Published online Jun 7, 2011. doi: 10.3748/wjg.v17.i21.2667
Revised: October 19, 2010
Accepted: October 26, 2010
Published online: June 7, 2011
AIM: To investigate the silencing effects of pAd-shRNA-pleiotrophin (PTN) on PTN in pancreatic cancer cells, and to observe the inhibition of pAd-shRNA-PTN on neurite outgrowth from dorsal root ganglion (DRG) neurons in vitro.
METHODS: PAd-shRNA-PTN was used to infect pancreatic cancer BxPC-3 cells; assays were conducted for knockdown of the PTN gene on the 0th, 1st, 3rd, 5th, 7th and 9th d after infection using immunocytochemistry, real-time quantitative polymerase chain reaction (PCR), and Western blotting analysis. The morphologic changes of cultured DRG neurons were observed by mono-culture of DRG neurons and co-culture with BXPC-3 cells in vitro.
RESULTS: The real-time quantitative PCR showed that the inhibition rates of PTN mRNA expression in the BxPC-3 cells were 20%, 80%, 50% and 25% on the 1st, 3rd, 5th and 7th d after infection. Immunocytochemistry and Western blotting analysis also revealed the same tendency. In contrast to the control, the DRG neurons co-cultured with the infected BxPC-3 cells shrunk; the number and length of neurites were significantly decreased.
CONCLUSION: Efficient and specific knockdown of PTN in pancreatic cancer cells and the reduction in PTN expression resulted in the inhibition of neurite outgrowth from DRG neurons.
- Citation: Yao J, Zhang M, Ma QY, Wang Z, Wang LC, Zhang D. PAd-shRNA-PTN reduces pleiotrophin of pancreatic cancer cells and inhibits neurite outgrowth of DRG. World J Gastroenterol 2011; 17(21): 2667-2673
- URL: https://www.wjgnet.com/1007-9327/full/v17/i21/2667.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i21.2667
Pancreatic cancer is one of the most aggressive and intractable human malignant tumors[1,2]. Perineural invasion extending into the pancreatic nerve plexus is a histopathologic characteristic of pancreatic cancer. However, the mechanisms contributing to the invasion of intrapancreatic nerves and spread of cancer cells along extrapancreatic nerves in the course of pancreatic cancer are still poorly understood. A family of proteins consisting of neurotrophic factors is of interest due to recent experimental data that showed their involvement in the neural invasion of pancreatic cancer[3,4].
Pleiotrophin (PTN) is a type of neurotrophic factor and is also known as a neurite growth-promoting factor. Human, mouse and rat PTN proteins are identical, and they share a 45% amino acid similarity with midkine-another member of this family[5,6]. PTN could promote neurite outgrowth in primary cultures of cortical neurons[7] and neuronal survival[8]. PTN is mainly expressed during early embryogenesis. In human adult tissues, PTN is markedly down-regulated and present only at minimal levels in very few tissues. It is not expressed in normal pancreatic tissues, but highly expressed in pancreatic cancer cells[9]. Kinnunen et al found a high expression level of PTN in 78% of the tumor samples from pancreatic cancer patients[10]. PTN and N-syndecan act as a receptor-ligand pair in neurite outgrowth. Anti-N-syndecan antibodies added to the culture media had an inhibitory effect on PTN-induced neurite outgrowth[11]. Therefore, PTN may play an important role in the neural invasion of pancreatic cancer.
RNA interference (RNAi) is a process during which double-stranded RNA induces the homology-dependent degradation of cognate mRNA[12]. In some organisms, the introduction of double-stranded RNA has been proven to be a powerful tool for suppressing gene expression through a process known as RNAi[13]. However, in most mammalian cells, it provokes a strong cytotoxic response[14]. This nonspecific effect could be circumvented using synthetic small interfering RNA (siRNA), which could mediate strong and specific suppression of gene expression[15]. Transfection of chemically synthesized siRNA is routinely used for gene silencing[16]. To circumvent the high cost of synthetic siRNA and establish stable gene knock-down cell lines by siRNA, several adenovirus vector systems, such as the BLOCK-iT™ Adenoviral RNAi Expression System, have been designed to produce siRNA intracellularly. The BLOCK-iT™ Adenoviral RNAi Expression System combines Invitrogen’s BLOCK-iT™ RNAi and ViraPower™ Adenoviral technologies to facilitate the creation of a replication-incompetent adenovirus that delivers a synthesized short hairpin RNA (shRNA) of interest to dividing or non-dividing mammalian cells for RNAi analysis. These RNAi expression vectors are highly efficient for in vitro and in vivo gene transfer into a variety of mammalian cells and tissues and have been used in functional and gene therapy studies[17,18].
