INTRODUCTION
Ischemia-reperfusion (IR) injury during liver transplantation (LT) is a complex, multi-factorial process in which numerous mediators and a variety of cells interact, leading to tissue damage. It is one of the major cause of both initial poor function and primary non-function of liver allograft, and is responsible for 81% of re-transplantations during the first week after surgery. An intricate network of hepatic and extra-hepatic mechanisms is involved in the genesis of hepatic IR[1-3].
Cold storage and warm reperfusion are unavoidable steps in transplantation and all grafts undergo some degree of IR injury. The cascade of events involves microvasculature (sinusoidal endothelial cells or SEC), Kupffer cells, Ito cells, parenchyma (hepatocytes) and bile ducts. Cold ischemia during organ storage, which is intentionally applied to reduce the metabolic activity of cells in order to preserve the graft before its transplantation, has a substantial effect on graft function. Also, warm ischemia that begins at implantation has an additional negative impact on graft function and outcome. Whatever the type of attack, liver graft damage initiated during the ischemic phases is exacerbated after reperfusion with oxygen and the reintroduction of blood elements.
Although our understanding of the pathophysiology of IR injury is only partial, many response elements indicate that vascular endothelium disruption, the immune response, oxidative mediators, and several cell-death pathways all play important roles. A variety of mediators have been implicated, including reactive oxygen species (ROS) and inflammatory mediators tumor necrosis factor (TNF)-α, interleukin (IL)-6, IL-1, transforming growth factor-β, interferon-γ and endotheline (ET)-1. Furthermore, complement and chemokines are also released, which leads to leukocyte recruitment and activation. Upregulated adhesion molecules, including intercellular adhesion molecule 1 and E-selectin, enhance endothelial-immune cell interactions. ROS directly injure many cytoskeletal and functional cellular components, causing cell damage. After IR, direct endothelial damage and abnormal vascular tone occurs as a result of an imbalanced sensitivity to mediators of vasoconstriction and vasodilation, such as ET-1/nitric oxide (NO). Endothelial injury also causes cell swelling and narrowing of the vascular lumen, further reducing blood flow. Finally, key regulators of apoptosis, such as caspases, are upregulated resulting in increased cell death.
It is now largely appreciated that IR associated with LT leads to a rapid endothelial dysfunction characterized by a marked decrease in NO production[4-6]. The decrease in NO bioavailability occurs within the first few minutes after reperfusion, and appears to be due to decreased synthesis of NO by NO synthase (NOS), enhanced inactivation of NO by the overproduction of superoxide anion (O2-), or both. Experimental studies emphasize that it is essential to minimize the deregulation of hepatic microcirculation during LT[7].
NO is a free-radical diatomic gas of low molecular weight with an unpaired electron[8]. It is highly lipophilic, allowing it to permeate quickly across the cell membranes. Its half-life in vivo is a few seconds, and it is rapidly converted to stable nitrites (NO2-) and nitrates (NO3-)[8]. Endogenous NO is synthesized from the amino acid precursor L-arginine in an oxidation reaction, catalyzed by the NOS enzymes. This complex reaction requires the presence of co-substrates O2 and NAD(P)H (nicotinamide adenine dinucleotide phosphate reduced), as well as many cofactors such as flavin adenine dinucleotide, flavin mononucleotide and BH4 (tetrahydrobiopterin)[8,9]. There are three isoforms of NOS expressed in the liver in different cells and in different conditions, namely endothelial NOS (e-NOS), neuronal NOS (n-NOS) and inducible NOS (i-NOS). Both e-NOS and n-NOS are constitutively present in liver. The exact role of n-NOS in the pathophysiology of IR injury during LT remains to be established[10]. n-NOS has been observed within neurons innervating the portal tracts by histochemical methods, and several authors believe that this protein is scarcely expressed in the liver[11,12]. In contrast to inducible NOS, constitutive NOS activation requires Ca2+.
Like any important signaling molecule, NO diffuses freely across cell membranes. Under physiological conditions, NO binds to soluble guanylate cyclase inside cells and then induces the production of large quantities of cGMP (guanosine 5’-monophosphate), which then triggers the signal (Figure 1)[13]. In vivo, NO is inactivated mainly by superoxide anion, but other pathways could be involved. The first pathway involves the autoxidation of NO to NO2- and then to NO3-. Other pathways may be mediated by metal-catalyzed oxidation reactions. The copper-containing protein ceruloplasmin (P-Cu2+) has been shown to rapidly oxidize NO to NO2- in physiological conditions[14,15]. In addition to the P-Cu2+-mediated reaction, ferrous deoxygenated hemoglobin (Hb-Fe2+O2; oxyhemoglobin) rapidly converts NO to NO3-.
