Th2 and Th1 immune response, respectively. Interestingly, Th1-biased IFN-γ-/- mice developed more tumors than wild type. Since in these mice there was high expression of IL-4 and IL-5, the authors concluded that Th2-derived cytokines promote tumor growth. Indeed, a Th2 response has been correlated with progression of experimental and human sporadic CRC[31,32] while a Th1 response has been associated with a better prognosis[33]. Moreover, the Th2-related cytokines IL-4 and IL-13 have been shown to induce the upregulation of activation-induced cytidine deaminase (AID), an enzyme involved in DNA mutation in epithelial cells in vitro[34]. Accordingly, AID levels are highly expressed in tumor samples from UC patients. The higher susceptibility to develop CAC shown by IFN-γ-/- mice might be related to a decreased immunosurveillance[34]. Indeed, IFN-γ has been shown to be involved in the activation of cytotoxic T cells and NK cells which play a central role in the antitumor immune response[35,36].
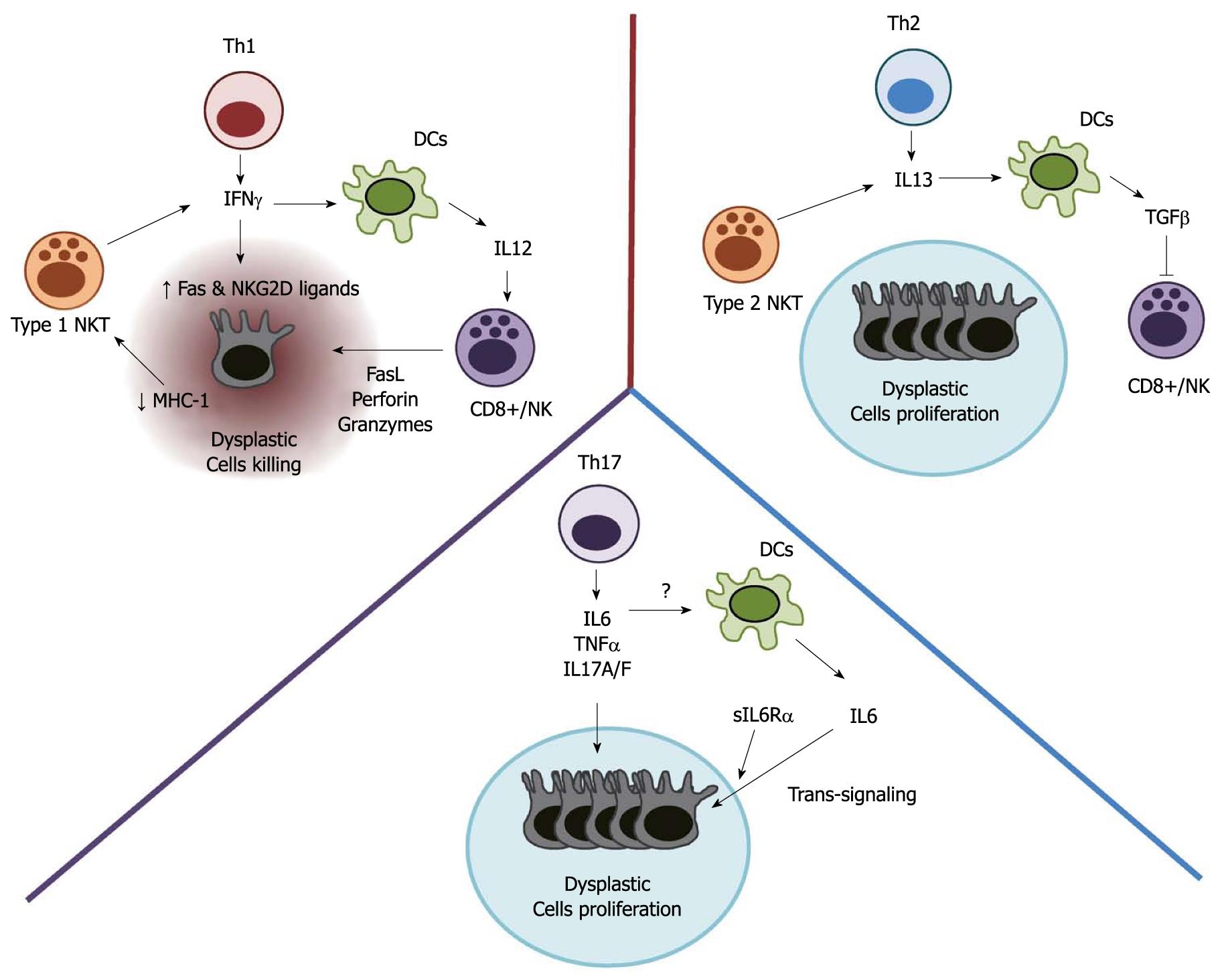
The initial observation that UC patients have a higher risk of CAC in comparison to CD fits well with the concept that the Th2 immune response, observed in UC, promotes cancer development while the CD-associated Th1 response is protective. However, as mentioned above, the initially observed lower incidence of CAC in CD in comparison to UC is considered to be due to the methodological approach used in these studies. An alternative explanation for this apparent contradiction could derive from recent advances in the pathogenesis of CD. CD has been long thought to be a Th1-mediated immune disease. This concept derives from initial studies on the role of IL-12, a cytokine involved in the differentiation of Th1 cells[13,37]. In these studies, neutralization of p40, a subunit of IL-12, was effective in preventing gut inflammation both in experimental models and in humans[38-40]. However, we now know that p40 is shared by another cytokine, IL-23, which is a heterodimer composed of p40 and p19. IL-23 has been shown to be involved in the maintenance of Th17 cells, a novel class of T helper cells[41]. Accumulating evidence suggests that Th17 cells might play a role in the pathogenesis of CD. Gain of function polymorphisms of the IL-23 receptor gene are associated with CD[42]. IL-23 p19-/- mice are less susceptible to colitis in comparison with IL-12 p35-/- mice which do not express IL-12[43]. Mice deficient in receptor-related organ receptor (ROR)γ t, the lineage commitment transcription factor of Th17 cells, are resistant to inflammation in different models of colitis[44]. Most of the proinflammatory effect of Th17 has been attributed to the expression of IL-17. IL-17 is known to induce the expression of proinflammatory factors such as TNF-α, IL-6, IL1, iNOS, metalloproteinases and chemokines, which also play a role in CAC[45]. Finally, IL-23 expression is increased in several types of human cancer including CRC[46], and IL-23 p19-/- mice are demonstrated to be more resistant to tumor development[47]. Overall, these data suggest that tumor-promoting Th17 cells rather than Th1 cells might sustain inflammation in CD, thus explaining the increased risk of CAC in CD patients (Figure 1).
Figure 1 Different T helper-mediated immune responses might be associated with distinct effects on dysplastic cell survival.
While Th2 and Th17 immune responses might promote dysplastic cell proliferation and tumor growth, Th1 cells could induce cell death thus preventing tumor progression. Th: T helper; NK: Natural killer; NKT: Natural killer T cells; IL: Interleukin; TGF-β: Transforming growth factor-β; DC: Dendritic cells; MHC: Major histocompatibility complex; Fas: Tumor necrosis factor receptor superfamily, member 6; NKG2D: Killer cell lectin-like receptor subfamily K, member 1; TNF-α: Tumor necrosis factor-α; sIL6R-α: Soluble IL-6 receptor-α.
Cytotoxic T cells
CD8+ T cells, NK and natural killer T (NKT) cells have been shown to play a role in cancer immunity. Their role, initially limited to the capacity to kill dysplastic target cells, has been recently extended demonstrating a more complex contribution to the antitumor immune response.
A central role in cancer immunosurveillance is attributed to CD8+ cytotoxic T cells. After presentation of tumor-related antigens by antigen-presenting cells, CD8+ T cells become activated and release different cytotoxic molecules responsible for target cell killing. Activated CD8+ T cells express high levels of IFN-γ and FasL[48]. While FasL, a membrane bound molecule, induces apoptosis by interacting with Fas expressed on the surface of dysplastic cells[49], IFN-γ has been shown to enhance the expression of Fas in colorectal cancer cell lines thus enhancing the killing process[50]. Perforin, granzyme A and granzyme B are also expressed by CD8+ T cells and their effect on target cells is to induce a permeabilization of the cell membrane and cell death[51,52].