In this study, we investigated the silencing effects of pAd-shRNA-PTN on the PTN gene in the pancreatic cancer cell line BxPC-3, and observed the inhibition of neurite outgrowth from dorsal root ganglion (DRG) neurons in vitro. Our results demonstrated efficient and specific knockdown of PTN in the BxPC-3 pancreatic carcinoma cell line, and suggested that PTN is an attractive new target for the study of neural invasion in pancreatic cancer gene therapy.
The BLOCK-iT ™ Adenoviral RNAi Kits (pAd/BLOCK-iT™-DEST GatewayR Vector Kit; GatewayR LR Clonase™ Enzyme Mix; 293A Cell Line; and BLOCK-iT™ U6 RNAi Entry Vector Kit) and Lipofectamine™ 2000 were purchased from Invitrogen Corp. (Carlsbad, California, USA). Monoclonal mouse antihuman PTN, anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH) antibodies, and the secondary antibody (peroxidase-coupled goat anti-mouse IgG) were purchased from Santa Cruz Biotechnology (Santa Cruz, California, USA). The human pancreatic cancer cell line BxPC-3 was purchased from the American Type Culture Collection. The BxPC-3 cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM) obtained from Life Technologies (Carlsbad, California, USA). Fetal calf serum (FCS) and Ham’s F12 medium were purchased from Gibco BRL (Carlsbad, California, USA).
The construction procedure for recombinant adenovirus pAd-shRNA-PTN has been described previously[19]. Briefly, according to data on the human pleiotrophin mRNA (GenBank accession no. NM002825), four pairs of complementary single-stranded oligonucleotides (ss oligos) were designed and synthesized using the Invitrogen’s RNAi Designer online. Then, the ss oligos were annealed to create double-stranded oligonucleotides (ds oligos). The four ds oligos were cloned into the pENTR/U6 vector to produce four shuttle plasmid pENTR/U6-shRNAs that were transduced into the BxPC-3 cells by Lipofectamine™ 2000 after sequencing, to identify and select the shuttle plasmids showing optimal silencing effect. Oligo-3 was proven to have an optimal silencing effect. The sense and antisense strands of oligo-3 were 5′-CACCGCCAGAAGACTGTCACCATCTCGAAAGATGGTGACAGTCTTCTGGC-3′ and 5′-AAAAGCCAGAAGACTGTCACCATCTTTCGAGATGGTGACAGTCTTCTGGC-3′. Then, the attL and attR (LR) recombination reaction was performed. Recombinant adenovirus pAd-shRNA-PTN was produced and amplified in HEK 293A cells; the viral titers were determined by TCID50 assays[20]. The construction of pAd-shRNA-PTN was confirmed via electron microscopic observation.
The BxPC-3 cells were placed at a concentration of 1 × 106 cells per well into a 6-well plate, and 2 mL of normal DMEM along with 10% FCS was added into each well. On the day of infection (Day 0), 5 μL adenoviral stock (1 × 1010 pfu/mL) was added into a 2 mL fresh culture medium with 2% FCS (at a multiplicity of infection (MOI), i.e. an MOI of 50). Then, we removed the previous culture medium from the cells, mixed the medium containing virus gently, added it to each cell, and incubated it at 37°C overnight. The following day (Day 1), we removed the medium containing virus and replaced it with fresh DMEM containing 2% FCS. The cells were harvested on the 0th, 1st, 3rd, 5th, 7th, and 9th d after infection and assayed for knockdown of the PTN gene by immunocytochemistry, real-time quantitative polymerase chain reaction (PCR), and Western blotting analysis. In this study, we regarded the cells harvested on the 0th d after infection as the control.
Immunostaining of the human pancreatic cancer cell line BxPC-3 was performed on the 0th (control), 3rd, and 5th d after infection. The BxPC-3 cells were grown on glass slides and fixed with acetone; endogenous peroxidase was blocked by incubation with 0.3% H2O2 in methanol. The sections were washed and incubated overnight with a 1:20 dilution of anti-PTN antibody at 4°C. After subsequent wash in phosphate buffered saline (PBS), the secondary antibody was added and incubated for 1 h at room temperature. After another wash in PBS, the peroxidase activity was localized by staining with diaminobenzidine as the substrate. Then, the sections were rinsed in water, dried, and covered.
