Published online Jun 28, 2011. doi: 10.3748/wjg.v17.i24.2945
Revised: February 15, 2011
Accepted: February 22, 2011
Published online: June 28, 2011
AIM: To analyze the association between the emergence of tyrosine-methionine-asparatate-asparatate (YMDD) mutants (reverse transcription; rtM204I/V) and deterioration of liver function during long-term lamivudine treatment of Japanese patients with chronic hepatitis B virus (HBV) infection.
METHODS: The data of 61 consecutive Japanese patients with chronic hepatitis B who underwent continuous lamivudine treatment for more than 24 mo and had a virological response were analyzed. Analysis of YMDD mutants was done by real-time polymerase chain reaction with LightCycler probe hybridization assay for up to 90 mo (mean, 50.8 mo; range, 24-90 mo).
RESULTS: A mixed mutant-type (YMDD + tyrosine-isoleucine-asparatate-asparatate: YIDD or tyrosine-valine-asparatate-asparatate: YVDD) or a mutant-type (YIDD or YVDD) were found in 57.4% of 61 patients at 1 year, 78.7% of 61 patients at 2 years, 79.6% of 49 patients at 3 years, 70.5% of 34 patients at 4 years, 68.4% of 19 patients at 5 years, 57.1% of 14 patients at 6 years, and 33.3% of 6 patients at 7 years. Of the 61 patients, 56 (92%) had mixed mutant- or a mutant-type. Only 5 (8%) had no mutants at each observation point. Virological breakthrough was found in 26 (46.4%) of 56 patients with YMDD mutants, 20 of whom had a hepatitis flare-up: the remaining 30 (53.6%) had neither a virological breakthrough nor a flare-up. All 20 patients who developed a hepatitis flare-up had a biochemical and virological response after adefovir was added to the lamivudine treatment.
CONCLUSION: Our results suggest that it is possible to continue lamivudine treatment, even after the emergence of YMDD mutants, up to the time that the patients develop a hepatitis flare-up.
- Citation: Murata M, Furusyo N, Unno M, Ogawa E, Toyoda K, Taniai H, Ohnishi H, Hayashi J. Long-term effects of lamivudine treatment in Japanese chronic hepatitis B patients. World J Gastroenterol 2011; 17(24): 2945-2952
- URL: https://www.wjgnet.com/1007-9327/full/v17/i24/2945.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i24.2945
Chronic hepatitis B virus (HBV) infection affects more than 350 million people worldwide, with 75% living in the Asia-Pacific region[1,2]. Although Japan was historically endemic for HBV infection, our previous studies have shown that the prevalence of hepatitis B surface antigen (HBsAg) carriage in Okinawa, Japan markedly decreased from 12.4% in 1970 to 4.2% in 1996[3,4]. However, chronic HBV infection continues to be a major health problem because it leads to the development of liver cirrhosis, hepatocellular carcinoma (HCC), and raises the risk of hepatic disease-related death. Because HBV replication is associated with liver injury, therapy for patients with chronic HBV infection aims to stop or reduce disease progression and to prevent the development of hepatic decompensation through the sustained suppression of HBV replication[5]. For this purpose, interferon or oral antiviral nucleos(t)ide analogues, such as lamivudine, adefovir, entecavir, telbivudine and tenofovir have been approved for the treatment of patients with chronic HBV infection. Previous studies have shown that HBV genotype influences liver disease progression[6,7], and our epidemiological study of the Japanese HBV genotype distribution showed that most patients were infected with genotype C[8]. Because genotype C has been reported to be associated with severe liver damage and to be resistant to non-pegylated interferon[8-10], Japanese patients with chronic hepatitis B are often given nucleos(t)ide analog treatment. Nucleos(t)ide analogs have fast and potent inhibitory effects on HBV polymerase and reverse transcriptase activity, are safe and easy to use, and can induce HBV DNA suppression, alanine aminotransferase (ALT) normalization and improvement of liver histology[11-14].
Lamivudine is the first oral nucleoside analogue to be approved for the treatment of chronic hepatitis B patients, and it has been shown to suppress HBV replication by interfering with HBV DNA polymerase and disease activity, to reduce the incidence of HCC, and to improve survival[15,16]. A study that included a large number of Japanese chronic hepatitis B patients showed lamivudine to have good virological and biochemical efficacy in long-term treatment[17].
Although there is much evidence supporting the effectiveness of lamivudine for chronic hepatitis B patients, the clinical benefit of lamivudine treatment has been eroded, in the case of long-term treatment, by the emergence of lamivudine-resistant HBV mutants with mutation of the reverse transcriptase domain of the polymerase gene. The emergence of lamivudine-resistant mutants is mainly based on point mutation from methionine to valine/isoleucine at rt204 (rt204V/I) in the tyrosine-methionine-asparatate-asparatate (YMDD) motif[18]. The emergence of lamivudine-resistant HBV has been linked to virological breakthrough, sometimes followed by biochemical breakthrough, and to flare-ups of hepatitis[19].
The detection of YMDD mutants has mainly been done by methods such as direct DNA sequencing or hybridization[20,21], but these methods are labor-intensive and time-consuming. Fluorometric real-time polymerase chain reaction (PCR) with the LightCycler probe hybridization assay is reported to be an easy, rapid and accurate method for the detection of YMDD mutants[22,23]. Few studies have sequentially assessed the emergence of YMDD mutants during long-term lamivudine treatment in Japan. The aim of the present study was to analyze the association between the emergence of YMDD mutants and deterioration of liver function during long-term lamivudine treatment of Japanese chronic hepatitis B patients by use of the LightCycler probe hybridization assay.