NK cells are large granular lymphocytes with both cytotoxicity against tumor and cytokine-producing effector function. NK cells are involved in the rejection of in vivo implanted tumors in a manner dependent on the presence or absence of signals on the target cells. The lack of MHC class I expression on the surface of target cells or the upregulation of NKG2D ligands can determine NK cells activation[53-55]. NKG2D ligands are expressed at various levels in CRC cell lines and the expression of one of them, MICA, was associated with a better prognosis in CRC patients[56]. Once activated, NK cells express high levels of IFN-γ, perforin and granzymes which induce apoptosis in target cells. Interestingly, IL-21, a cytokine highly expressed in both UC and CD, has been shown to activate NK cells[57]. However, whether IL-21-induced activation of NK cells plays a role in anti-tumor immunity is still unclear[58-60].
In contrast to NK cells, which lack the T cell receptor (TCR), NKT cells express a limited variety of TCRs. Although NKT cells have NK-like cytolytic activity, they are considered regulators of the immune response, being able to express both Th1- and Th2-related cytokines. In tumor immunity, NKT cells have been considered as both enhancers and suppressors of the anti-tumor activity. A subset of NKT cells (type I NKT), characterized by the expression of V-α-14-Jα-18 TCR-α chain, has been shown to enhance tumor immunity by IFN-γ expression and NK cell activation[61,62]. Moreover, IFN-γ indirectly promotes the activation of CD8+ T cells by inducing the expression of IL-12 in antigen-presenting cells[63]. Tumor infiltration by type I NKT cells in CRC patients has been positively correlated with the disease-free survival[64]. In contrast to type I NKT cells, type II NKT cells, which do not express the V-α-14-Jα-18 TCR-α chain, have been associated with suppression of antitumor immunity. Type II NKT cells express IL-13, which has been shown to induce the expression of the immunosuppressive cytokine TGF-β in myeloid cells[56,65]. Interestingly, the selective activation of type II NKT cells enhanced CT26 cell growth in a mouse model of CRC metastasis[66]. In UC, activation of type II NK cells and expression of IL-13 characterize the “atypical” Th2 immune response observed in these patients[11]. It is tempting to speculate that activation of type II NKT cells in UC might contribute to CAC development by selectively dampening the antitumor immune response while sustaining mucosal inflammation and cancer development.
Innate immune cells
The role of innate immune cells in sporadic CRC progression has started to be unveiled (For review, see Mantovani et al[67]). However, whether these cells are also important in the development of CAC is still unclear.
The role of innate immunity in the development of CAC is suggested by recent findings that Toll-like receptors (TLRs) are important in inflammation and CAC. TLRs form a family of membrane-bound receptors expressed by cells of different lineages such as epithelial cells, macrophages and dendritic cells. TLRs “sense” the presence of bacterial compound present in the extracellular space. The interaction between the microbiota and the intestinal mucosa through TLRs is required to maintain intestinal homeostasis. Recent genetic studies suggest that polymorphisms in the genes encoding TLRs are associated with increased risk of IBD and disease extension[68,69]. Moreover, TLR4 is demonstrated as being upregulated in intestinal epithelial cells of patients with active IBD[70]. With regard to CAC, it was shown that intestinal bacteria are required for tumor development in models of CAC. Furthermore, deficiency of MyD88, a molecule involved in TLR intracellular signaling, significantly exacerbated chemically-induced colitis[71] and reduced tumor number and size in sporadic and colitis-associated CRC models[72,73].
CAC: the role of cytokines and chemokines
As mentioned above, immune cells actively contribute to CAC by expressing soluble factors (e.g. cytokines and chemokines) and the role of some of these has been extensively investigated.
