Published online Jun 28, 2011. doi: 10.3748/wjg.v17.i24.2965
Revised: November 28, 2010
Accepted: December 5, 2010
Published online: June 28, 2011
AIM: To identify cancer stem cells (CSCs) in human gallbladder carcinomas (GBCs).
METHODS: Primary GBC cells were cultured under serum-free conditions to produce floating spheres. The stem-cell properties of the sphere-forming cells, including self-renewal, differentiation potential, chemoresistance and tumorigenicity, were determined in vitro or in vivo. Cell surface expression of CD133 was investigated in primary tumors and in spheroid cells using flow cytometry. The sphere-colony-formation ability and tumorigenicity of CD133+ cells were assayed.
RESULTS: In vitro culture experiments revealed that floating spheroids were generated from primary GBC cells, and these sphere-forming cells could generate new progeny spheroids in serum-free media. Spheroid cells were differentiated under serum-containing conditions with downregulation of the stem cell markers Oct-4, Nanog, and nestin (P < 0.05). The differentiated cells showed lower spheroid-colony-formation ability than the original spheroid cells (P < 0.05). Spheroid cells were more resistant to chemotherapeutic reagents than the congenetic adherent cells (P < 0.05). Flow cytometry showed enriched CD133+ population in sphere-forming cells (P < 0.05). CD133+ cells possessed high colony-formation ability than the CD133- population (P < 0.01). CD133+ cells injected into nude mice revealed higher tumorigenicity than their antigen-negative counterparts (P < 0.05).
CONCLUSION: CD133 may be a cell surface marker for CSCs in GBC.
- Citation: Shi CJ, Gao J, Wang M, Wang X, Tian R, Zhu F, Shen M, Qin RY. CD133+ gallbladder carcinoma cells exhibit self-renewal ability and tumorigenicity. World J Gastroenterol 2011; 17(24): 2965-2971
- URL: https://www.wjgnet.com/1007-9327/full/v17/i24/2965.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i24.2965
Gallbladder carcinoma (GBC) is the most common malignant neoplasm of the biliary tract and the seventh most common gastrointestinal cancer[1]. Its clinical presentation is nonspecific and may include abdominal pain, weight loss, fever, and jaundice. Current evidence suggests that radical surgery is the only curative treatment for GBC. However, despite development in surgery, the 5-year survival rate in patients with advanced stage GBC is still only around 10%[2,3]. Pooling of carcinogens under conditions causing biliary stasis, or malignant degeneration of metaplastic changes after chronic inflammation have been suggested as possible factors, but the precise pathogenetic mechanisms of GBC remain unclear[1]. The biology of GBC therefore needs further investigation.
Emerging evidence has shown that the abilities for tumor growth and propagation reside in a small population of tumor cells, termed cancer stem cells (CSCs) or tumor-initiating cells. These cells possess properties of self-renewal, differentiation potential, resistance to chemotherapy, and high tumorigenicity[4-8]. Based on this hypothesis, CSCs were initially isolated from human acute myeloid leukemia[9]. Regarding solid tumors, the existence of CSCs in breast cancer was reported in 2003, when as few as 200 CD44+CD24-/low ESA+ breast cancer cells were shown to be adequate to produce new tumors in nonobese diabetic/severe combined immunodeficient mice, whereas a significantly higher number of other cell populations failed to form tumor xenografts[10]. Tumor-initiating cells with distinct cell surface markers have recently been identified in various solid tumors, such as brain[11], prostate[12], pancreatic[13], and ovarian cancer[14], and in Ewing’s sarcoma[15]. It is generally considered that the identification of the CSCs could have a significant impact on the understanding of tumor biology and therapy.
Several different methods have previously been used to identify CSCs[16,17], including the culture of cancer cells under non-adherent conditions in serum-free media containing epidermal growth factor (EGF) and basic fibroblast growth factor (bFGF). The growth of spherical colonies is considered to reflect the self-renewal ability and phenotype of CSCs. In the present study, we cultured primary GBC cells to generate spherical colonies and estimated their differentiation potential in the serum medium. We compared the chemoresistance of spheroid cells and differentiated cells in vitro. We also examined the expression of the CSC surface marker CD133, and investigated its use as a candidate marker to further identify the CSC phenotype in GBC, including comparing the in vitro spheroid-colony-formation and in vivo tumorigenicity of CD133+ and CD133- cells. The results of this study may clarify the phenotype of CSCs in GBC, thus contributing to the development of more effective therapeutic approaches.
Two samples of human GBC were obtained after surgical excision in accordance with Institutional Review Board-approved guidelines. Tumor tissue specimens were dissociated using scissors and scalpels, mixed with collagenase IV (Invitrogen, USA) in medium199 (collagenase 200 U/mL, Invitrogen), and incubated at 37°C for 2.5-3 h. At the end of the incubation, cells were filtered through a 40-μm nylon mesh and washed twice with phosphate-buffered saline (PBS)/10% fetal bovine serum (FBS, Gibco, USA).
