Published online Jun 28, 2011. doi: 10.3748/wjg.v17.i24.2933
Revised: February 15, 2011
Accepted: February 22, 2011
Published online: June 28, 2011
AIM: To investigate the role of α-fetoprotein (AFP), a cancer-associated fetal glycoprotein, in glucocorticoid-induced precocious maturation in rat colon.
METHODS: Colons from suckling Sprague-Dawley rats were used in this study. Corticosterone acetate at a dose of 100 μg/g body weight was given to normal pups on days 7, 9 and 11 after birth to induce hypercorticoidism. Control animals were injected with identical volumes of normal saline. Some rats receiving corticosterone 7 d after birth were also treated with mifepristone (RU38486), a glucocorticoid cytoplasm receptor antagonist to investigate the effects of glucocorticoids (GCs). The morphological changes of the crypt depth and villous height of the villous zone in colon were observed as indices of colon maturation. Expression levels of AFP in colons were detected by reverse transcriptase polymerase chain reaction and Western blotting. To identify the cellular localization of AFP in developing rat colons, double-immunofluorescent staining was performed using antibodies to specific mesenchymal cell marker and AFP.
RESULTS: Corticosterone increased the crypt depth and villous height in the colon of 8- and 10-d-old rats with hypercorticoidism compared with that in the control animals (120% in 8-d-old rats and 118% in 10-d-old rats in villous height, P = 0.021; 145% in 8-d-old rats and 124% in 10-d-old rats in crypt depth, P = 0.017). These increases were accompanied by an increase of AFP expression in both mRNA and protein (2.5-folds in 8-d-old and 2.5-folds in 10-d-old rats higher than in control animals, P = 0.035; 1.8-folds in 8-d-old and 1.3-folds in 10-d-old rats higher than in control animals, P = 0.023). Increased crypt depth and villous height and increased expression of AFP in the colon of rats with hypercorticoidism were blocked by mifepristone. Both had positive staining for AFP or vimentin, and overlapped in mesenchymal cells at each tested colon.
CONCLUSION: GCs promote the development of rat colon. AFP appears to be involved, in part, in mediating the effects of GCs in the developmental colon.
- Citation: Chen M, Sun P, Liu XY, Dong D, Du J, Gu L, Ge YB. α-fetoprotein involvement during glucocorticoid-induced precocious maturation in rat colon. World J Gastroenterol 2011; 17(24): 2933-2940
- URL: https://www.wjgnet.com/1007-9327/full/v17/i24/2933.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i24.2933
Glucocorticoids (GCs) have been routinely used in clinical practice to prevent diseases of prematurity such as respiratory distress syndrome. GCs treatment may reduce the incidence of necrotizing enterocolitis[1-3], in which a typical immaturity of the intestine[4-6] indicated that GCs may enhance intestinal maturation. Although this regulation seems to be mediated by the GCs receptor pathway, the precise regulatory mechanisms have not yet been documented.
The effect of GCs may require interactions with another tissue or cell type, such as mesenchyme[7]. During late embryogenesis, the mouse colon develops from a pseudostratified, undifferentiated endoderm to a single-layered columnar epithelium accompanying with mesenchymal maturation. Mammalian α-fetoprotein (AFP) is a single-chain glycoprotein with a molecular mass ranging from 66 to 72 kDa and 3%-5% carbohydrate (glycan) content. Recent reports showed that the AFP was expressed and produced in mesenchymal cells and was considered as an important growth factor with a specific function in gastrointestinal development, including pancreas and colon[8-10]. In contrast, AFP enhancer segment contains a sequence resembling the steroid hormone response element and the enhancer activity was mediated by dexamethasone in a dose-dependent manner[11]. Thus, AFP seems to play a role in GCs-induced precocious gastrointestinal maturation.
Numerous investigators have reported the ability of GCs to stimulate intestinal maturation in postnatal rodents[12-14]. However, little is known about the involvement of GCs in rat colon during development.
Corticosterone acetate (CA), gel mount aqueous mounting medium (G0918), and mifepristone were purchased from Sigma Chemical (St. Louis, USA). TRIZOL reagent was purchased from Invitrogen Life Technologies (Burlington, Ontario, Canada). Takara RNA polymerase chain reaction (PCR) 3.0 Kit was from Takara (Dalian, China). Rabbit anti-goat IgG conjugates and goat polyclonal anti-AFP were from Santa Cruz Biotech (USA). Monoclonal antibody of anti-vimentin was from Chemicon International (Temecula, CA, USA). Protease inhibitor cocktail was from Roche (Mannheim, Germany). Nitrocellulose membranes were from Bio-Rad (Hercules, CA, USA), BCA Protein Assay Kit and enhanced chemiluminescence reagents were from Pierce (Rockford, IL, USA) and CBL202 from Chemicon International, Inc. Temecula (CA, USA).