IL-6
IL-6 is a multifunctional cytokine important for immune responses, cell survival, apoptosis, and proliferation. IL-6 has been linked to IBD pathogenesis. Atreya et al[74] have demonstrated that IL-6 expression in the mucosa of IBD-affected patients induces T cell resistance to apoptosis, thus contributing to chronic inflammation. Accordingly, a correlation between IL-6 levels and the clinical activity of IBD has been demonstrated[75,76]. With regard to CAC, IL-6 expressed during colitis was shown to promote tumor growth in mice[26]. In this model, selective inhibition of the TGF-β signaling in T cells was associated with an enhanced expression of IL-6. In turn, IL-6 trans-signaling, mediated by the interaction of IL-6 and the soluble form of the IL-6 receptor α (sIL-6α) with the gp130 receptor expressed on the surface of dysplastic cells, enhanced tumor cell proliferation. Moreover, in a similar model, Grivennikov et al[77] demonstrated that IL-6 expressed by lamina propria myeloid cells protects normal and transformed epithelial cells from apoptosis in a STAT-3-dependent manner, demonstrating the critical oncogenic function of this cytokine-activated transcription factor. Recently, higher expression of IL-6 and STAT3 was observed in both patients with active UC and those who had progressed to CAC, compared with patients with inactive disease or control patients[78]. In the same study, patients with either inactive or active UC, compared with control individuals, showed increased expression of suppressor of cytokine signaling 3 (SOCS3), which limits the ability of IL-6 to activate STAT3. On the other hand, the expression of SOCS3 was decreased in patients with UC who had progressed to CRC. In the AOM/DSS mouse model of CAC, IEC-specific SOCS3 gene disruption led to increased size, number and load of colonic tumors and this was associated with increased STAT3 and nuclear factor-κB (NF-κB) activation in colon[79].
TNF-α
Tumor necrosis factor (TNF)-α is a pivotal cytokine in the pathogenesis of IBD and anti-TNF-α monoclonal antibody (MAb) therapy is routinely used in UC and CD patients[80]. Although TNF-α has been classically considered as an anticancer agent, it is currently recognized that chronically elevated TNF-α in tissues may promote tumor growth, invasion and metastasis[81]. Indeed, mice deficient for the p55 TNF-α receptor subunit were protected from tumor development in the AOM/DSS model of CAC[82]. Moreover, in the same study, repetitive anti-TNF-α treatment not only suppressed colitis in mice but also prevented CAC.
The effect of TNF-α signaling in CAC is mostly due to the intracellular activation of NF-κB. NF-κB is a pleiotropic transcription factor with a key role in innate and adaptive immunity and is required for the expression of various proinflammatory factors[83]. In addition to its critical function in inflammation, NF-κB activation can support carcinogenesis by increasing cell proliferation and angiogenesis, inhibiting cell death, and promoting cell invasion and metastasis[84]. Greten et al[85] have shown that blocking NF-κB activation in the intestinal epithelium dramatically reduced the incidence of CAC, and this was associated with enhanced epithelial cell apoptosis during early tumor development. Interestingly, no reduction of intestinal inflammation was observed in these mice, thus indicating that prosurvival signals provided by NF-κB in epithelial cells play a role in CAC initiation independent of the inflammation severity.
IL-10
IL-10 is an immunomodulatory cytokine and its main biological function is to limit and terminate inflammatory responses. Experimental data indicate that IL-10 might play a role in the pathogenesis of IBD and CAC. Indeed, patients carrying mutations of IL-10 receptor that abrogate IL-10 signaling develop more aggressive disease. Moreover, IL-10-deficient mice spontaneously develop colitis[86] and CAC[87] when infected with certain enteric bacteria such as H. hepaticus. In this model, colitis and CAC could be prevented by administering exogenous IL-10, thus indicating that IL-10 is pivotal in the control of inflammation and inflammation-related cancer in the gut. Analogous to IL-6, IL-10 activates STAT3 in target cells. However, the final effect is inhibition of NF-κB activation[88,89] and reduction of the expression of proinflammatory cytokines such as TNF-α, IL-6 and IL-12[90]. IL-10 has also been shown to act as an antiangiogenic factor[91].