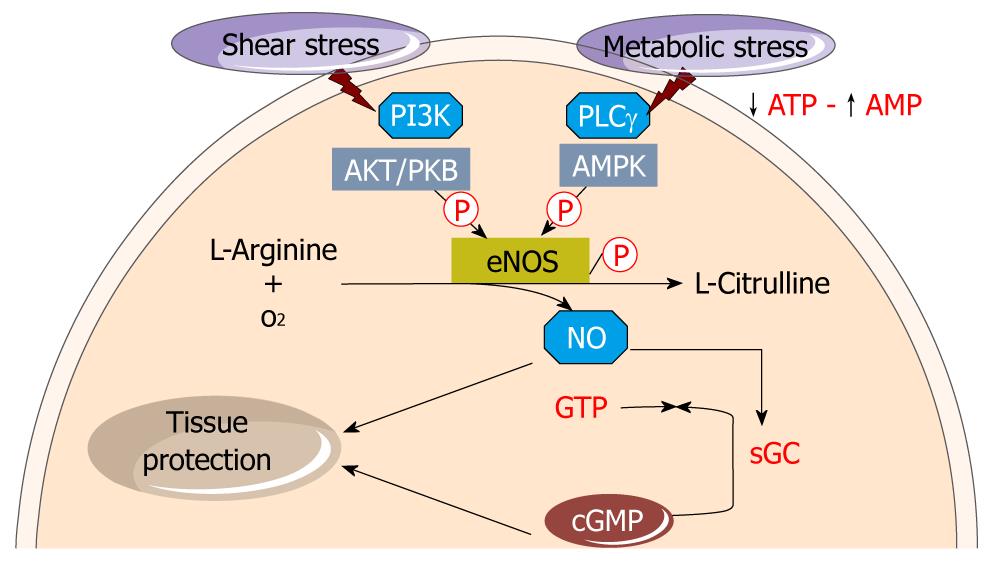
Figure 1 Endothelial nitric oxide synthase-derived nitric oxide synthesis.
Shear stress leads to endothelial nitric oxide synthase (e-NOS) phosphorylation and through pathway involving phosphoinositide 3-kinase (PI3K) and Akt. Metabolic stress also phosphorylates e-NOS through the adenosine monophosphate kinase (AMPK) route. The coordination of signalling through these converging pathways allows for e-NOS activation. L-arginine is converted in the endothelium monolayer by the constitutive e-NOS to nitric oxide (NO) and L-citrulline. NO diffuses into both the vessel lumen and the vessel wall, thereby activating soluble guanylate cyclase (sGC) to produce cyclic guanosine monophosphate (cGMP) from guanosine 5’-triphosphate (GTP). NO in concert with cGMP involve tissue protection.
e-NOS ACTIVITY AND e-NOS-DERIVED NO PRODUCTION IN LT: PATHOPHYSIOLOGICAL ASPECTS
e-NOS is constitutively expressed in venous and arterial endothelial cells and it produces small quantities of NO (at picomolar levels)[16]. It is the main source of NO in endothelial cells under physiologic conditions[9]. e-NOS is also induced in response to specific extracellular stimuli, such as shear stress[17] and metabolic stress (Figure 1)[18]. e-NOS is localized to the caveolae[19,20], which are micro-domains of the plasma membrane that have been implicated in a variety of cellular functions, including signal transduction. Caveolin proteins are the major coat proteins of caveolae, and in endothelial cells e-NOS binds to caveolin-1. Caveolin-1 and other peptides from the caveola region directly inhibit e-NOS activity[21,22]. This complex membrane structure is sensitive to the fluid pressure on the membrane.
The e-NOS activation can also be triggered through the signalling pathway involving serine/threonine kinase Akt [or protein kinase B (PKB)], which in turn is stimulated by the phosphoinositide 3-kinase (PI3K)[23,24]. e-NOS is one of the targets of Akt. An important step in this activation is the phosphorylation by Akt of the serine residue in position 1179 (bovine sequence) or serine 1177 (human sequence) of the e-NOS enzyme[25,26]. Therefore, Akt-dependent e-NOS phosphorylation may be an important mechanism in the attenuation of IR injury after LT. Treatment of liver grafts with adenovirus encoding for myr-Akt improves biochemical and cytoprotective parameters after orthotopic LT in pigs, in comparison to uninfected groups[27].
Recent reports demonstrate that metabolic stress can elicit adenosine monophosphate protein kinase (AMPK), which stimulates phosphorylation of e-NOS protein and increases NO bioavailability in endothelial cells[18,28,29]. The e-NOS-derived NO, in turn, decreases hepatic levels of ET-1, improves hepatic microcirculation and significantly attenuates TNF-α hepatic expression and, remarkably, reduces the activation of caspase-8 and caspase-3 after OLT[30].
In addition to its key role in vascular tone regulation, studies have shown that NO is involved in several other protective routes (Figure 2). Animal studies have shown that early in the reperfusion period, tissue damage appears to be associated with decreased NO availability related to e-NOS down-regulation[27,31]. Similarly, the use of NOS inhibitors leads to IR damage[32]. It has been demonstrated that e-NOS-derived NO inhibits the production and release of several endothelial vasoconstrictor factors, including ET-1[33,34]. NO also interferes with the adhesion and aggregation of platelets, adhesion of leukocytes and monocytes to endothelial cells in vessel walls[35,36]. It also modulates the expression of pro-inflammatory molecules, such as vascular cell adhesion molecule-1 and monocyte chemoattractant protein 1. Furthermore, NO is emerging as an endogenous inhibitor of TNF-α. NO decreases endothelial permeability and also exerts anti-mitogenic effects in vascular smooth muscle cells by inhibiting their growth and proliferation.