Pregnant female Sprague-Dawley rats in late gestation were purchased from Nanjing Medical University Animal Centre. After arrival, cages were checked twice daily for pups. The day of birth was designated as day 0, and experiments were conducted at various ages thereafter. Litter size was restricted to 12 pups per dam that was performed at day 2 postpartum. All animals were kept in a temperature-controlled room (22 ± 1°C) and maintained on a 12-h light-dark cycle. To prevent the stress of removing one animal from a cage on sequential days, all the animals in a cage were killed on a single day. Young rats were never separated from their mothers except during the period of fasting. During this period, the pups were placed in cages artificially warmed by electric light bulbs. Before studies, all rats were fasted for various times. To prevent mortality from prolonged fasting, the 8- and 10-d-old rats were fasted for 18 h, and 14-d-old rats were fasted for 24 h. Experiments were designed using littermate controls disregarding the sex of the pups. All animals were killed between 8 and 10 am[15]. CA was given at a dose of 100 μg/g body weight by intraperitoneal injection to normal pups on days 7, 9, and 11 to induce hypercorticoidism. Control animals were given identical volumes of normal saline[16]. To observe the effect of GCs on suckling rats, another experiment was designed as follows: 100 μg/g body weight of CA was given once to pairs of 7-d-old pups (HC group) by intraperitoneal injection. Control pups (C group) were injected with identical volumes of normal saline at the same day. Pups were treated with 50 μg/g body weight mifepristone (17β-hydroxy-11β-4-dimethyl-aminophenyl-17α-propynylestra-4,9-diene-3-one, RU38486), a glucocorticoid cytoplasm receptor antagonist alone (M group), or 100 μg/g hydrocortisone plus 50 μg/g mifepristone (H+M group)[17].
Colons from control and hypercorticoidism rats at 8, 10 and 14 d of age were used in this study and five rats were used in each age stage. Samples were fixed in 4% paraformaldehyde overnight at 4°C followed by a standard protocol of dehydration and paraffin embedding. Five-micrometer sections were prepared for morphological and fluorescence immunohistochemical studies. Total RNA and lysate were extracted from tissues at each time point for the reverse transcriptase PCR (RT-PCR) and Western blotting analysis. The study protocol was approved by the Nanjing Medical University Animal Care and Use Committee.
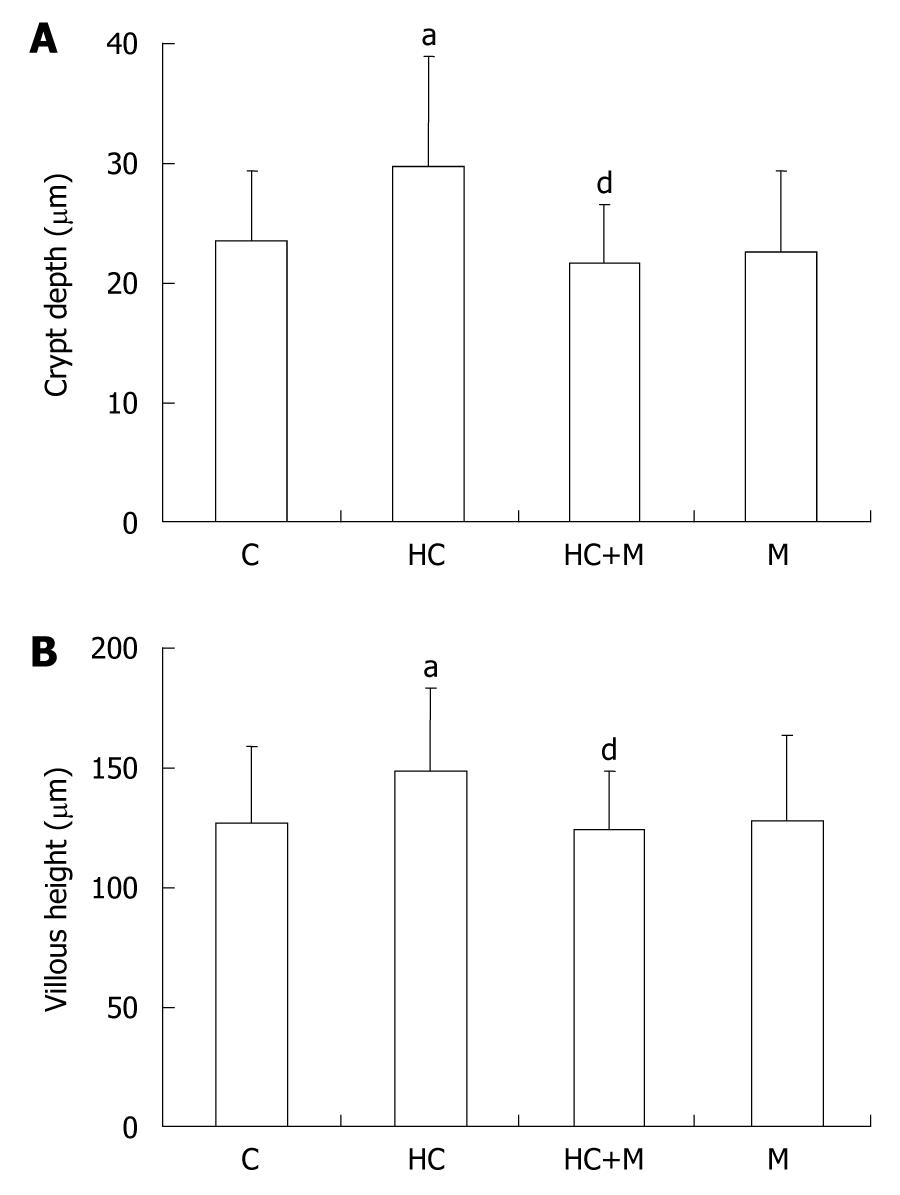
Routine hematoxylin and eosin-stained sections for light microscopic (LM) evaluation were used to study the hormonal effects in the morphological development of colon. Slides were viewed under an Olympus BX51 microscope (Olympus Optical, Tokyo, Japan). Morphologic measurement made by the same technician was blinded to the different treatment groups. Under LM, the crypt depth and villous height of the villous zone were measured using an image analysis system (NYD100). The group means were obtained based on 10 villi and 20 crypts per slide, with a minimum of five animals in each group.