The single tumor cells were suspended in serum-free DMEM/F12 (1:1 volume, Gibco) consisting of 20 ng/mL human recombinant EGF (PeproTech, USA), 20 ng/mL bFGF (PeproTech), 5 μg/mL insulin (Sigma, USA), and cultured in 24-well culture plates at a density of 1 × 104/well. Fresh serum-free DMEM/F12 (described above) was added into the wells at 0.05 mL/well every day. Spheroids were collected and dissociated 2 wk after primary culture. The resulting single cells were placed into stem cell culture medium to generate progeny spheres. Images of the spheroid colonies were recorded using an inverted microscope (Nikon, Type 108) equipped with a Nikon 2000-S Inverted Photomicroscope and Nikon NIS-Elements F2.30 software.
To assess their differentiation potential, spheres were collected and placed into DMEM/F12 supplemented with 10% FBS without growth factors, as described previously, and cell morphology was observed. After 14 d of culture in differentiating medium, tumor cells were collected and suspended in serum-free DMEM/F12 (described above), and cultured in 96-well culture plates at a density of 10 cells per well. Fresh serum-free DMEM/F12 was added into the wells at 0.025 mL per well every day. After 2 wk, each well was examined under light microscope and the total number of spheroid colonies in the 96-well plates was counted.
Total RNA was extracted from spheroid cells or adherent cells using RNeasy Mini kit (Qiagen), and was reverse transcribed into cDNA using M-MLV reverse transcriptase enzyme (Sigma). Real-time quantitative reverse transcription-polymerase chain reaction (RT-PCR) was performed using an ABI PRISM 7900HT sequence detection system (Applied Biosystems), according to the manufacturer’s instructions. The relative mRNA expression levels of the tested genes were normalized to the level of endogenous control gene, glyceraldehyde-3-phosphate dehydrogenase.
Cells were seeded in 96-well plates at 3000 cells per well. Each well was supplied with DMEM medium containing 10% FBS, together with either gemcitabine (1 μg/mL, Sigma) or 5-fluorouracil (0.1 μg/mL, Sigma), or no drug as control. The culture medium was changed 3 d after initial treatment and the number of viable cells was determined using the 3-(4,5-dimethylthiazol-2)-2,5-diphenyltetrazolium bromide (MTT) method. Briefly, 20 μL of MTT (5 mg/mL in PBS, Sigma) was added to the medium for 4 h. Medium and MTT were removed, dimethylsulfoxide (Sigma) was added, and the absorbance was measured at 490 nm using a plate reader Multiskan EX (Thermo Fisher Scientific Inc., Waltham, MA).
Cells derived from primary tumors or spheres were separately resuspended in PBS with 2% FBS at a concentration of 106/100 μL. Anti-CD133/1-phycoerythrin (eBioscience, USA) was added to the samples, and incubated on ice for 30 min. After incubation, the samples were washed twice with 2% FBS/PBS and resuspended in 2% FBS/PBS. Flow cytometric analysis was performed using a FACSAria (BD Immunocytometry Systems, Franklin Lakes, NJ, USA).
CD133+ and CD133- populations were sorted from sphere-forming cells using fluorescence-activated cell sorting (FACS). For FACS, cells were collected and stained, and sorted using a FACSAria. The sorted tumor cells were suspended in serum-free DMEM/F12, and cultured in 96-well culture plates at a density of 10/well. After 2 wk, the total number of spheroid colonies in the 96-well plate was counted, as described above.
Female nude mice (BALB/C), 4-6 wk old, were purchased from Hunan Slack King of Laboratory Animal Co., Ltd. (Changsha, China). CD133+ and CD133- populations were sorted from two primary tumors (tumor 3 and tumor 4), and from sphere-forming cells using FACS. Cells were routinely sorted twice, and reanalyzed for a purity, which was typically > 90%. Sorted cells were resuspended in PBS/Matrigel mixture (1:1 volume). The mice were anesthetized using ethyl ether and 10 000 tumor cells were injected subcutaneously into the abdominal region, using a gauge needle. The mice were maintained under standard conditions according to the institutional guidelines for animal care. Tumor appearance was inspected weekly by visual observation and palpation. Animal experiments were terminated 3 mo after cell injection.
Primary tumor tissues and mouse xenografts were fixed in 10% formalin and embedded in paraffin. Sections were cut and stained with hematoxylin and eosin (HE) to assess tumor type. The sections were incubated with anti-human CA19-9 antibody (Abcam, UK) and secondary antibodies using an ImmunoPure ABC Staining kit (Santa Cruz, USA), according to standard immunohistochemical procedures. Negative controls containing no primary antibody were prepared. All microscopic images were captured as above.
Data were expressed as mean ± SD and Student’s t test was used to compare the differences between groups. Values of P < 0.05 were considered significant.