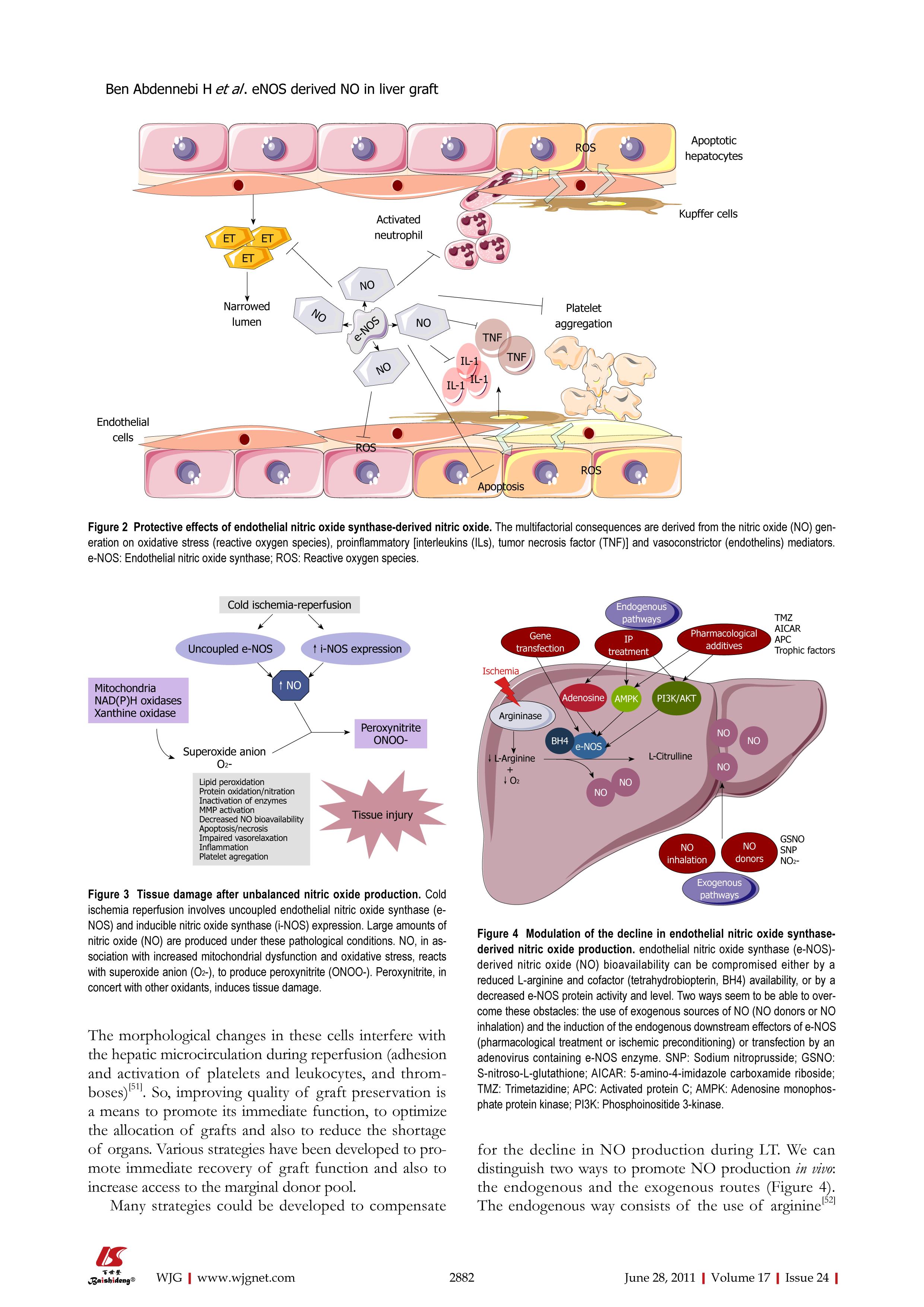
Figure 2 Protective effects of endothelial nitric oxide synthase-derived nitric oxide.
The multifactorial consequences are derived from the nitric oxide (NO) generation on oxidative stress (reactive oxygen species), proinflammatory [interleukins (ILs), tumor necrosis factor (TNF)] and vasoconstrictor (endothelins) mediators. e-NOS: Endothelial nitric oxide synthase; ROS: Reactive oxygen species.
However, other lines of evidence suggest that ROS production after LT reduces e-NOS activation, which becomes uncoupled and perturbs e-NOS-derived NO homeostasis[37] (Figure 3). In parallel, i-NOS is transcriptionally up-regulated in all liver cells, leading to the production of large amounts of NO for persistent periods[37]. Excessive NO generation can be detrimental, because it may alter systemic vascular tone and reactivity, leading to hypotension and circulatory shock[38]. In addition, the generation of peroxynitrite (ONOO-), a potent oxidant formed by reacting NO with O2-[39], could also cause cell injury through lipid peroxidation, direct inhibition of the mitochondrial respiratory chain[40,41], inhibition of membrane Na+/K+ ATPase activity, or oxidative protein modification such as the formation of nitrotyrosine[4,38]. Thus, NO acts as a double-edged sword since it has neither harmful or beneficial effects, depending on its source and the experimental conditions.