Blood samples of rats were centrifuged at 2000 ×g for 20 min, and sera were stored at -20°C until analyzed. AFP levels in the rat serum were measured by the routine standard radioactive method used in the Nanjing Clinical Nuclear Medicine Center (Nanjing, China).
Total RNA was isolated from tissues by Trizol according to the protocol supplied by the manufacturers. cDNA was synthesized using Takara RNA PCR 3.0 Kit in a total volume of 10 μL, containing 0.5 μL avian myeloblastosis virus RT, 0.5 μL random 9 primer, 2 μL 25 mmol/L MgCL2, 1 μL 10 × RT buffer, 1 μL dNTP mixture (each 10 mmol/L), 0.25 μL RNase inhibitor, 1 μL RNA, and 3.75 μL dH2O. Conditions for RT were: 30°C for 10 min, 42°C for 25 min, 99°C for 5 min, and 5°C for 5 min. PCR was performed in 50 μL reactions containing 2.5 ng cDNA, 1 μL each primer pair, and 25 μL Premix Taq in the Takara RNA PCR kit. PCR was carried out in a T-gradient Biometra PCR thermal cycler (Montreal Biotech Inc., Kirkland, Quebec, Canada) to determine the annealing temperature for each paired primers. The following AFP primer pairs were used: 5'-GCTGAACCCAGAGTACTGCAC-3' (forward), and 5'-GACACGTCGTAGATGAACGTG-3' (reverse). Amplification reactions were carried out for 30 cycles at 94°C for 30 s, 58.4°C for 30 s, and at 72°C for 1 min.
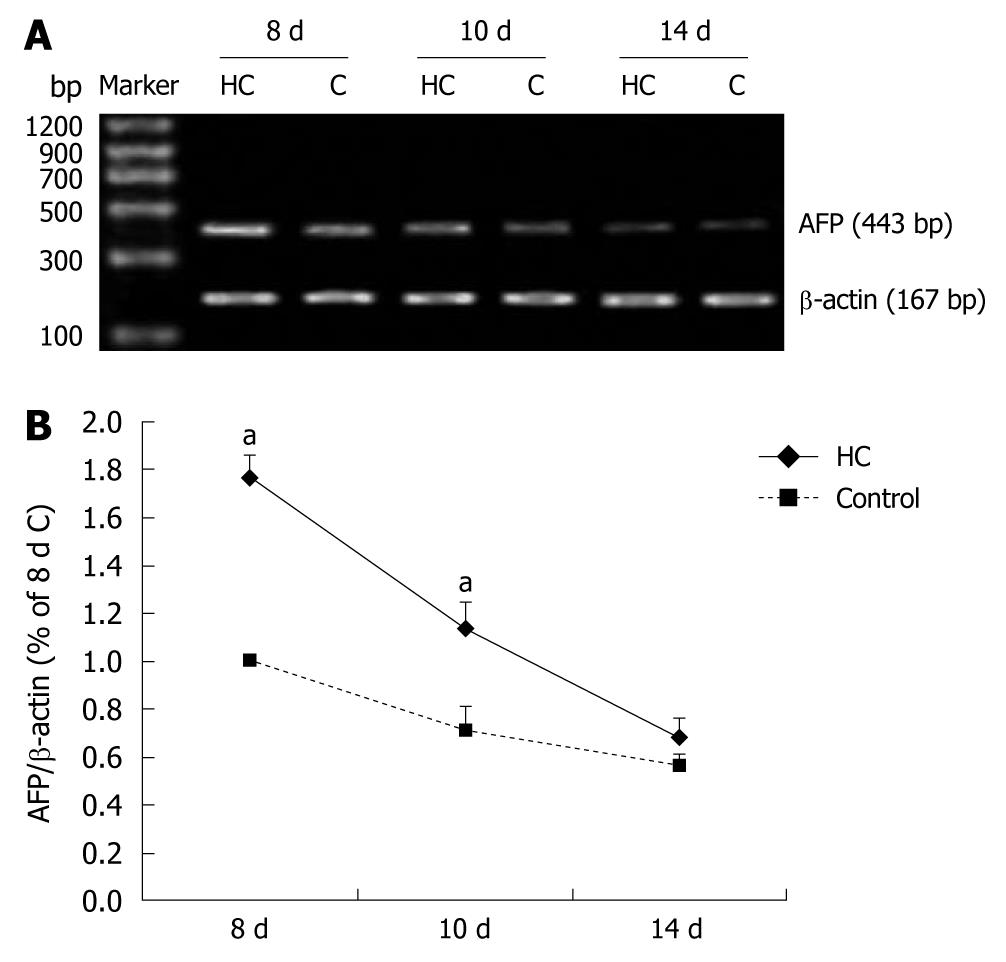
The amplified products were 443 bp and analyzed on 1% agarose gels and visualized by ethidium bromide staining. Omitting RT, cDNA or DNA polymerase were adopted in the controls, and showed no reaction bands. The data were normalized by actin.