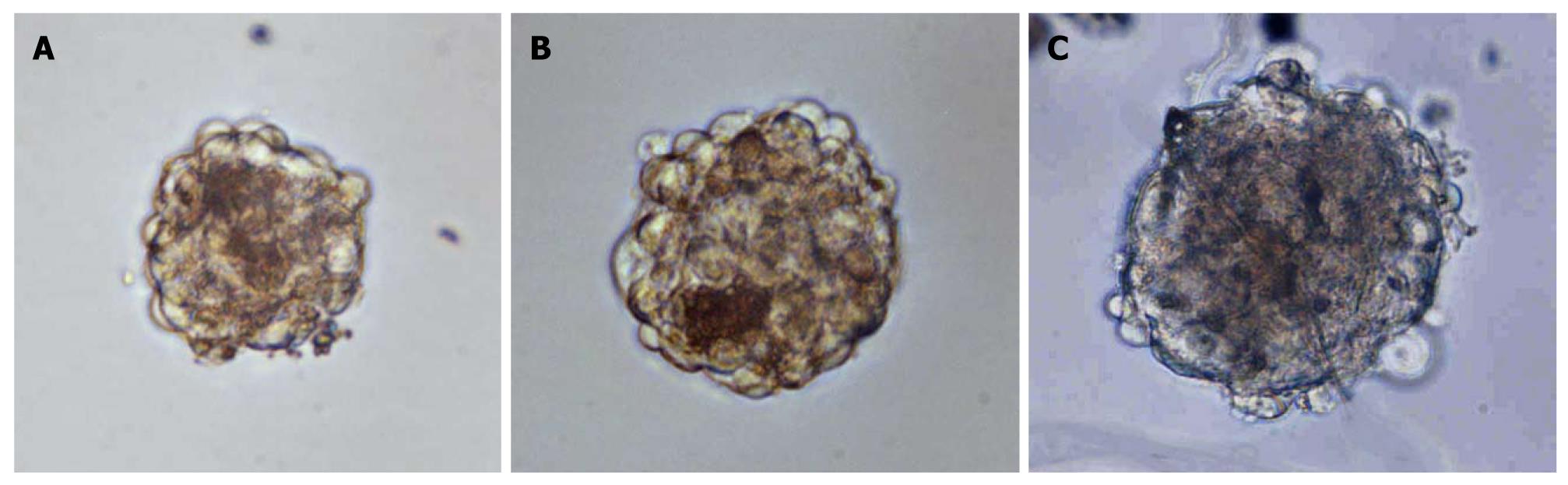
Previous studies indicated that CSCs could produce floating three-dimensional tumor spheroids under stem-cell-selective conditions[18-21]. Based on these studies, we cultured primary human GBC cells in serum-free DMEM/F12 in an attempt to expand human GBC CSCs. Non-adherent spheres derived from human GBCs were observable after in vitro culture for 1 wk (Figure 1A), and these continued to expand for 2-3 wk in serum-free media. The spheres were dissociated and the resulting single cells were plated in the same stem-cell-selective medium; similar progeny spheres emerged after 2 wk (Figure 1B and C). This demonstrated that tumor sphere cells had self-renewing characteristics.
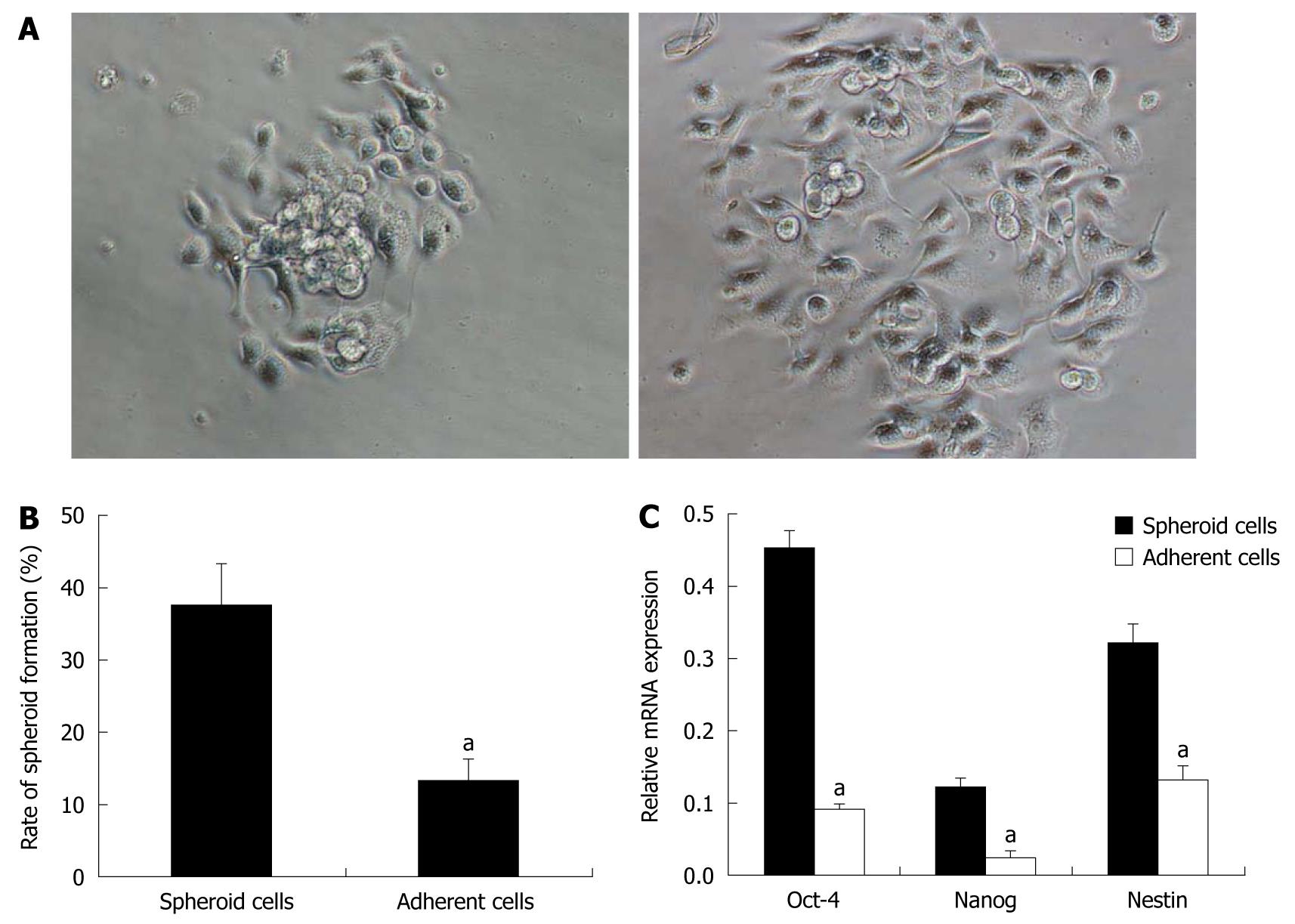
Spheres were cultivated under differentiating conditions to determine the differentiation potential of the tumor sphere cells. After 6 h of culture, the floating tumor spheres attached to the bottom of the culture plates and cells migrated from the spheres and became adherent (Figure 2A). After 14 d of culture in differentiating