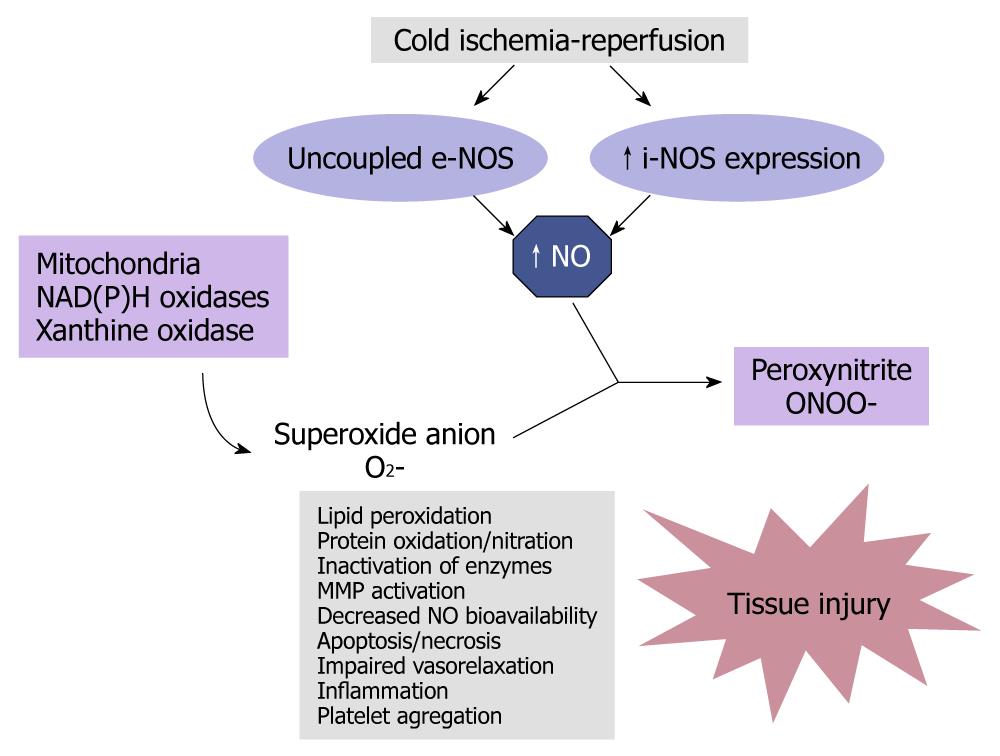
Figure 3 Tissue damage after unbalanced nitric oxide production.
Cold ischemia reperfusion involves uncoupled endothelial nitric oxide synthase (e-NOS) and inducible nitric oxide synthase (i-NOS) expression. Large amounts of nitric oxide (NO) are produced under these pathological conditions. NO, in association with increased mitochondrial dysfunction and oxidative stress, reacts with superoxide anion (O2-), to produce peroxynitrite (ONOO-). Peroxynitrite, in concert with other oxidants, induces tissue damage.
The importance of e-NOS for hepatic injury after cold storage/warm reperfusion in transplanted liver grafts has been investigated. The functions of mouse liver grafts retrieved from e-NOS-deficient donors and those from wild-type donors were compared after orthotopic transplantation[31]. e-NOS-deficient liver grafts intensified IR injury, as shown by increased ALT, necrosis and apoptosis, and elevated graft infiltration of monocytes/macrophages. In addition, both flow rate and sinusoidal diameter were diminished after transplantation of e-NOS-deficient grafts. All these alterations are detected from 4 h after LT. In another study, decreased hepatic bioavailability of NO was detected as early as 1 h after reperfusion of human liver transplants[4]. This decline was attributed to a reduction of e-NOS protein levels after reperfusion, rather than to a change in e-NOS mRNA transcription. The concerned mechanism would be a rapid turnover/degradation of the e-NOS enzyme[42]. The extent of the alteration of e-NOS protein expression depended on the duration of preservation, because the loss of e-NOS was exacerbated after 6 h of cold ischemia[43]. The stimulus for the rapid decrease in e-NOS expression is not known.
LT depletes serum arginine due to a massive release of arginase I from cold-injured liver parenchymal cells[44,45]. The depletion of arginine decreases tissue arginine availability, with subsequent down regulation of e-NOS[46,47]. In contrast, enhancement of arginine availability through arginase blockade can protect against hepatic IR injury. The results demonstrate that inhibition of arginase with nor-NOHA can partially reverse the arginine depletion seen in IR injury and improve the histopathological damage following transplantation[48].
MODULATION OF e-NOS-DERIVED NO PRODUCTION FOR LT
The quality of cold preservation is a major detriment of initial graft function and survival. While cold ischemia is considered necessary to slow tissue metabolism, it causes well-documented lesions in the SEC[49]. The SEC is the main target cell of reperfusion injury, at least during the early phase[50]. Endothelial dysfunction leads to NO deficiency, which has been implicated in many disorders. The morphological changes in these cells interfere with the hepatic microcirculation during reperfusion (adhesion and activation of platelets and leukocytes, and thromboses)[51]. So, improving quality of graft preservation is a means to promote its immediate function, to optimize the allocation of grafts and also to reduce the shortage of organs. Various strategies have been developed to promote immediate recovery of graft function and also to increase access to the marginal donor pool.