The study included 61 consecutive Japanese patients with chronic hepatitis B who underwent continuous lamivudine treatment for more than 24 mo, and had a virological response (defined as a decline of more than 4.0 log copies/mL in HBV DNA level during treatment). The patients started lamivudine treatment between February 2001 and May 2007 at the Department of General Internal Medicine, Kyushu-University Hospital in Fukuoka, Japan. Before the start of lamivudine treatment, all patients had HBsAg and detectable levels of HBV DNA by PCR assay. The diagnosis of chronic hepatitis and cirrhosis was based on a liver biopsy for most patients, but if unavailable it was based on clinical laboratory and ultrasonography data. None of the patients tested positive for antibody to hepatitis C virus or human immunodeficiency virus type 1, nor was there evidence of other forms of liver diseases, such as alcoholic liver disease, drug-induced liver disease, or autoimmune hepatitis.
All patients received lamivudine (Zeffix, Glaxo Smith Kline, UK) in a single oral daily dose of 100 mg. Observation was for up to 90 mo (mean, 50.8 mo; range, 24-90 mo) after the start of lamivudine administration, and the emergence of YMDD mutants during treatment was identified using the LightCycler probe hybridization assay. Serum ALT, hepatitis B e antigen (HBeAg), anti-HBe, and HBV DNA were measured every 1-2 mo. Sera were tested for mutation of the HBV polymerase gene every 6-12 mo during treatment.
Virological breakthrough was defined as an increase in serum HBV DNA of more than 1 log copies/mL from the nadir of the initial response[19]. A flare-up of hepatitis was defined as an increase in ALT level to more than 3 times the upper limit of normal.
Biochemical tests were performed using standard procedures before and at least once monthly during treatment. HBsAg, HBeAg and anti-HBe were determined by a chemiluminescence enzyme immunoassay (Abbott Japan Co., Tokyo, Japan). HBV genotype analysis was performed by a previously reported method[24]. Serum HBV DNA level was measured by a PCR-based method (Roche Amplicor HBV Monitor; Roche Diagnostics, Mannheim, Germany). The detection range of the assay was between 2.6 (corresponding to 400 copies/mL) and 8.7 log copies/mL.
Serum samples were obtained from all patients before and during lamivudine treatment and stored at -20°C until use. HBV DNA was extracted from serum using the QIAamp DNA mini kit (Qiagen Ltd., Crawley, United Kingdom) according to the manufacturer’s instructions.
Lamivudine-resistant mutation was detected by rapid PCR amplification across the YMDD-encoding gene locus and analysis of the hybridization kinetics of an integrated probe to infer its sequence was done using the LightCycler (Roche Diagnostics) according to the method reported by Whalley et al[22].
Briefly, HBV DNA was extracted from serum, and a 399 bp region of the polymerase gene was amplified by hemi-nested PCR assay. The amplified PCR product was denatured and hybridized to the Bi-probe system, which uses Cy5-labeled probes in conjunction with SYBR Green I (Bio/Gene, Kimbolton, United Kingdom). The LightCycler was used for amplification of PCR clones and to determine the melting characteristics of the probe-amplification hybrid. A melting curve analysis of the data was performed using the LightCycler analysis software v3.5 (Roche Diagnostics). Melting curves were converted to melting peaks by plotting the negative derivative of fluorescence with respect to temperature (-dF/dT). This analysis gave the melting temperature (Tm) at which 50% of the hybridizing probe was annealed to the PCR product. Because the presence of a single-base mismatch results in a shift in the melting temperature to a temperature lower than that for the probe-specific sequence, analysis of the probe melting curves allows differentiation of the wild-type YMDD from the YMDD mutants including YIDD, YVDD, and the YMDD/YIDD and YMDD/YVDD mixed types. The detection limit of this assay was about 10%-20% of the total virus pool (data not shown).
The distribution of continuous variables was analyzed by the Mann-Whitney U test. Differences in proportions were tested by Fisher’s exact test. A two-tailed P value of less than 0.05 was considered statistically significant. Statistical analysis was performed with the SPSS statistical package (version 11.0, SPSS, Inc., Chicago, IL, USA).
Of the 61 patients, 45 were male with a median age of 50 years (range, 28-65 years) and 16 female with a median age of 49 years (range, 38-69 years). All patients were infected with genotype C, 36 (59%) were negative for HBeAg, 15 (25%) were cirrhotic, and the median HBV DNA level was 6.5 log copies/mL (range, 2.7-8.7 log copies/mL).