conditions, the sphere-formation ability of the adherent cells was assayed. The spheroid-colony-forming ability decreased, compared with that of the original sphere-forming cells (P < 0.05, Figure 2B). The expression of stem cell markers, including Oct-4, Nanog and nestin, were examined using real-time RT-PCR. These markers indicate an undifferentiated stem cell phenotype[22,23]. Spheroid cells showed higher expression of these markers than adherent cells (P < 0.05, Figure 2C), strongly supporting the idea that spheroid cells were differentiated in serum-containing medium.
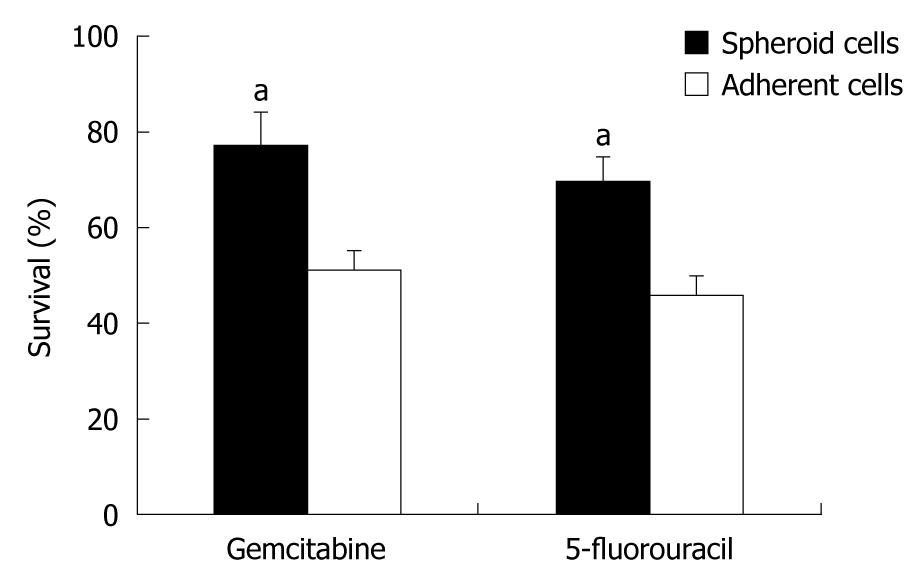
Previous studies suggested that CSCs in several solid tumors possessed higher chemoresistance than non-CSCs[24-26]. To examine if our spheroid cells also possessed a CSC chemoresistant phenotype, the chemosensitivities of these cells were assessed under stem-cell-selective vs differentiating conditions. Spheroid cells under stem-cell-selective conditions displayed a greater resistance to gemcitabine and 5-fluorouracil than those under differentiating conditions (P < 0.05, Figure 3).