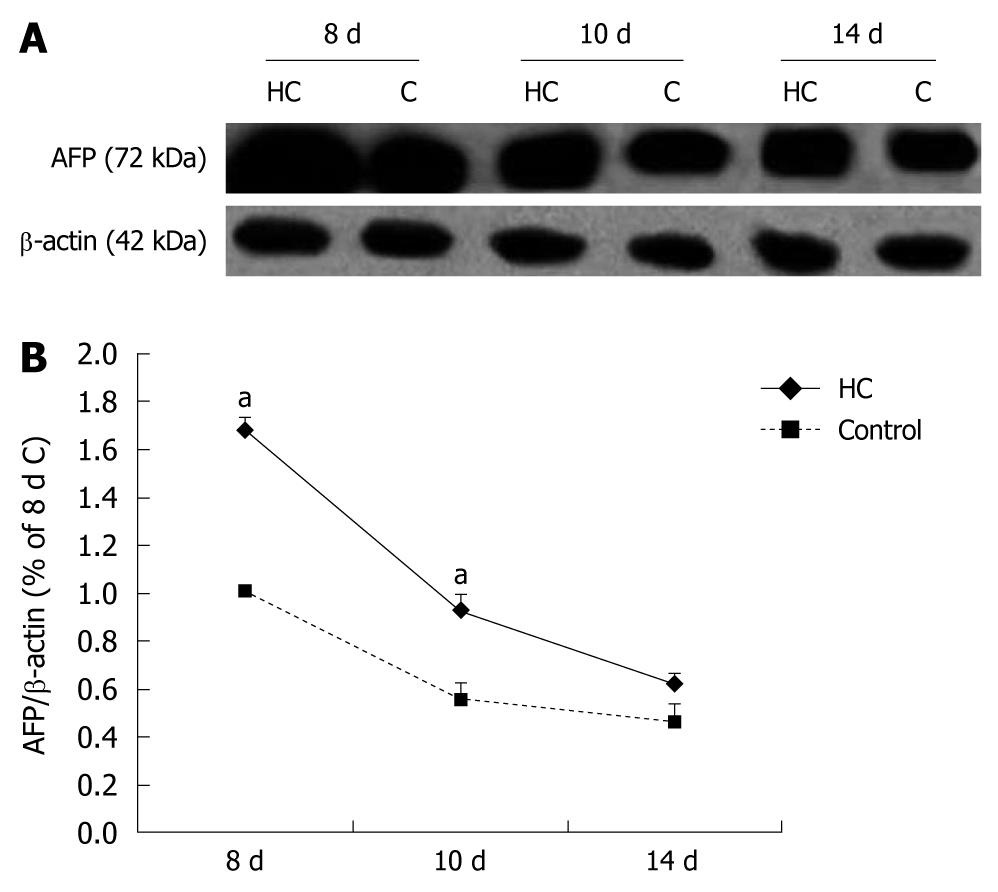
The tissues were homogenized in a sample buffer containing 50 mmol/L Tris-HCl (pH 7.5), 150 mmol/L NaCl, 5 mmol/L EDTA, 10 mmol/L NaF, 1 mmol/L sodium orthovanadate, 1% Triton X-100, 0.5% sodium deoxycholate, 1 mmol/L phenylmethylsulfonyl fluoride and protease inhibitor cocktail. An equal amount of protein samples were separated by 12.5% SDS-polyacrylamide gel electrophoresis (SDS-PAGE). After transferred to nitrocellulose membranes and blocked with 5% fat-free milk in Tris-buffered saline plus 0.05% Tween 20 overnight at 4°C, polyclonal antibody for AFP and the corresponding secondary antibody were applied. Blots were visualized with enhanced chemiluminescence reagents and exposed to X-Omat BT film. Signal intensity was quantified using a Bio-Rad image analysis system and the results were normalized to band intensities at e18.5. The β-actin was used as an internal control and the primary antibody was omitted for negative controls.