Many strategies could be developed to compensate for the decline in NO production during LT. We can distinguish two ways to promote NO production in vivo: the endogenous and the exogenous routes (Figure 4). The endogenous way consists of the use of arginine[52] to compensate for the deficiency of this NO precursor. Other methods consist of directly influencing the activity of the e-NOS enzyme by adding to the preservation solution one of its cofactors (BH4)[53], or one of its inducers, to increase downstream effectors of e-NOS such as Akt and AMPK[54-56]. Transfection of donor liver by an adenovirus containing the e-NOS enzyme could also be envisaged before graft extraction[57,58]. Ischemic preconditioning (IP) could be an endogenous source of NO[59]. The exogenous route requires the use of an exogenous source of NO, which may be given directly by inhalation or indirectly by an NO donor such as sodium nitroprusside (SNP) or S-nitroso-L-glutathione (GSNO)[60-63].
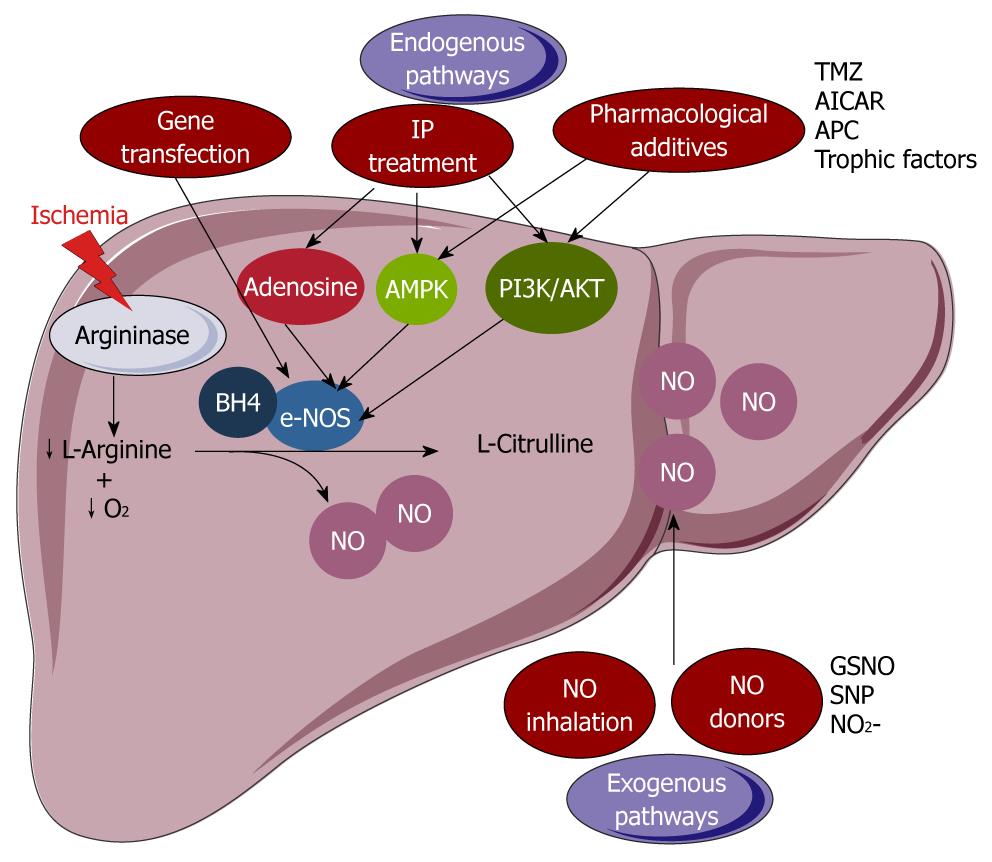
Figure 4 Modulation of the decline in endothelial nitric oxide synthase-derived nitric oxide production.
endothelial nitric oxide synthase (e-NOS)-derived nitric oxide (NO) bioavailability can be compromised either by a reduced L-arginine and cofactor (tetrahydrobiopterin, BH4) availability, or by a decreased e-NOS protein activity and level. Two ways seem to be able to overcome these obstacles: the use of exogenous sources of NO (NO donors or NO inhalation) and the induction of the endogenous downstream effectors of e-NOS (pharmacological treatment or ischemic preconditioning) or transfection by an adenovirus containing e-NOS enzyme. SNP: Sodium nitroprusside; GSNO: S-nitroso-L-glutathione; AICAR: 5-amino-4-imidazole carboxamide riboside; TMZ: Trimetazidine; APC: Activated protein C; AMPK: Adenosine monophosphate protein kinase; PI3K: Phosphoinositide 3-kinase.
These strategies have shown their effectiveness experimentally, but are not always applicable in human clinical contexts. NO can react with superoxide anions to form ONOO-, a highly reactive oxidant, which can induce apoptosis and cause structural and functional disorders of the graft. This explains why some authors claim that the contribution of NO during cold ischemia may be unnecessarily risky, especially if given at a high dose[64], and the use of NO in humans is controversial at present.