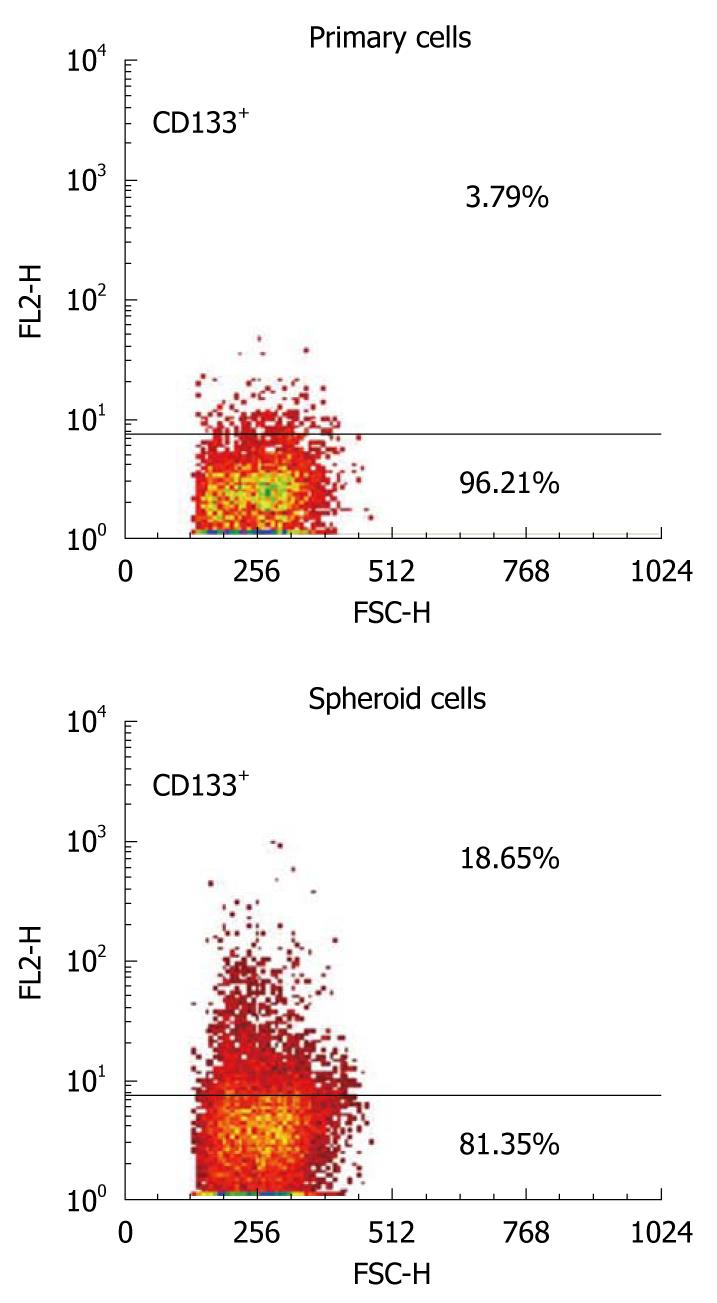
The expression pattern of a possible candidate cell surface marker for CSCs was examined in primary human GBC and in sphere-forming cells, using flow cytometry. CD133 was selected as a potential marker, based on the results of previous studies of CSCs in solid tumors. Flow cytometric analysis revealed that CD133+ cells were present at relatively low percentages in samples from both primary tumors (3.79% in tumor 1 and 3.15% in tumor 2). The CD133+ populations, however, were significantly increased to 18.65% (tumor 1) and 21.54% (tumor 2) in the tumor spheres (P < 0.05, Figure 4). These results suggest that CD133 could be a candidate cellular surface marker for GBC progenitors.
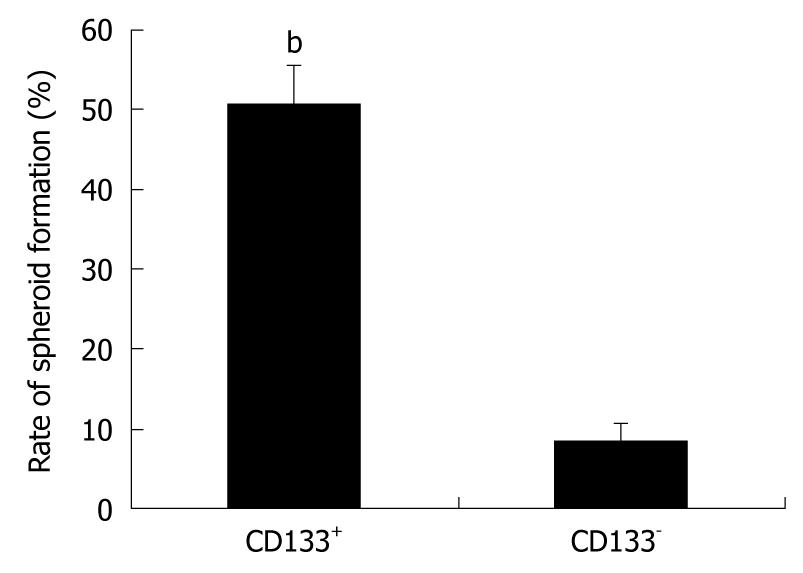
The growth of spherical colonies is considered to reflect the self-renewal ability and phenotype of CSCs[16]. CD133+ cells were isolated from spheres and placed into stem-cell-selective conditions. After in vitro culture for 2 wk, the total number of spheroid colonies containing more than 20 cells was counted, and CD133+ cells generated more spheroid colonies than the CD133- fractions (P < 0.01, Figure 5). These results suggest that the CD133+ subset plays a dominant role in the spheroids.