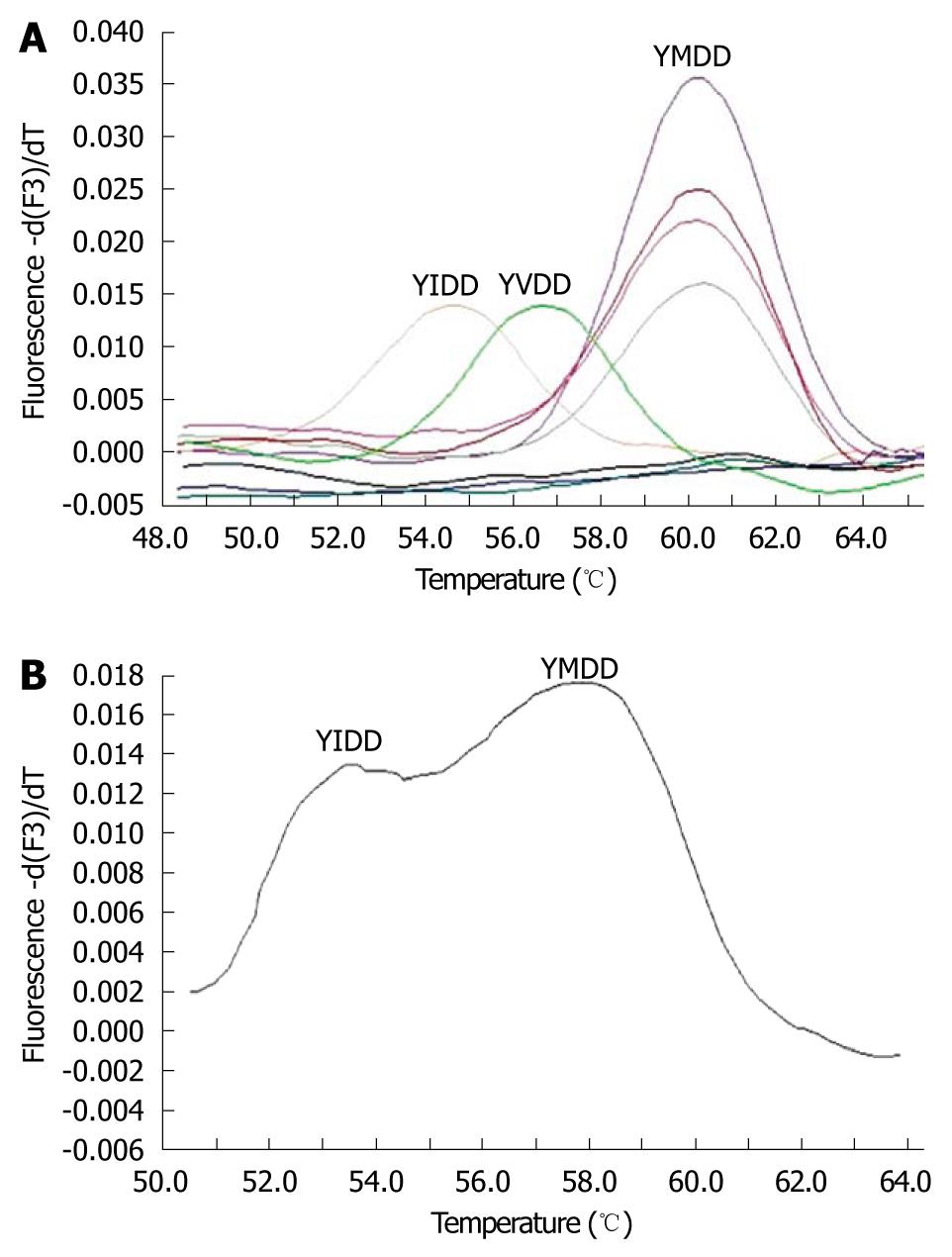
YMDD mutants were analyzed by melting curve analysis. The melting peaks of the wild-type and mutant HBV strains were typically observed at different temperatures, as shown in Figure 1A. The melting temperatures of the YIDD and YVDD mutants were approximately 9°C and 2.5°C lower than that of the wild-type, respectively. Because the melting curve showed a double peak in the case of YIDD or YVDD mutant mixed with the wild-type YMDD, as shown in Figure 1B, this type of melting curve was considered to be the mixed mutant-type.
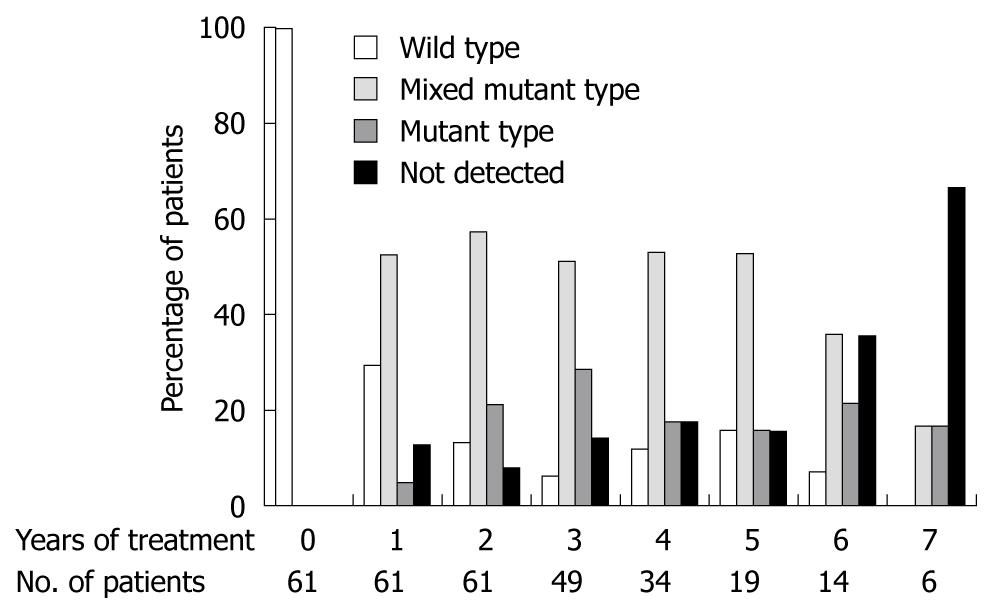
The mixed mutant- or mutant-type was found in 57.4% of 61 patients at 1 year, 78.7% of 61 patients at 2 years, 79.6% of 49 patients at 3 years, 70.5% of 34 patients at 4 years, 68.4% of 19 patients at 5 years, 57.1% of 14 patients at 6 years, 33.3% of 6 patients at 7 years, as shown in Figure 2.