NO GENERATION INDUCED BY THE ENDOGENOUS PATHWAY
Pharmacological e-NOS induction during cold preservation
The potentially protective role of endogenous NO in IR-induced injury to the liver is supported by recently published studies demonstrating enhanced hepatocellular injury in post-ischemic animals that had been rendered deficient in e-NOS[65]. In addition, researchers have demonstrated that over-expression of liver e-NOS protects mice from IR-induced liver injury[27,66-68].
Endogenous e-NOS activation and e-NOS-derived NO might be a promising approach to limiting organ injury. Several pro-survival pathways such as PI3K/Akt and AMPK, which are activated following hepatic injury, are involved in the regulation of e-NOS. Pharmacological up-regulation of these pro-survival kinase cascades may provide an approach to limiting cold ischemic insult. In this regard, 5-amino-4-imidazole carboxamide riboside (AICAR), carvedilol (CVD), trimetazidine (TMZ), activated protein C (APC) and insulin-like growth factor (IGF)-1 could be promising additives in preservation solutions to improve the outcome of liver grafts after cold storage and reperfusion.
Recent studies have shown that the enrichment of University of Wisconsin (UW) with AICAR, an activator of AMPK, ameliorated long-term liver preservation[55]. The protective effect of AICAR on liver injury and function seems to be mediated by enhanced e-NOS activation and NO generation following AMPK phosphorylation, which induces vasodilatation in liver grafts[55]. The relevance of AMPK as an e-NOS upstream regulator was evidenced by the administration of AraA, a phospho-AMPK inhibitor, previous to liver graft preservation, demonstrating that AMPK inhibition reduces e-NOS activation and NO production[55]. In line with these results, Ben Mosbah et al[54] demonstrated that the use of CVD, a β- and α-adrenergic blocking drug, when added to UW preservation solution, protected livers through the same mechanism[54]. Livers preserved in this solution show decreased transaminases levels, improved vascular resistance, reduced mitochondrial damage and enhanced ATP levels after reperfusion.
However, the AMPK pathway is not the only e-NOS modulator. Other authors have attempted to improve the histidine-tryptophan-ketoglutarate (HTK) preservation solution by supplementation with an anticoagulant and anti-inflammatory agent, such as APC[30]. They found that the modified HTK preservation solution decreased portal pressure and improved hepatic microcirculation through increased NO hepatic levels via up-regulated e-NOS. In addition, the solution attenuated TNF-α expression and markedly reduced the activation of caspase-3 and caspase-8[30]. Although the exact mechanism by which APC activated e-NOS remains unclear, other in vitro reports postulated that APC activated e-NOS via phosphatidylinositol 3-kinase-dependent phosphorylation, followed by activation of PKB[69].
Recently, Institut Georges Lopez-1 (IGL-1) solution has been proposed as an effective alternative to UW liquid in clinical kidney transplantation, and in experimental orthotopic LT models[70,71]. In addition, we recently demonstrated that IGL-1 is more suitable than UW solution for fatty liver preservation and that the benefits from IGL-1 were linked to an increment of NO synthesis through e-NOS activation which, in turn, reduced oxidative stress and liver injury[72].
TMZ, which has been used as an anti-ischemic drug in the heart for over 35 years, reduced liver injury and improved liver regeneration and survival rate in an experimental model of partial hepatectomy under hepatic blood inflow occlusion[56]. Studies examining the underlying protective mechanisms of TMZ as an additive to UW solution for liver preservation suggest that AMPK up-regulation is the mechanism by which TMZ activates e-NOS and exerts its cytoprotective effect[55]. Another study demonstrated that TMZ attenuated myocardial IR injury via PI-3K/AKT kinase pathway activation[73]. We recently found that the addition of TMZ to IGL-1 solution has a synergistic effect on e-NOS-derived NO generation that favours HIF-1α accumulation during normothermic reperfusion[32]. Preserved HIF-1α levels contribute to the increase in the over-expression of cytoprotective proteins such as HO-1 in fatty liver grafts.
It has been established that IGF-1 up-regulates e-NOS activity by interacting with a tyrosine kinase membrane receptor which activates the AKT signalling pathway[74,75]. Furthermore, trophic factors, including IGF-1, have been added to UW solution in an attempt to improve the survival of pig orthotopic liver allografts after cold storage[76]. To this end, we explored the effects of the addition of IGF-1 to IGL-1 solution on fatty liver preservation during cold IRI. We examined the mechanisms responsible for such effects, including AKT phosphorylation and NO generation. We have demonstrated that the beneficial action of IGF-1 as an additive to IGL-1 is mediated by AKT activation and NO generation, with concomitant prevention of pro-inflammatory cytokines, such as TNF-α[77].