A source of IL-10 is represented by regulatory T cells (Tregs), a class of immunosuppressive T cells. Interestingly, in a model of sporadic CRC, Erdman et al[92] showed that the transfer of wild type Tregs, but not IL-10-/- Tregs, in CRC-susceptible Apcmin-/+ mice prevented the development of adenomas and induced rapid tumor regression. These data suggest that, besides its role as a negative controller of the immune system, IL-10 might directly suppress the growth of transformed epithelial cells. Accordingly, Tregs transfer was associated with induction of epithelial cell apoptosis and downregulation of Cox-2, a molecule involved in dysplastic cell survival and proliferation.
TGF-β
Another important immunosuppressive cytokine is transforming growth factor (TGF)-β. TGF-β tightly controls the activation of the immune system and the inhibition of TGF-β signaling causes autoimmune diseases involving several organs including the gut. Moreover, the inhibition of TGF-β signaling operated by the intracellular inhibitory molecule Smad7 in gut lamina propria cells has been shown to contribute to chronic gut inflammation observed in IBD[90].
TGF-β plays an important role in epithelial cell differentiation and growth arrest. Accordingly, TGF-β signaling is found to be altered in sporadic CRC[93]. In contrast, the role of TGF-β in CAC is still unclear. Using a T cell-specific dominant negative TGF-β receptor II transgenic mouse, Becker et al[26] demonstrated that TGF-β signaling-mediated negative control of IL-6 expression in T cells is required to inhibit dysplastic epithelial cell proliferation. Conversely, IL-6 has been shown to inhibit TGF-β signaling by inducing Smad7 expression[94]. Smad3 is a key intracellular mediator of the anti-inflammatory and immunosuppressive activity of TGF-β in the colon. Accordingly, Smad3-deficient mice develop CAC that is dependent on the presence of enteric bacteria[95]. Despite the role of TGF-β as immunosuppressant and inhibitor of dysplastic cell growth, TGF-β signaling acts, under certain conditions, as a tumor promoter. Indeed, TGF-β-induced suppression of tumor-specific CD8+ T cells might favor tumor growth and progression[96].
Chemokines
Chemokines and their receptors play an integral role in IBD by regulating the accumulation of immune cells at the site of intestinal inflammation[97].
Monocyte chemoattractant protein 1 (MCP-1, CCL2), a member of the CCβ family of chemokines, is a known chemotactic factor regulating the recruitment of monocytes/macrophages and other inflammatory cells to sites of inflammation via activation of the CCR2 receptor[98]. The expression of MCP-1 is increased in the mucosa of patients with IBD[99]. Popivanova et al[100], using a model of CAC, showed that CCL2 blockade reduces the infiltration of COX-2-expressing F4/80-positive cells and suppresses COX-2 expression by infiltrating macrophages, resulting in retardation of cancer progression.
The role of chemokines in CAC is further supported by recent studies on chemokine decoy receptor D6. D6, like all decoy receptors, does not induce a conventional intracellular signal but mediates high-affinity ligand binding and efficient ligand degradation. D6 expression is increased in patients with IBD and with CAC compared with healthy subjects. In the AOM/DSS model of CAC, D6-deficient mice showed increased expression of chemokines and higher accumulation of inflammatory cells in comparison to the wild type, resulting in the development of more severe colitis and higher incidence of CAC[101].
CONCLUSION
Clinical and experimental data indicate that chronic inflammation increases the risk of developing CAC, acting at different stages of the carcinogenesis process. The constant release of free radicals is known to be genotoxic leading to the dysregulation of important oncogenes and onco-suppressors. Moreover, it is also known that the release of cytokines such as IL-6 and TNF-α during chronic colitis can promote tumor growth and that low expression of immunosuppressive cytokines such as TGF-β and IL-10 can exacerbate this process. However, it is also clear that what we call macroscopically chronic inflammation may be the result of very different kinds of immune responses and their impact on CAC development is still unclear.