Of the 61 patients, 56 (92%) had the mixed mutant- or mutant-type: Only 5 (8%) had none of the tested mutations during the observation period. Virological breakthrough or a flare-up of hepatitis was observed in 26 (46.4%) and 20 (35.7%), respectively, of the 56 patients with YMDD mutants. None of the 5 patients without YMDD mutants had virological breakthrough or a flare-up of hepatitis. Virological breakthrough or a flare-up of hepatitis was experienced significantly more often by patients with than without YMDD mutants (P < 0.0001).
No significant differences in sex, age, pretreatment ALT level, serum albumin, platelet count, frequency of HBeAg positivity, pretreatment HBV DNA level, presence of cirrhosis, or history of HCC were observed between these groups. Of the 56 patients with YMDD mutants, 30 (53.6%) had no virological breakthrough. However, no significant differences in sex, age, pretreatment ALT level, serum albumin, platelet count, frequency of HBeAg positivity, pretreatment HBV DNA level, presence of cirrhosis, or history of HCC were observed for the patients with mutants, with or without virological breakthrough (data not shown) (Table 1).
| Characteristics | Mutant- or mixed mutant-type (n = 56) | Not detected or wild-type (n = 5) | P-value |
| No. of men (%) | 42 (75.0) | 3 (80.0) | NS |
| Age (yr) | 50 (28-69) | 52 (34-55) | NS |
| ALT level (U/L) | 79 (15-1593) | 63 (44-108) | NS |
| Albumin (g/dL) | 4.1 (2.9-5.0) | 4.3 (4.0-4.3) | NS |
| Platelet count (× 104/mL) | 13.1 (3.3-43.3) | 17.8 (15.2-26.0) | NS |
| HBeAg positivity (%) | 23 (41.1) | 2 (40.0) | NS |
| HBV-DNA level (log copies/mL) | 6.5 (2.7-8.7) | 7.0 (5.4-8.7) | NS |
| Cirrhosis (%) | 22 (39.3) | 1 (20.0) | NS |
| History of HCC (%) | 8 (14.3) | 1 (20.0) | NS |
| Virological breakthrough (%) | 26 (46.4) | 0 | < 0.0001 |
| Patients with hepatitis flare-ups (%) | 20 (35.7) | 0 | < 0.0001 |
Of the 61 patients, 36 (59%) were HBeAg negative. Although about 90% of patients with or without HBeAg had YMDD mutants, HBeAg negative patients had a tendency to have a lower frequency of virological breakthrough and hepatitis flare-ups than HBeAg positive patients. However, no significant between group differences were found in sex, age, number of patients with YMDD mutants, the frequency of virological breakthrough and hepatitis flare-ups; only pretreatment HBV DNA level showed a significant difference (Table 2).
| Characteristics | HBeAg negative (n = 36) | HBeAg positive (n = 25) | P-value |
| No. of men (%) | 28 (78) | 17 (68) | NS |
| Age (yr) | 50 (29-69) | 50 (28-60) | NS |
| HBV DNA level (log copies/mL) | 5.8 (2.7-7.6) | 7.6 (4.1-8.7) | < 0.0001 |
| Patients with YMDD mutants (%) | 33 (92) | 23 (92) | NS |
| Virological breakthrough (%) | 12 (33) | 14 (56) | NS |
| Patients with flare-ups of hepatitis (%) | 9 (25) | 11 (44) | NS |
Of the 61 patients, a flare-up of hepatitis was experienced by 20 (32.8%), 15 (75%) males, 5 (25%) females, median age 56 years (range, 44-65 years), 11 (55%) with cirrhosis, and 14 (70%) with HBeAg. All patients who developed flare-ups of hepatitis following an increase in the HBV DNA level had YMDD mutation. The peak HBV DNA level (median 6.7 log copies/mL; range, 5.7-8.0 log copies/mL) at the time of a flare-up was significantly lower than at pretreatment (median 7.6 log copies/mL; range, 6.0-8.7 log copies/mL) (P < 0.05) (Table 3).