Surgical e-NOS induction by IP
IP is a technique described firstly in the heart by Murry et al[78] in 1986, which consists of the application of short and repetitive periods of I/R before a sustained one. The protective effect of IP is not specific to the myocardial muscle, since it is observed in other organs such as skeletal muscle[79], brain[80], intestines[81], lungs[82], kidneys[83] and liver[34]. In any case, the protection induced by IP against IR injury seems to be specific to each organ and animal species, depending on the number of IR cycles applied before the sustained IR. For example, 3-4 cycles are needed for the protection of myocardium[78], whereas in the liver just one cycle of 10 min of ischemia and 10 min of reperfusion is sufficient for maximal protection[59].
In the heart, IP offers an initial protection of 2-3 h after reperfusion, and a remote protection after 12-24 h that lasts for 2 to 3 d. A similar pattern was observed in the liver, although remote protection is not yet well established. Moreover, a differential IP protection was observed in the liver, also depending on the animal species.
Molecular mechanisms responsible for hepatoprotection
The molecular basis of IP is a sequence of episodes triggered by a rapid signal, which leads to an intracellular message and to the amplification of the effector mechanisms of protection[84]. The benefits of IP are caused by the release of several inflammatory mediators such as adenosine and NO, which is followed by the activation of multiple cellular signals. NO is generated by the adenosine released (activation of adenosine A2 receptors), which in turn activates the endothelial constitutive form of the e-NOS enzyme[34,85], a few minutes after IP. The window of liver protection induced by IP is defined by two factors: (1) the concentration of adenosine must be high enough to induce NO; and (2) the concentration of xanthine must be low enough to avoid its prejudicial effects. It is well established that a high concentration of xanthine would support significant increases in superoxide anion. This would react with NO to generate peroxynitrite[86], which would cancel the beneficial effects of IP. Vasodilator effects of NO release improved liver oxygenation and microcirculation[87], and also inhibited the generation of ET, powerful vasoconstrictors generated during liver reperfusion[34].
In addition, IP preserves energy metabolism during sustained ischemia[88,89]. This is confirmed by the maintenance of ATP levels, as well as the depletion of lactate accumulated during the ischemic period. This beneficial effect is mediated by the increase of AMPK, whose activation can be mediated by NO[89].
The activation of the G protein-bound A2 receptor by adenosine stimulates the activity of many intracellular kinases, such as protein-kinase C (PKC) and p38 MAPK[90,91]. In addiction, recent studies implicate PKC in some of the beneficial effects of IP in liver. They show that PKC activation depends on the phosphorylation of different effector molecules, such as the tyrosine kinases[92] and MAPK (including p38 and MAPK[93]), and increases the tolerance of hepatocytes and endothelial cells to the ischemic insult.
Many transcription factors are involved in PKC activation, such as nuclear factor (NF)-κB, which is responsible for the protective effects of PI[94,95]. These transcription factors modulate the expression of particular genes, resulting in the synthesis of proteins such as heat shock proteins (HSP), which are understood to be effectors for the benefits of IP[96]. In the liver, IP is associated with the synthesis of many inducible forms of HSP: HSP70, HSP2 and HSP73 and heme oxygenase (HO-1/HSP32). The induction of HSP depletes the binding between pro-inflammatory transcriptional factors and improves the oxidant capacity of the cells[96-98]. Both effects could contribute to the decrease in TNF-α and to the attenuation of the inflammatory response of preconditioned livers[99,100]. It was also suggested that IP could reduce the transcription of genes, such as c-fos and c-jun, implicated in the development of the hepatic IRI, and that NFκB activation could induce the activation of signal translator and transcription activator 3, implicated in hepatoprotection and cell proliferation[89,94,96,101,102].
The beneficial role of NFκB in IP is controversial. Whereas Funaki et al[103] demonstrate that the protective role of IP is associated with the inhibition of NFκB activation, other authors suggest the opposite, showing that these effects are due to NFκB activation[94,95]. These differences could be attributed to differences in the models used. Besides these cellular signalization pathways, recent studies show that IP could induce the release of small quantities of ROSs[104] and TNF-α, contributing to the protective mechanisms.
Applications to LT
Several studies in animal models have demonstrated the usefulness of IP, but its application to clinical transplantation needs to be clarified. The first clinical application was carried out by Koneru et al[105], who used IP in deceased donor LT. They showed that deceased donor liver tolerated 5 min of hilar clamping, but IP did not decrease graft injury. More recently, Azoulay et al[106] demonstrated that the effects of 10 min of IP of the liver graft in the donor are associated with better tolerance to ischemia, as well as a worsened early liver function. These studies are consistent with those by Jassem et al[107], who demonstrated the protection of cadaver donor livers subjected to IP prior to retrieval by clamping of the hepatic pedicle for 10 min at 24 h after transplantation. This was evidenced by a significant decrease in transaminase levels, and a concomitant reduction of the non-specific inflammatory response. Similar results were obtained by Cescon et al[108], in a prospective randomized study on cadaver donors, by the use of 10 min of IP followed by 15 min of reperfusion. These authors also demonstrated a significant reduction in AST, ALT and i-NOS expression levels after transplantation.
Taking all this into account, new research work is needed to establish the “effective protection” window in human LT and confirm the usefulness of IP in clinical transplantation, including the marginal graft donors, which at present are discarded for clinical transplantation purposes.