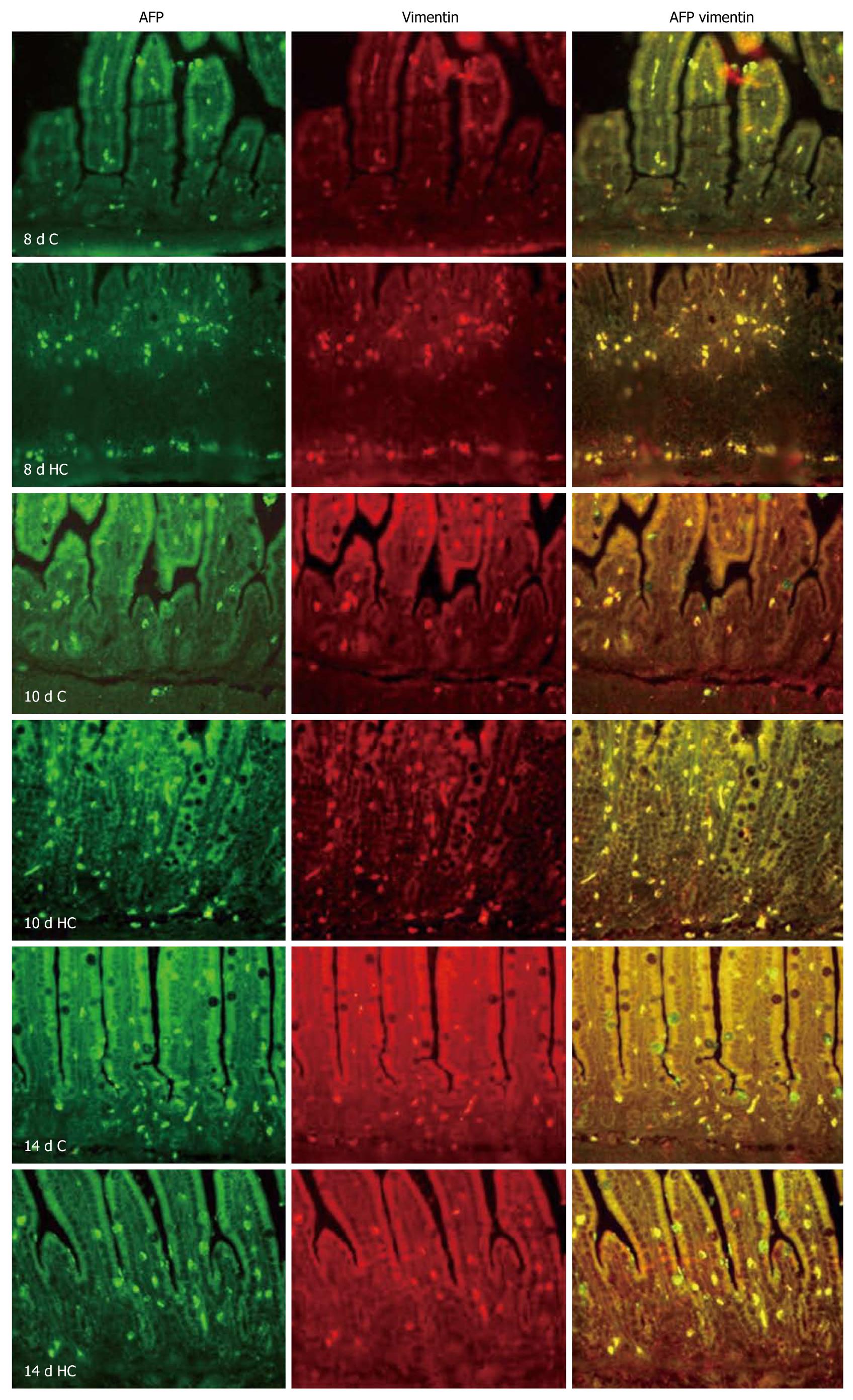
Double-immunofluorescent staining of AFP and vimentin, a specific marker of mesenchymal cell, were used to determine the regional and cellular localization of AFP in rat colons. Staining was performed according to the standard procedures. Briefly, the sections were deparaffinized in xylene, cleared with graded ethanol in phosphate buffered saline (PBS), and then placed in 10 mmol/L citrate buffer (pH 6.0) for 15 min at 100°C for antigen retrieval. The sections were applied to goat anti-AFP polyclonal antibody overnight at 4°C and then linked with FITC-labeled rabbit anti goat-IgG. After washing by Tris buffered saline (TBS), mouse anti-vimentin monoclonal antibody and rhodamine-labeled anti-mouse IgG were applied. Sections were placed in Gel Mount aqueous mounting medium with a cover glass and were examined under an Olympus BX51 microscope. Controls were treated by omitting the primary or secondary antibodies. No staining was observed under the negative control conditions. Images were taken at a magnification of ×200. An image analysis system (NYD100) was used for quantitative analysis of cell density (cell number/view field) of the AFP-positive cells in the rat colon. Four sections from four rats in each group were used. AFP-positive cells were counted in five randomly selected view fields per section at a magnification of × 400. At least 20 fields in each group were analyzed.
All experiments were done in triplicate. The experimental data was analyzed using PDQuest 7.0 software (Bio-Rad Laboratories, Hercules, CA, USA) and one-way analysis of variance and paired t test were used. Data were presented as mean ± SD. P < 0.05 was considered statistically significant.
Injecting corticosterone into the suckling animals for 1, 2, and 3 d showed no effect on the animal’s intestinal and body weights (data not shown). The crypt depth and villous height after corticosterone treatment were higher in the colon of 8- and 10-d-old rats with hypercorticoidism than in the control animals and did not influence those in the 14-d-old rats with hypercorticoidism (Table 1).