| Patient | Age (yr) | Sex | Cirrhosis | HBeAg | Change of HBV DNA level after treatment (log copies/mL) | Mutant type | ||
| Pre-treatment | Nadir | Virological breakthrough with hepatitis flare-ups | ||||||
| 1 | 54 | F | + | + | 8.7 | 4.7 | 7.6 | YIDD/YVDD |
| 2 | 56 | M | + | + | 8.1 | 4.1 | 7.9 | Mixed |
| 3 | 59 | M | + | - | 6.6 | < 2.6 | 6.8 | YIDD |
| 4 | 60 | M | + | + | 7.9 | < 2.6 | 8 | Mixed |
| 5 | 65 | M | + | + | 6.5 | < 2.6 | 6.7 | Mixed |
| 6 | 49 | M | + | + | 7.9 | 3.9 | 7 | YIDD |
| 7 | 58 | M | - | + | 8.7 | 3.9 | 7.2 | YVDD |
| 8 | 51 | M | - | + | 7.8 | 3.8 | 7.6 | YIDD/YVDD |
| 9 | 58 | M | - | + | 8.7 | 4.3 | 7.9 | Mixed |
| 10 | 61 | M | + | - | 6 | < 2.6 | 6.6 | Mixed |
| 11 | 51 | F | - | + | 7.2 | < 2.6 | 5.9 | YIDD |
| 12 | 62 | F | - | + | 7.7 | < 2.6 | 6.6 | Mixed |
| 13 | 44 | M | + | + | 6.7 | < 2.6 | 5.9 | YVDD |
| 14 | 56 | M | - | + | 8.7 | 5 | 7 | YVDD |
| 15 | 50 | F | - | + | 7.6 | 5.6 | 6.2 | YIDD |
| 16 | 47 | M | + | + | 7.9 | 3.9 | 6.4 | YIDD |
| 17 | 59 | F | + | - | 6.9 | < 2.6 | 6.1 | YVDD |
| 18 | 45 | M | - | - | 6.9 | < 2.6 | 6.1 | Mixed |
| 19 | 61 | M | - | - | 6.7 | < 2.6 | 5.9 | Mixed |
| 20 | 56 | M | + | - | 6.6 | < 2.6 | 5.7 | YVDD |
All 20 patients who had a flare-up were prescribed 10 mg of adefovir dipivoxil daily, in addition to lamivudine treatment, and all had a biochemical and virological response.
Early detection and monitoring of HBV genotypic resistance in patients with chronic hepatitis B using nucleoside analogues allows clinicians to avoid virological breakthroughs followed by flare-ups of hepatitis. In this retrospective study, we found that the LightCycler probe hybridization assay was useful for monitoring the emergence of YMDD mutants. Furthermore, our results provide important data on the YMDD mutation of Japanese chronic hepatitis B patients undergoing long-term lamivudine treatment.
Continuous lamivudine treatment is associated with an increased percentage of patients with YMDD mutants. Lamivudine-genotypic resistance was reported in 24% of patients after 1 year of treatment, 41% after 2 years, 53% after 3 years and 70% after 4 years by PCR using a restriction fragment-length polymorphism assay (PCR-RFLP)[25]. Similarly, the present study showed an increased percentage of patients with YMDD mutants within 3 years of treatment, although the percentage was higher than that of the previous report, probably a reflection of different sensitivities of the assay used for the detection of YMDD mutants. Direct DNA sequencing, PCR-RFLP, and reverse hybridization line probe assay are common methods of detecting lamivudine-genotypic resistance[20]. The detection limits of these assays are about 20%, 5%, and down to 10% of the total viral pool, respectively[21]. These methods are labor-intensive, time-consuming, and have the risk of contamination because they require s separate set of endonuclease reactions for each of the mutants, or a specific probe for each mutant. The LightCycler probe hybridization assay can detect YMDD mutants within 1 h and the risk of carryover contamination is minimal because PCR is performed in a closed glass capillary. The detection limit of this assay is about 10%-20% of the total virus pool, and minor subpopulations can be detected (those constituting about 20% of the total population). Our results may more accurately reflect the actual rates of YMDD mutants than were found in previous reports. Because of the quasi-species nature of HBV, YMDD mutants have been detected in patients with chronic hepatitis B who never received lamivudine treatment using a more sensitive method than our method[26-28]. Unfortunately, the present study could not determine if preexisting mutants prevailed after the initiation of lamivudine treatment because this assay could not detect YMDD mutants before treatment.
The present study showed that virological breakthrough and flare-ups of hepatitis occurred after the emergence of YMDD mutants, as reported previously[29]. Therefore, monitoring ALT and HBV DNA levels after the emergence of YMDD mutants is clinically important for the management of patients treated with lamivudine. It has been reported that a short latency to the emergence of YMDD mutants, mixed type YMDD mutant (YIDD + YVDD type), and a low ALT level in patients with YMDD mutants were associated with virological breakthrough or flare-up of hepatitis[30]. In the present study, 20 (35.4%) of 56 patients with YMDD mutants developed flare-ups after virological breakthrough during the treatment. As reported in previous studies[17,31], our results also showed that a flare-up of hepatitis was frequently observed in patients with cirrhosis, or HBeAg positive patients, which may be related to the more active liver disease of HBeAg positive patients. However, our results showed no virological breakthrough by about half of our patients with YMDD mutants during long-term lamivudine treatment. It is important to consider the prognosis of the patients who continued lamivudine treatment after the emergence of YMDD mutants. It has been reported that there was no benefit for patients who continued lamivudine treatment after the emergence of YMDD mutants compared with patients who discontinued treatment, based on a comparison of the rates of flare-ups of hepatitis, hepatic decompensation, and HBe seroconversion over a 12-mo period[32]. Another report, however, showed a benefit of long-term lamivudine treatment, for 8 years, in Asian patients with YMDD mutants without advanced disease, who had a lower risk of development of cirrhosis and HCC, a greater reduction of HBV DNA level, and a similar rate of flare-ups of hepatitis compared with untreated patients[33]. Our data from the present study suggests that it is possible to continue lamivudine treatment even after the emergence of YMDD mutants if clinicians note the above risk factors associated with virological breakthrough or flare-ups of hepatitis.