NO GENERATION INDUCED BY THE “EXOGENOUS PATHWAY” DURING COLD PRESERVATION
Some considerations
Many teams have sought to improve the performance of storage solutions by supplementation with cytoprotective agents. The bulk of the work on these specific modifications was performed on cellular models in rodents. Unfortunately, the benefits observed were not always confirmed in humans and these changes have often led to inconclusive results in clinical practice. Several NO donors have been tested experimentally for their protective effects on cold IR injury. However, due to the numerous possible reactions and related biological consequences, inappropriate NO levels can cause a series of disease states. On the other hand, insufficient NO production also has serious medical consequences. NO donor therapy should aim to achieve the production of the correct quantity of NO in the correct place for the correct length of time. The exogenous NO should acts primarily as a local mediator to respond to specific stimuli, and then it should simply dissipate through diffusion and oxidation to NO2- and NO3-, without the need for complex catabolism. The chemical versatility of NO has led to the synthesis of a wide range of NO donors, each with different modes and rates of NO release. NO donors are pharmacologically active substances that spontaneously release NO (direct donors) or are metabolized to NO (donors requiring metabolism)[109].
The selection of one among these NO donors for therapeutic uses is not easy, since it must meet certain requirements. The compound must be highly soluble in aqueous solutions and diffuse easily into cells, where it produces NO. It must be a proven NO donor, remain in a subtoxic range, have a prolonged half-life and mimic the effect of the endogenous NO. Several authors have amply evaluated the consequences of the use of NO donors in liver under warm ischemia conditions, but little work has been done on the effects on cold ischemia and graft preservation. In our opinion the use of such donors as additive to preservation solution is limited by their short half-life.
Kuroki et al[110] studied the effects of SNP on liver warm IR injury. They reported improvement of liver microcirculation and hepatocyte injury in the early period of reperfusion. In another study supporting this finding, SNP infusion after a short period of liver ischemia also decreased liver IR injury[111]. Interestingly, deleterious effects observed during cold storage conditions (like vacuolated hepatocytes, increase in intrahepatic resistance and diminution of bile production) were significantly removed after addition of 500 mmol/L SNP to the UW preservation solution[60]. The authors assumed that beneficial effects of SNP were mediated by NO release[63].
In addition, GSNO has been evaluated in the liver. GSNO may serve as an endogenous long-lived adduct or carrier of NO[112]. GSNO possesses significant anti-platelet action at doses that cause mild hemodynamic effects[113]. Thus, one possible mechanism by which the NO-donor can protect the graft against IR-induced damage could be based on its ability to block platelet aggregation, thus avoiding the intravascular coagulation that can occur during reperfusion. Quintana et al[60,61] evaluated the benefit of the addition in UW solution of GSNO as a NO donor. After assaying four GSNO concentrations (50, 100, 250 and 500 mmol/L), they reported an improvement in the properties of UW solution when it contained 100 mmol/L GSNO. It preserved the morphology of hepatocytes and endothelial cells and prevented the alteration of the hemodynamics and function of livers after cold preservation/reperfusion.
Some authors conclude that nitrite (NO2-) therapy might prove beneficial in protecting organ function and integrity during periods of IR such as those encountered in organ transplantation[114-116]. Under physiological conditions of pH and oxygen pressure, NO2- has been shown to be a non-active metabolic end product of NO oxidation with limited intrinsic biological activity[117]. However, under ischemic conditions, when e-NOS activity is strongly decreased as a result of its essential dependence on oxygen and the depletion on arginine, an increasing body of evidence indicates alternative NO production by NOS-independent routes. For instance, nitrite may be reduced back to NO by the nitrite reductase action of deoxygenated hemoglobin[118], acidic disproportionation[119], or xanthine oxidoreductase (XOR)[120,121]. In summary, the use of NO2- in the field of cold preservation could be considered for the following reasons: (1) it is a highly stable substance with no potentially toxic effect; (2) it selectively releases NO under conditions that subsist in stored tissue, namely ischemia, hypoxia, or low pH; (3) it increases XOR activity, which may compensate for compromised constitutive e-NOS activity in terms of NO production, during hypoxia and acidosis; and (4) sodium nitrite is an FDA-approved compound. However, the use of nitrite in preservation solutions that contain allopurinol (such as UW or IGL-1 solutions) would be ineffective, since this latter inhibits XOR.
NEW DIRECTIONS FOR THE FUTURE
Along these lines we have evidenced the relevance of e-NOS system as a useful tool for liver preservation against cold ischemia reperfusion injury. However, new potential strategies should be established in the future to increase e-NOS activity and modulate the NO availability, as well as to define the appropriate “therapeutic window” to provide the most suitable graft protection during cold storage and further during LT. A better knowledge of understanding the molecular pathways involved may lead to more efficient protective strategies to prevent early cold reperfusion injury during transplantation based on multifactorial activation of the endogenous cytoprotective e-NOS existing in preserved liver grafts.
Peer reviewer: Fabio Grizzi, PhD, Laboratories of Quantitative Medicine, Istituto Clinico Humanitas IRCCS, Via Manzoni 56, 20089 Rozzano, Milan, Italy
S- Editor Sun H L- Editor Rutherford A E- Editor Zheng XM