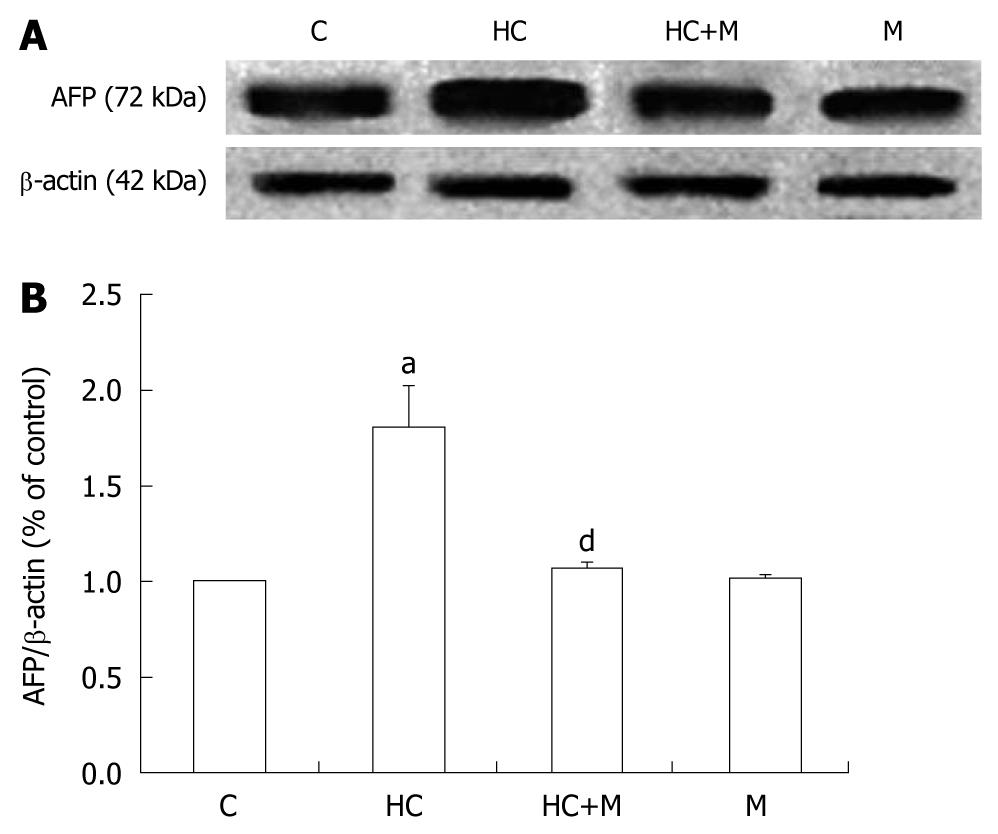
The serum AFP levels in the rats were near 18 μmol/L in all measured rats and had no difference between hypercorticoidism and control animals (data not shown). In this study, we analyzed the effect of corticosterone on the level of AFP mRNA by RT-PCR. The AFP mRNA concentration increased significantly after corticosterone treatment, by 2.5-folds in the 8-d-old and 2.5-folds in the 10-d-old rats compared with those in the control animals (Figure 1). The transcriptional regulation of AFP expression of corticosterone was confirmed by the Western blotting analysis (Figure 2).
The AFP positive cells were scattered on the epithelium in either corticosterone treated or untreated rats. The cell density of AFP-positive cells was higher in 8- and 10-d-old corticosterone-treated rats than in control animals (Table 2). Double-immunofluorescent staining for the vimentin and AFP showed that there was complete overlap between the AFP-positive cells and the antibody staining for vimentin in all the tested animals (Figure 3).
The effect of increased crypt depth and villous height in the colon of 8-d-old rats with hypercorticoidism was blocked by mifepristone (Figure 4). The increased expression of AFP was also inhibited in the rats treated with mifepristone (Figure 5).
Although rodent colon may differ from human colon in the breadth of its responses to GCs, it is nevertheless a useful model to address issues related to morphological maturation, since the development of gastrointestinal functions is similar in all mammals. The aim of this study was to assess the role of exogenous GCs in the maturation of the colon. Accordingly, we used the rats in the first postnatal week whose circulating concentrations of natural GCs were very low (< 0.5 μg/mL)[18].
Exogenous administration of GCs causes precocious maturation of the intestine. In this study, GCs did result in an increase in villous height and crypt depth of the colon (Table 1) in 8- and 10-d-old rats compared with the age-matched controls. This effect was completely blocked by the mifepristone, a glucocorticoid cytoplasm receptor antagonist (Figure 4). It is possible that GCs had a precocious effect on the development of rat colon in the first postnatal week, and in the rat small intestine as well[19,20].
In contrast, the GCs had no effect on the colon in 14-d-old rats. It has been suggested that immature gastrointestinal tract seems to have more sensitive responsiveness to the regulation of GCs[21]. It is possible that prepartum cellular differentiation progresses until day 10 in rats after birth[22]. On the other hand, cytoplasm receptors of GCs are activated after the binding of GCs that allows their translocation to the nucleus. Of note is the pattern of ontogeny changes in the concentration of GCs receptors in the small intestine as cytoplasm GCs receptors are present in the intestine at all ages, but at higher concentrations during the two postnatal weeks than in older rats[23]. Moreover, GCs receptors themselves have been shown to down-regulate by GCs through GCs enhanced expression of a factor (repressor), which binds the regulatory sequence in the GCs receptor gene promoter[24]. This might serve as a negative feedback control in the GCs response systems under developmental conditions. In 14-d-old rats, the plasma corticosterone of GCs was raised to 5 μg/mL[18], and a loss of responsiveness to GCs for colon development might occur in these rats. No effect of exogenous GCs administration could be also explained by a fall in GCs receptors in 14-d-old rats with a natural rise in corticosterone level, although this was not tested in this study.