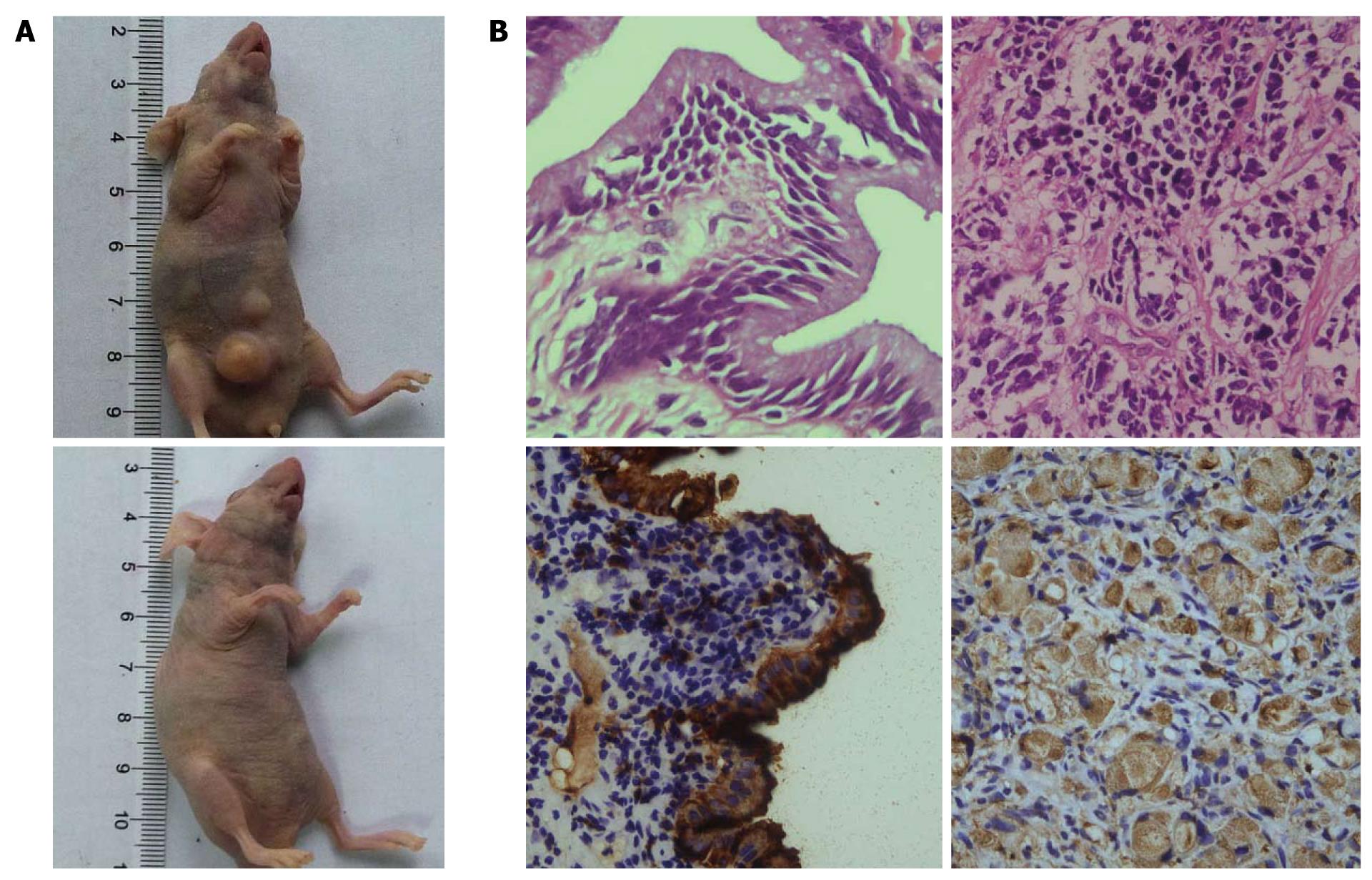
To authenticate the in vitro findings, sorted GBC cells were transplanted into nude mice. An apparent difference in tumorigenicity was observed between the cell populations (P < 0.05, Table 1, Figure 6A). It was found that 104 CD133+ GBC cells were able to generate tumors in six out of six or three out of three nude mice after 9-12 wk, while the same number of CD133- cells induced tumors in only one out of six or one out of three nude mice, with smaller mass and longer latency. HE staining and immunohistochemistry demonstrated that the xenografts in the immunodeficient mice were generated from the injected human GBC cells. The xenograft tumors revealed similar histologic characteristics and expression of CA19-9 to those of the primary GBC (Figure 6B). Taken together, these results indicate that CD133+ GBC cells exhibited cancer stem-cell-like characteristics, strongly supporting the existence of tumor-initiating cells in this population.