The present study showed that about half of patients with YMDD mutants did not encounter virological breakthrough during long-term lamivudine treatment. It has previously been shown that YMDD mutants (rtM204V or rtM204I) have preexisting polymorphisms in HBV-infected patients because of the quasi-species nature of HBV in infected individuals, and that these mutants appeared randomly in viral populations, which had a replication disadvantage to the YMDD wild-type in the absence of lamivudine[34]. A previous study showed that HBV mutants with mutations in the YMDD motif in patients before treatment would not be selected by lamivudine or induce breakthrough hepatitis[27]. Furthermore, the rtM204V mutant in domain C frequently accompanies rtL180M mutants in domain B[26]. In vitro studies showed that rtM204I alone had lower replication competency than rtL180M/rtM204V[35]. These data suggest that the gain of replication capacity of YMDD mutants during lamivudine treatment may be associated with multiple factors, including intrinsic replicative advantages potentially conferred by mutations accumulating outside domain C, the fluctuating environment in which these mutants replicate, and the host immune response.
In the present study, 5 (8%) of 61 patients had no emergence of YMDD mutation during the treatment. Hashimoto et al[30] reported that factors associated with YMDD mutants not appearing during 5-year lamivudine therapy for patients with chronic HBV infection are HBeAg negativity, lack of cirrhosis, and high γ GTP level. We were not able to confirm their results because there were too few patients free of YMDD mutants to draw a significant conclusion.
Adding adefovir dipivoxil, which is without cross-resistance to lamivudine, is effective for achieving a virological and biochemical response in patients with lamivudine-resistance[36,37]. The American Association for the study of Liver Disease guidelines on HBV recommend the addiction of a second drug in the event of lamivudine resistance[38,39]. It has been reported for patients with lamivudine resistance that the virological and biochemical response rates were similar between a group being switched to adefovir monotherapy and a group for which adefovir was added to lamivudine treatment, but adefovir resistance more frequently occurred in the patients who had combined adefovir and lamivudine treatment[37,40]. Therefore, the add-on treatment is thought to be superior to switching treatment with regard to the prevention of subsequent multi-drug resistance. The above data supported our result of a biochemical and virological response by all patients who had adefovir added to lamivudine treatment after a flare-up of hepatitis.
In conclusion, no virological breakthrough was observed in about half of the patients with YMDD mutants during long-term lamivudine treatment. Patients who developed flare-ups of hepatitis were successful in achieving a virological and biochemical response by addition of adefovir to lamivudine treatment. These data suggest that it is possible to continue lamivudine treatment even after emergence of YMDD mutants, at least until the patients develop a flare-up of hepatitis.
Although there is much evidence of the effectiveness of lamivudine in chronic hepatitis B patients, the number of patients with tyrosine-methionine-asparatate-asparatate (YMDD) motif mutation, which is linked to virological breakthrough, sometimes followed by a flare-up of hepatitis, is higher with prolonged lamivudine treatment. There is little information about the association between YMDD mutants and deterioration of liver function during long-term lamivudine treatment.
The detection of YMDD mutants has mainly been by methods such as direct DNA sequencing, polymerase chain reaction (PCR)-restriction fragment length polymorphism, or reverse hybridization line probe assay, but these methods are labor-intensive, time-consuming, and have the risk of contamination. In this study, the authors demonstrated that fluorometric real-time PCR with the LightCycler probe hybridization assay was an easy, rapid and accurate method for the detection of YMDD mutants.
Regardless of hepatitis B e antigen positivity, the present study showed, by use of the LightCycler probe hybridization assay, that about half of the patients with YMDD mutants did not encounter virological breakthrough during long-term lamivudine treatment. Furthermore, all patients who developed flare-ups of hepatitis had a biochemical and virological response after adefovir was added to the lamivudine treatment.
The results suggest that it is possible to continue lamivudine treatment, even after the emergence of YMDD mutants, up to the time that the patients develop flare-ups of hepatitis.
In this study, Murata et al retrospectively analyzed 61 HBV patients for up to 90 mo to find out the association between lamivudine resistance from its emergence with the hepatic deterioration. The positive finding of this study is the indication of continuation of lamivudine therapy in patients of genotype C having YMDD mutation until the stage of hepatic flare-up.The potential of Light Cycler probe hybridization for detection and monitoring of such mutants has also been elucidated. This is an important issue because lamivudine resistance is associated with progressive liver disease.