AFP is known to be associated with the successful completion of term pregnancies in mammals and even minute amounts of AFP may still be necessary during human pregnancy[25]. The capability of both up and down modulation of growth and differentiation as a dose-dependent function of AFP has been demonstrated in a multitude of cell types, including placental, ovarian, uterine, and lymphoid, epidermal, endothelial, testicular, breast, and liver[26-30]. In our previous studies, we indicated that AFP was expressed and produced in mesenchymal cells, which might act as a potent paracrine regulator of colonic cell proliferation and organ maturation[10]. In this study, exogenous administration of GCs demonstrated an increased expression of AFP, this effect was also inhibited by mifepristone (Figures 2 and 3). Double-immunofluorescent staining for the vimentin, a mesenchymal cell marker, and AFP showed that the AFP was localized in the mesenchymal cells both in GCs-treated and control colons, similar to our previous study[10].
In present study, the AFP levels in the rat serum were near 18 μmol/L in all measured rats and had no difference between hypercorticoidism and control animals (data not shown). High cortisone concentration in serum prompts the acceleration of colonic ontogenesis, and is not accompanied by an increased level of serial AFP. In the current study, we did observe an increased expression of AFP in both RNA and protein levels in the GCs-treated premature colon of rats. Therefore, mesenchymal cell-derived AFP acted as a potent paracrine regulator in rat developmental colon.
The effect of GCs may require interactions with another tissue or cell type, such as mesenchyme[7]. The epithelial-mesenchymal interactions play an essential role in the control of gastrointestinal epithelial growth and differentiation not only in fetal stages, but also in adults[31,32]. There is no report that GCs could regulate the growth colonic mucosa directly. Our in vitro study also showed a negative result for GCs stimulating the proliferation of colonic epidermis (data not shown). The exogenous GCs were blocked, and the effect of accelerating maturation and the increased expression of AFP were also inhibited. Therefore, mesenchymal cell-derived AFP appears to be responsible, in part, in mediating the effects of GCs on developmental colon. Our present study may help us discern whether the epithelial-mesenchymal interactions and the effect of GCs enhance intestinal maturation in the gastrointestinal tract.
The involvement of GCs in AFP expression in the gut still remains an open question. Studies are now in progress in our laboratory using animal and tissue culture models to test it.
In summary, our present study for the first time demonstrated that GCs promote the development of rat colon. AFP appears to be responsible, in part, in mediating the effects of GCs on developmental colon. The exact function of AFP in rat colon development remains to be determined.
Glucocorticoids (GCs) are considered to play an important role in the maturation of the gastrointestinal (GI) tract. However, the mechanism of GCs on GI system development has not been fully elucidated.
Although rodent colon may differ from human colon in the breadth of its responses to GCs, it is nevertheless a useful model to address issues related to morphological maturation, since the development of gastrointestinal functions is similar in all mammals. This study assessed the role of exogenous GCs in the maturation of the colon in rats.
This study for the first time demonstrated that GCs promote the development of rat colon. α-fetoprotein (AFP) appears to be responsible, in part, in mediating the effects of GCs on developmental colon. The exact function of AFP in rat colon development remains to be determined.
This animal model is a useful tool for the study of mechanism of gastrointestinal mucosal proliferation and differentiation in vivo.
Corticosterone acetate is a chemical compound of GCs. Mammalian AFP is a single-chain glycoprotein with a molecular mass ranging from 66 to 72 kDa and 3%-5% carbohydrate (glycan) content.
This is an excellent study.
Peer reviewer: Dr. Frank V Schiødt, MD, Clinic of Internal Medicine I, Bispebjerg Hospital, Bispebjerg Bakke 23, DK-2400 Copenhagen, Denmark
S- Editor Sun H L- Editor Ma JY E- Editor Zheng XM
| 1. | Bauer CR, Morrison JC, Poole WK, Korones SB, Boehm JJ, Rigatto H, Zachman RD. A decreased incidence of necrotizing enterocolitis after prenatal glucocorticoid therapy. Pediatrics. 1984;73:682-688. |
| 2. | Crowley P, Chalmers I, Keirse MJ. The effects of corticosteroid administration before preterm delivery: an overview of the evidence from controlled trials. Br J Obstet Gynaecol. 1990;97:11-25. |
| 3. | Tapia JL, Ramírez R, Cifuentes J, Fabres J, Hübner ME, Bancalari A, Mercado ME, Standen J, Escobar M. The effect of early dexamethasone administration on bronchopulmonary dysplasia in preterm infants with respiratory distress syndrome. J Pediatr. 1998;132:48-52. |