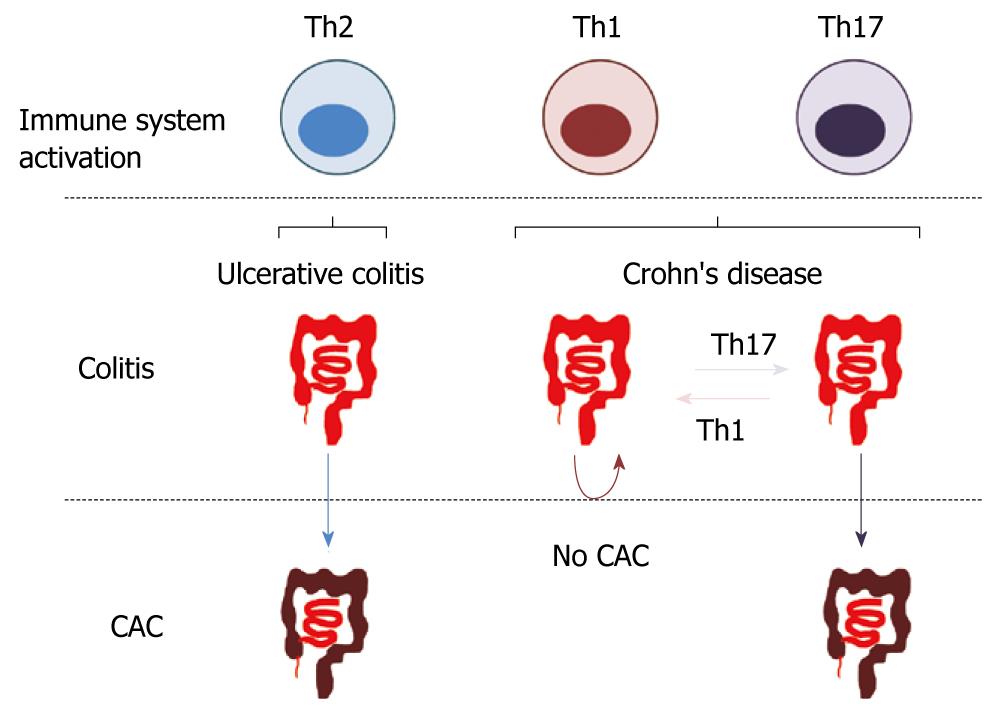
Many lines of evidence indicate that IFN-γ expressed by Th1 cells protects from tumorigenesis in different experimental models. Indeed, IFN-γ is critical in the activation of cytotoxic cells and antitumor activity. Moreover, IFN-γ renders dysplastic cells more susceptible to cell-mediated cytotoxicity. In contrast, Th2- and Th17-mediated immune responses in the gut seem to promote CAC development. Therefore, it is tempting to speculate that different Th-driven “chronic inflammations” of the gut could be associated with different risk of CAC (Figure 2). If this concept will turn out to be true, not only generic immunosuppressive therapy but also modulation of the ongoing intestinal immune response could be considered as an approach in the prevention of CAC in IBD patients.
Figure 2 Hypothetical relationship between inflammatory bowel disease and colitis associated colorectal cancer.
The T helper (Th)2 immune response characterizing ulcerative colitis determines an elevated risk of developing colitis associated colorectal cancer (CAC). In Crohn's disease, while a Th17-mediated immune response could cause inflammation and enhance CAC risk, the shift towards a Th1-mediated colitis could lower the incidence of CAC.
In clinical practice, many anti-inflammatory drugs and immune-modulators are routinely used in the therapy of IBD. Their efficacy is based on the capacity to reduce clinical manifestations related to disease and to reduce the in situ macroscopic/microscopic inflammation. However, in most of the cases little is known about their impact on the immune response at a molecular level and the consequent effect on colon carcinogenesis. An exception is represented by 5-ASA. Clinical data indicate that the long term use of 5-ASA might prevent CAC in UC patients, acting as an anti-inflammatory agent and interfering with cancer cell growth[102]. However, whether 5-ASA might act in part by modulating the activity of the immune system, sustaining immunosurveillance, has not yet been investigated. The impact of other anti-inflammatory drugs and immune-modulators on UC-related colon carcinogenesis, and their capacity to improve or dampen the immunosurveillance against dysplastic cells, are still unknown. The long term evaluation of patients undergoing different therapeutic regimens will help address this issue.