| Cell type | Fraction | 1st mo | 2nd mo | 3rd moa |
| Spheroid cells | CD133+ | 1/6 | 3/6 | 6/6 |
| CD133- | 0/6 | 0/6 | 1/6 | |
| Tumor 3 | CD133+ | 0/3 | 1/3 | 3/3 |
| CD133- | 0/3 | 0/3 | 1/3 | |
| Tumor 4 | CD133+ | 1/3 | 2/3 | 3/3 |
| CD133- | 0/3 | 0/3 | 0/3 |
A number of studies have demonstrated the presence of CSCs in solid tumors. These cells possess the abilities of self-renewal and differentiation, high tumorigenicity, and resistance to current treatments[10-15]. In this study, we described the characterization of CSCs in human GBC. Previous studies showed that tumor spheres could be generated from tumor cells in serum-free medium and that the constituent cells exhibited the properties of CSCs, including self-renewal, differentiation potential, chemotherapy resistance, and high tumorigenicity[11,14]. In our experiments, primary GBC cells formed tumor spheres when cultivated under stem-cell-selective conditions similar to those reported previously. The self-renewal and differentiation potentials, proliferation ability and chemosensitivity of the sphere-forming cells were assessed. These cells displayed CSC properties by regenerating new tumor spheres in serum-free medium, overexpressing stem cell markers and showing a higher resistance to chemotherapeutic reagents, while these features were diminished under differentiating conditions. These results indicate that CSCs were enriched in these floating GBC spheres.
Cell surface markers of CSCs can help distinguish, isolate and purify these tumor-initiating cells for further biological investigation. The protein CD133 is cell surface marker for CSCs in brain tumor[11], Ewing’s sarcoma[15] and liver cancers[26]. The development and differentiation of human bile ducts and liver are closely related; both start from hepatic endodermal cells and hepatoblasts just after liver primordium formation. We therefore selected CD133 as a potential CSC marker in the current study, and detected its expression in primary GBC and in sphere-forming cells. CD133+ cells comprised a small fraction of the total tumor population in all three samples studied, but represented an increased percentage of the sphere-forming cells. This suggests that CD133 could act as a cell surface marker for CSCs in GBC. We also investigated the use of this cell surface protein as a candidate marker to further identify the CSC phenotype in GBC. The self-renewal ability of CD133+ cells was tested using spheroid-forming assays in serum-free medium. CD133+ cells possessed higher clonogenicity than their antigen-negative counterparts. Subsequent in vivo tumorigenesis experiments demonstrated that CD133+ cells possessed higher tumorigenicity than the CD133- subpopulation. Furthermore, the tumors generated in nude mice displayed the same phenotype as the primary GBC tissue. Taken together, these results firmly suggest that CD133+ cells possess the potentials for self-renewal and high tumorigenicity, exhibiting cancer stem-cell-like characteristics in human GBC.
The internal relationship between the expression of CD133 and the characteristics of CSCs remains unclear. Previous studies suggested that CD133 expression was associated with cell motility in melanoma[27] and colorectal cancer cells[28], and a high level of CD133 was also associated with increased resistance to staurosporine-inducing apoptosis[28]. These associations may be due to the interaction between CD133 and the canonical Wnt pathway[27]. However, the role of CD133 in these biological activities remains to be further clarified.
In summary, the results of this study demonstrate that CSCs are enriched in non-adherent spheres derived from GBC cells, and that CD133 protein may represent a cell surface marker for this cell population.
Gallbladder carcinoma (GBC) is the most common malignant neoplasm of the biliary tract and the seventh most common gastrointestinal cancer. Emerging evidence has shown that the abilities for tumor growth and propagation reside in a small population of tumor cells, termed cancer stem cells (CSCs) or tumor-initiating cells.
Tumor-initiating cells with distinct cell surface markers have recently been identified in various solid tumors. In this study, the authors demonstrate that primary human GBC cells also contain tumor-initiating cells and that CD133 protein may be a cell surface marker for this cell population.
Previous studies have suggested that CD133 expression is associated with cell motility and is a cell surface marker for tumor-initiating cells in some tumors. This is the first study to report upregulation of CD133 in spheroids derived from primary human GBC cells. Furthermore, the in vitro and in vivo studies suggest that CD133 protein may represent a cell surface marker for CSCs in GBC.
This study may provide a novel approach to the diagnosis and treatment of GBC.
The presented manuscript deals with extremely thrilling issue of cancer development. It should be of great interest for the readers reflecting progress in our comprehension of carcinogenesis.