| 4. | Crissinger KD. Animal models of necrotizing enterocolitis. J Pediatr Gastroenterol Nutr. 1995;20:17-22. |
| 6. | Israel EJ. Neonatal necrotizing enterocolitis, a disease of the immature intestinal mucosal barrier. Acta Paediatr Suppl. 1994;396:27-32. |
| 7. | Tsukada S, Ichinose M, Yahagi N, Matsubara Y, Yonezawa S, Shiokawa K, Furihata C, Miki K, Fukamachi H. Induction of precocious pepsinogen synthesis by glucocorticoids in fetal rat gastric epithelium in organ culture: importance of mesenchyme for epithelial differentiation. Differentiation. 1998;62:239-247. |
| 8. | Angeletti RH. Chromogranins and neuroendocrine secretion. Lab Invest. 1986;55:387-390. |
| 9. | Liu L, Guo J, Yuan L, Cheng M, Cao L, Shi H, Tong H, Wang N, De W. Alpha-fetoprotein is dynamically expressed in rat pancreas during development. Dev Growth Differ. 2007;49:669-681. |
| 10. | Liu XY, Dong D, Sun P, Du J, Gu L, Ge YB. Expression and location of alpha-fetoprotein during rat colon development. World J Gastroenterol. 2009;15:1738-1743. |
| 11. | Houart C, Szpirer J, Szpirer C. The alpha-foetoprotein proximal enhancer: localization, cell specificity and modulation by dexamethasone. Nucleic Acids Res. 1990;18:6277-6282. |
| 12. | Zhou YJ, Gao J, Yang HM, Zhu JX, Chen TX, He ZJ. Morphology and ontogeny of dendritic cells in rats at different development periods. World J Gastroenterol. 2009;15:1246-1253. |
| 13. | Galand G. Brush border membrane sucrase-isomaltase, maltase-glucoamylase and trehalase in mammals. Comparative development, effects of glucocorticoids, molecular mechanisms, and phylogenetic implications. Comp Biochem Physiol B. 1989;94:1-11. |
| 14. | Yeh KY, Yeh M, Holt PR. Thyroxine and cortisone cooperate to modulate postnatal intestinal enzyme differentiation in the rat. Am J Physiol. 1991;260:G371-G378. |
| 15. | Tseng CC, Johnson LR. Does corticosterone affect gastric mucosal cell growth during development? Am J Physiol. 1986;250:G633-G638. |
| 16. | Tseng CC, Schmidt KL, Johnson LR. Hormonal effects on development of the secretory apparatus of chief cells. Am J Physiol. 1987;253:G274-G283. |
| 17. | Fiancette JF, Balado E, Piazza PV, Deroche-Gamonet V. Mifepristone and spironolactone differently alter cocaine intravenous self-administration and cocaine-induced locomotion in C57BL/6J mice. Addict Biol. 2010;15:81-87. |
| 18. | Henning SJ. Plasma concentrations of total and free corticosterone during development in the rat. Am J Physiol. 1978;235:E451-E456. |
| 19. | Biol-N’garagba MC, Niepceron E, Mathian B, Louisot P. Glucocorticoid-induced maturation of glycoprotein galactosylation and fucosylation processes in the rat small intestine. J Steroid Biochem Mol Biol. 2003;84:411-422. |
| 20. | Quaroni A, Tian JQ, Göke M, Podolsky DK. Glucocorticoids have pleiotropic effects on small intestinal crypt cells. Am J Physiol. 1999;277:G1027-G1040. |
| 21. | Solomon NS, Gartner H, Oesterreicher TJ, Henning SJ. Development of glucocorticoid-responsiveness in mouse intestine. Pediatr Res. 2001;49:782-788. |
| 22. | Helander HF. Morphological studies on the development of the rat colonic mucosa. Acta Anat (Basel). 1973;85:155-176. |
| 23. | Henning SJ, Ballard PL, Kretchmer N. A study of the cytoplasmic receptors for glucocorticoids in intestine of pre- and postweanling rats. J Biol Chem. 1975;250:2073-2079. |
| 24. | LeClerc S, Palaniswami R, Xie BX, Govindan MV. Molecular cloning and characterization of a factor that binds the human glucocorticoid receptor gene and represses its expression. J Biol Chem. 1991;266:17333-17340. |
| 25. | Sher C, Shohat M. Congenital deficiency of AFP and Down syndrome screening. Prenat Diagn. 1997;17:884-885. |
| 26. | Keel BA, Eddy KB, Cho S, May JV. Human alpha-fetoprotein purified from amniotic fluid enhances growth factor-mediated cell proliferation in vitro. Mol Reprod Dev. 1991;30:112-118. |
| 27. | Wang XW, Xie H. Alpha-fetoprotein enhances the proliferation of human hepatoma cells in vitro. Life Sci. 1999;64:17-23. |
| 28. | Cingolani N, Shaco-Levy R, Farruggio A, Klimstra DS, Rosai J. Alpha-fetoprotein production by pancreatic tumors exhibiting acinar cell differentiation: study of five cases, one arising in a mediastinal teratoma. Hum Pathol. 2000;31:938-944. |
| 30. | De Mees C, Laes JF, Bakker J, Smitz J, Hennuy B, Van Vooren P, Gabant P, Szpirer J, Szpirer C. Alpha-fetoprotein controls female fertility and prenatal development of the gonadotropin-releasing hormone pathway through an antiestrogenic action. Mol Cell Biol. 2006;26:2012-2018. |
| 31. | Kedinger M, Simon-Assmann PM, Lacroix B, Marxer A, Hauri HP, Haffen K. Fetal gut mesenchyme induces differentiation of cultured intestinal endodermal and crypt cells. Dev Biol. 1986;113:474-483. |
| 32. | Sanderson IR, Ezzell RM, Kedinger M, Erlanger M, Xu ZX, Pringault E, Leon-Robine S, Louvard D, Walker WA. Human fetal enterocytes in vitro: modulation of the phenotype by extracellular matrix. Proc Natl Acad Sci USA. 1996;93:7717-7722. |