Peer reviewers: Nageshwar D Reddy, Professor, Asian Institute of Gastroenterology, 6-3-652, Somajiguda, Hyderabad 500 082, India; Hanna Gregorek, PhD, Assistant Professor, Department of Microbiology and Clinical Immunology, The Children’s Memorial Health Institute, Al. Dzieci Polskich 20, Warsaw 04-730, Poland
S- Editor Sun H L- Editor Cant MR E- Editor Zheng XM
| 2. | Lavanchy D. Hepatitis B virus epidemiology, disease burden, treatment, and current and emerging prevention and control measures. J Viral Hepat. 2004;11:97-107. |
| 3. | Kashiwagi S, Hayashi J, Ikematsu H, Nomura H, Kusaba T, Shingu T, Hayashida K, Kaji M. An epidemiologic study of hepatitis B virus in Okinawa and Kyushu, Japan. Am J Epidemiol. 1983;118:787-794. |
| 4. | Furusyo N, Hayashi J, Sawayama Y, Kawakami Y, Kishihara Y, Kashiwagi S. The elimination of hepatitis B virus infection: changing seroepidemiology of hepatitis A and B virus infection in Okinawa, Japan over a 26-year period. Am J Trop Med Hyg. 1998;59:693-698. |
| 5. | Liaw YF. Hepatitis B virus replication and liver disease progression: the impact of antiviral therapy. Antivir Ther. 2006;11:669-679. |
| 6. | Sumi H, Yokosuka O, Seki N, Arai M, Imazeki F, Kurihara T, Kanda T, Fukai K, Kato M, Saisho H. Influence of hepatitis B virus genotypes on the progression of chronic type B liver disease. Hepatology. 2003;37:19-26. |
| 7. | Yuen MF, Fung SK, Tanaka Y, Kato T, Mizokami M, Yuen JC, Wong DK, Yuan HJ, Sum SM, Chan AO. Longitudinal study of hepatitis activity and viral replication before and after HBeAg seroconversion in chronic hepatitis B patients infected with genotypes B and C. J Clin Microbiol. 2004;42:5036-5040. |
| 8. | Furusyo N, Nakashima H, Kashiwagi K, Kubo N, Hayashida K, Usuda S, Mishiro S, Kashiwagi S, Hayashi J. Clinical outcomes of hepatitis B virus (HBV) genotypes B and C in Japanese patients with chronic HBV infection. Am J Trop Med Hyg. 2002;67:151-157. |
| 9. | Nakashima H, Furusyo N, Kubo N, Kashiwagi K, Etoh Y, Kashiwagi S, Hayashi J. Double point mutation in the core promoter region of hepatitis B virus (HBV) genotype C may be related to liver deterioration in patients with chronic HBV infection. J Gastroenterol Hepatol. 2004;19:541-550. |
| 10. | Akuta N, Kumada H. Influence of hepatitis B virus genotypes on the response to antiviral therapies. J Antimicrob Chemother. 2005;55:139-142. |
| 11. | Dienstag JL, Goldin RD, Heathcote EJ, Hann HW, Woessner M, Stephenson SL, Gardner S, Gray DF, Schiff ER. Histological outcome during long-term lamivudine therapy. Gastroenterology. 2003;124:105-117. |
| 12. | Hadziyannis SJ, Tassopoulos NC, Heathcote EJ, Chang TT, Kitis G, Rizzetto M, Marcellin P, Lim SG, Goodman Z, Ma J. Long-term therapy with adefovir dipivoxil for HBeAg-negative chronic hepatitis B for up to 5 years. Gastroenterology. 2006;131:1743-1751. |
| 13. | Gish RG, Lok AS, Chang TT, de Man RA, Gadano A, Sollano J, Han KH, Chao YC, Lee SD, Harris M. Entecavir therapy for up to 96 weeks in patients with HBeAg-positive chronic hepatitis B. Gastroenterology. 2007;133:1437-1444. |
| 14. | Lai CL, Gane E, Liaw YF, Hsu CW, Thongsawat S, Wang Y, Chen Y, Heathcote EJ, Rasenack J, Bzowej N. Telbivudine versus lamivudine in patients with chronic hepatitis B. N Engl J Med. 2007;357:2576-2588. |
| 15. | Liaw YF, Sung JJ, Chow WC, Farrell G, Lee CZ, Yuen H, Tanwandee T, Tao QM, Shue K, Keene ON, Dixon JS, Gray DF, Sabbat J. Lamivudine for patients with chronic hepatitis B and advanced liver disease. N Engl J Med. 2004;351:1521-1531. |
| 16. | Papatheodoridis GV, Dimou E, Dimakopoulos K, Manolakopoulos S, Rapti I, Kitis G, Tzourmakliotis D, Manesis E, Hadziyannis SJ. Outcome of hepatitis B e antigen-negative chronic hepatitis B on long-term nucleos(t)ide analog therapy starting with lamivudine. Hepatology. 2005;42:121-129. |
| 17. | Furusyo N, Takeoka H, Toyoda K, Murata M, Tanabe Y, Kajiwara E, Shimono J, Masumoto A, Maruyama T, Nomura H. Long-term lamivudine treatment for chronic hepatitis B in Japanese patients: a project of Kyushu University Liver Disease Study. World J Gastroenterol. 2006;12:561-567. |
| 18. | Allen MI, Deslauriers M, Andrews CW, Tipples GA, Walters KA, Tyrrell DL, Brown N, Condreay LD. Identification and characterization of mutations in hepatitis B virus resistant to lamivudine. Lamivudine Clinical Investigation Group. Hepatology. 1998;27:1670-1677. |
| 19. | Lok AS, Zoulim F, Locarnini S, Bartholomeusz A, Ghany MG, Pawlotsky JM, Liaw YF, Mizokami M, Kuiken C. Antiviral drug-resistant HBV: standardization of nomenclature and assays and recommendations for management. Hepatology. 2007;46:254-265. |