Peer reviewer: Giedrius Barauskas, Professor, Department of Surgery, Kaunas University of Medicine, Eiveniu str. 2, Kaunas, LT-50009, Lithuania
S- Editor Tian L L- Editor Ma JY E- Editor Zheng XM
| 1. | Reid KM, Ramos-De la Medina A, Donohue JH. Diagnosis and surgical management of gallbladder cancer: a review. J Gastrointest Surg. 2007;11:671-681. |
| 2. | Levy AD, Murakata LA, Rohrmann CA. Gallbladder carcinoma: radiologic-pathologic correlation. Radiographics. 2001;21:295-314; questionnaire, 549-555. |
| 3. | Roa I, de Aretxabala X, Araya JC, Villaseca M, Roa J, Guzmán P. [Incipient gallbladder carcinoma. Clinical and pathological study and prognosis in 196 cases]. Rev Med Chil. 2001;129:1113-1120. |
| 4. | Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105-111. |
| 5. | Scadden DT. Cancer stem cells refined. Nat Immunol. 2004;5:701-703. |
| 6. | Wicha MS, Liu S, Dontu G. Cancer stem cells: an old idea--a paradigm shift. Cancer Res. 2006;66:1883-1890; discussion 1895-1896. |
| 7. | O’Brien CA, Kreso A, Dick JE. Cancer stem cells in solid tumors: an overview. Semin Radiat Oncol. 2009;19:71-77. |
| 8. | Milas L, Hittelman WN. Cancer stem cells and tumor response to therapy: current problems and future prospects. Semin Radiat Oncol. 2009;19:96-105. |
| 9. | Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997;3:730-737. |
| 10. | Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA. 2003;100:3983-3988. |
| 11. | Singh SK, Clarke ID, Terasaki M, Bonn VE, Hawkins C, Squire J, Dirks PB. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003;63:5821-5828. |
| 12. | Collins AT, Berry PA, Hyde C, Stower MJ, Maitland NJ. Prospective identification of tumorigenic prostate cancer stem cells. Cancer Res. 2005;65:10946-10951. |
| 13. | Li C, Heidt DG, Dalerba P, Burant CF, Zhang L, Adsay V, Wicha M, Clarke MF, Simeone DM. Identification of pancreatic cancer stem cells. Cancer Res. 2007;67:1030-1037. |
| 14. | Zhang S, Balch C, Chan MW, Lai HC, Matei D, Schilder JM, Yan PS, Huang TH, Nephew KP. Identification and characterization of ovarian cancer-initiating cells from primary human tumors. Cancer Res. 2008;68:4311-4320. |
| 15. | Suvà ML, Riggi N, Stehle JC, Baumer K, Tercier S, Joseph JM, Suvà D, Clément V, Provero P, Cironi L. Identification of cancer stem cells in Ewing’s sarcoma. Cancer Res. 2009;69:1776-1781. |
| 16. | Ailles LE, Weissman IL. Cancer stem cells in solid tumors. Curr Opin Biotechnol. 2007;18:460-466. |
| 17. | Vermeulen L, Sprick MR, Kemper K, Stassi G, Medema JP. Cancer stem cells--old concepts, new insights. Cell Death Differ. 2008;15:947-958. |
| 18. | Ricci-Vitiani L, Lombardi DG, Pilozzi E, Biffoni M, Todaro M, Peschle C, De Maria R. Identification and expansion of human colon-cancer-initiating cells. Nature. 2007;445:111-115. |
| 19. | Wilson H, Huelsmeyer M, Chun R, Young KM, Friedrichs K, Argyle DJ. Isolation and characterisation of cancer stem cells from canine osteosarcoma. Vet J. 2008;175:69-75. |
| 20. | Agrama HA, Houssin SF, Tarek MA. Cloning of AFLP markers linked to resistance to Peronosclerospora sorghi in maize. Mol Genet Genomics. 2002;267:814-819. |
| 21. | Fang D, Nguyen TK, Leishear K, Finko R, Kulp AN, Hotz S, Van Belle PA, Xu X, Elder DE, Herlyn M. A tumorigenic subpopulation with stem cell properties in melanomas. Cancer Res. 2005;65:9328-9337. |
| 22. | Loh YH, Wu Q, Chew JL, Vega VB, Zhang W, Chen X, Bourque G, George J, Leong B, Liu J. The Oct4 and Nanog transcription network regulates pluripotency in mouse embryonic stem cells. Nat Genet. 2006;38:431-440. |
| 23. | Wiese C, Rolletschek A, Kania G, Blyszczuk P, Tarasov KV, Tarasova Y, Wersto RP, Boheler KR, Wobus AM. Nestin expression--a property of multi-lineage progenitor cells? Cell Mol Life Sci. 2004;61:2510-2522. |
| 24. | Vargo-Gogola T, Rosen JM. Modelling breast cancer: one size does not fit all. Nat Rev Cancer. 2007;7:659-672. |
| 25. | Takaishi S, Okumura T, Tu S, Wang SS, Shibata W, Vigneshwaran R, Gordon SA, Shimada Y, Wang TC. Identification of gastric cancer stem cells using the cell surface marker CD44. Stem Cells. 2009;27:1006-1020. |
| 26. | Zhu Z, Hao X, Yan M, Yao M, Ge C, Gu J, Li J. Cancer stem/progenitor cells are highly enriched in CD133+CD44+ population in hepatocellular carcinoma. Int J Cancer. 2010;126:2067-2078. |
| 27. | Rappa G, Fodstad O, Lorico A. The stem cell-associated antigen CD133 (Prominin-1) is a molecular therapeutic target for metastatic melanoma. Stem Cells. 2008;26:3008-3017. |