| 20. | Zoulim F, Locarnini S. Hepatitis B virus resistance to nucleos(t)ide analogues. Gastroenterology. 2009;137:1593-1608.e1-2. |
| 21. | Poordad F, Chee GM. Viral resistance in hepatitis B: prevalence and management. Curr Gastroenterol Rep. 2010;12:62-69. |
| 22. | Whalley SA, Brown D, Teo CG, Dusheiko GM, Saunders NA. Monitoring the emergence of hepatitis B virus polymerase gene variants during lamivudine therapy using the LightCycler. J Clin Microbiol. 2001;39:1456-1459. |
| 23. | Umeoka F, Iwasaki Y, Matsumura M, Takaki A, Kobashi H, Tatsukawa M, Shiraha H, Fujioka S, Sakaguchi K, Shiratori Y. Early detection and quantification of lamivudine-resistant hepatitis B virus mutants by fluorescent biprobe hybridization assay in lamivudine-treated patients. J Gastroenterol. 2006;41:693-701. |
| 24. | Osiowy C, Giles E. Evaluation of the INNO-LiPA HBV genotyping assay for determination of hepatitis B virus genotype. J Clin Microbiol. 2003;41:5473-5477. |
| 25. | Lai CL, Dienstag J, Schiff E, Leung NW, Atkins M, Hunt C, Brown N, Woessner M, Boehme R, Condreay L. Prevalence and clinical correlates of YMDD variants during lamivudine therapy for patients with chronic hepatitis B. Clin Infect Dis. 2003;36:687-696. |
| 26. | Lee CZ, Lee HS, Huang GT, Yang PM, Sheu JC. Detection of YMDD mutation using mutant-specific primers in chronic hepatitis B patients before and after lamivudine treatment. World J Gastroenterol. 2006;12:5301-5305. |
| 27. | Matsuda M, Suzuki F, Suzuki Y, Tsubota A, Akuta N, Hosaka T, Someya T, Kobayashi M, Saitoh S, Arase Y. YMDD mutants in patients with chronic hepatitis B before treatment are not selected by lamivudine. J Med Virol. 2004;74:361-366. |
| 28. | Kirishima T, Okanoue T, Daimon Y, Itoh Y, Nakamura H, Morita A, Toyama T, Minami M. Detection of YMDD mutant using a novel sensitive method in chronic liver disease type B patients before and during lamivudine treatment. J Hepatol. 2002;37:259-265. |
| 29. | Lee CH, Kim SO, Byun KS, Moon MS, Kim EO, Yeon JE, Yoo W, Hong SP. Predominance of hepatitis B virus YMDD mutants is prognostic of viral DNA breakthrough. Gastroenterology. 2006;130:1144-1152. |
| 30. | Hashimoto Y, Suzuki F, Hirakawa M, Kawamura Y, Yatsuji H, Sezaki H, Hosaka T, Akuta N, Kobayashi M, Saito S. Clinical and virological effects of long-term (over 5 years) lamivudine therapy. J Med Virol. 2010;82:684-691. |
| 31. | Sun J, Wang Z, Ma S, Zeng G, Zhou Z, Luo K, Hou J. Clinical and virological characteristics of lamivudine resistance in chronic hepatitis B patients: a single center experience. J Med Virol. 2005;75:391-398. |
| 32. | Liaw YF, Chien RN, Yeh CT. No benefit to continue lamivudine therapy after emergence of YMDD mutations. Antivir Ther. 2004;9:257-262. |
| 33. | Yuen MF, Seto WK, Chow DH, Tsui K, Wong DK, Ngai VW, Wong BC, Fung J, Yuen JC, Lai CL. Long-term lamivudine therapy reduces the risk of long-term complications of chronic hepatitis B infection even in patients without advanced disease. Antivir Ther. 2007;12:1295-1303. |
| 34. | Pallier C, Castéra L, Soulier A, Hézode C, Nordmann P, Dhumeaux D, Pawlotsky JM. Dynamics of hepatitis B virus resistance to lamivudine. J Virol. 2006;80:643-653. |
| 35. | Ono SK, Kato N, Shiratori Y, Kato J, Goto T, Schinazi RF, Carrilho FJ, Omata M. The polymerase L528M mutation cooperates with nucleotide binding-site mutations, increasing hepatitis B virus replication and drug resistance. J Clin Invest. 2001;107:449-455. |
| 36. | Lampertico P, Viganò M, Manenti E, Iavarone M, Lunghi G, Colombo M. Adefovir rapidly suppresses hepatitis B in HBeAg-negative patients developing genotypic resistance to lamivudine. Hepatology. 2005;42:1414-1419. |
| 37. | Rapti I, Dimou E, Mitsoula P, Hadziyannis SJ. Adding-on versus switching-to adefovir therapy in lamivudine-resistant HBeAg-negative chronic hepatitis B. Hepatology. 2007;45:307-313. |
| 38. | Lok AS, McMahon BJ. Chronic hepatitis B: update 2009. Hepatology. 2009;50:661-662. |
| 39. | Keeffe EB, Dieterich DT, Han SH, Jacobson IM, Martin P, Schiff ER, Tobias H. A treatment algorithm for the management of chronic hepatitis B virus infection in the United States: 2008 update. Clin Gastroenterol Hepatol. 2008;6:1315-1341; quiz 1286. |
| 40. | Lampertico P, Viganò M, Manenti E, Iavarone M, Sablon E, Colombo M. Low resistance to adefovir combined with lamivudine: a 3-year study of 145 lamivudine-resistant hepatitis B patients. Gastroenterology. 2007;133:1445-1451. |