On the 0th, 1st, 3rd, 5th, 7th, and 9th d after infection, the medium was removed from each well. Total RNA from each plate of BxPC-3 cells was extracted (RNeasy Mini Kit, Qiagen). The extracted RNA was quantified by measuring the absorbance at 260 nm. The purity of the RNA was verified by calculating the ratio between the absorbance values at 260 and 280 nm. This ratio ranged between 1.83 and 2.00, demonstrating the high quality of the RNA. Reverse transcription reactions were performed at 50°C for 30 min. The reaction mixtures were heated to 95°C for 15 min to activate HotStarTaq (Qiagen). The forward and reverse primers of PTN were 5′-TCCTAGTATTTTTTTCCTCAG-3′ and 5′-CTTGTTTTCTGCCAATAG-3′, respectively; the forward and reverse primers of GAPDH were 5′-TCATCCCTGCCTCTACTG-3′ and 5′-TGCTTCACCACCTTCTTG-3′, respectively. PCR amplification was performed in a total volume of 20 μL: 4.4 μL PCR master mix (TaKaRa Ex Taq R-PCR Version, TaKaRa), 10 pmol of each primer, 2 mmol MgCl2, 2 μL (1:15 000 dilution) SYBr Green I (SYBr Green I Nucleic Acid Gel Stain, Takara), and distilled water. PCR was performed using the ABI PRISM 7700 Sequence Detection System under the following conditions: 95°C for 30 s and 35 cycles at 95°C for 0 s for instrument setting to 0 s, 57°C for 5 s, and 72°C for 10 s. Cycle threshold (CT) values were obtained from the BIO-RAD iQ5 2.0 Standard Edition Optical System Software (BIO, RAD, USA). Data were analyzed using the ΔΔCT method and GAPDH served as an internal control.
The cells were harvested on the 0th, 1st, 3rd, 5th, 7th, and 9th d after infection, washed once with cold PBS (pH 7.0), and lysed in a lysis buffer (150 mmol/L NaCl, 50 mmol/L Tris-HCl (pH 7.4), 2 mmol/L EDTA, and 1% NP-40) containing protease inhibitors (Boehringer Mannheim, Germany). The analysis was performed on all the lysates with equal amounts of protein (20 μg per lane), quantified by colorimetric detection based on the bicinchoninic acid (BCA) test. The samples were heated at 95°C for 5 min and loaded on a 12% sodium dodecyl sulfate (SDS)-polyacrylamide gel. Following electrophoresis, the separated protein fractions were transferred onto a methanol-activated polyvinylidene fluoride (PVDF) membrane and incubated with anti-PTN and anti-GAPDH antibodies, followed by incubation with the corresponding secondary antibodies. The bands were visualized using the enhanced chemiluminescence system.
DRG neurons were dissociated from a 16-d rat fetus. An average of approximately 300 DRG neurons were plated on the poly-l-lysine (200 μg/mL)-coated glass cover slips (13 mm in diameter) placed in a 6-well plate and maintained overnight at 37°C in a humidified incubator gassed with 5% CO2 in air. Following this overnight setting period, co-cultures of the DRG neurons and BxPC-3 cells were prepared. BxPC-3 cells plated at a concentration of 1 × 105 cells per well in a 6-well plate and 2 mL of DMEM/F12 medium with 10% FCS were added into each well. On the day of infection (Day 0), 50 μL adenoviral stock and 2 mL fresh DMEM/F12 medium containing 2% FCS (at an MOI of 50) were added to the infected groups and 2 mL fresh DMEM/F12 medium containing 2% FCS but without adenoviral stock was added to the control (uninfected) group. The previous culture medium was removed from the cells, and the fresh medium was added to each cell. The plate was swirled gently to disperse the medium. After incubating at 37°C for 6 h, the glass cover slips with 300 DRG neurons were plated on it. The two cell types were cocultured for 7 d in the DMEM/F12 medium containing 2% FCS and maintained at 37°C in a humidified incubator with 5% CO2 in air.
The images obtained from the Western blotting were scanned and analyzed using the Quantity One software. The ratios of PTN to GAPDH were calculated. The statistical significance between the control and infected groups was calculated using one-way ANOVA test. P < 0.05 was considered to indicate statistical significance.
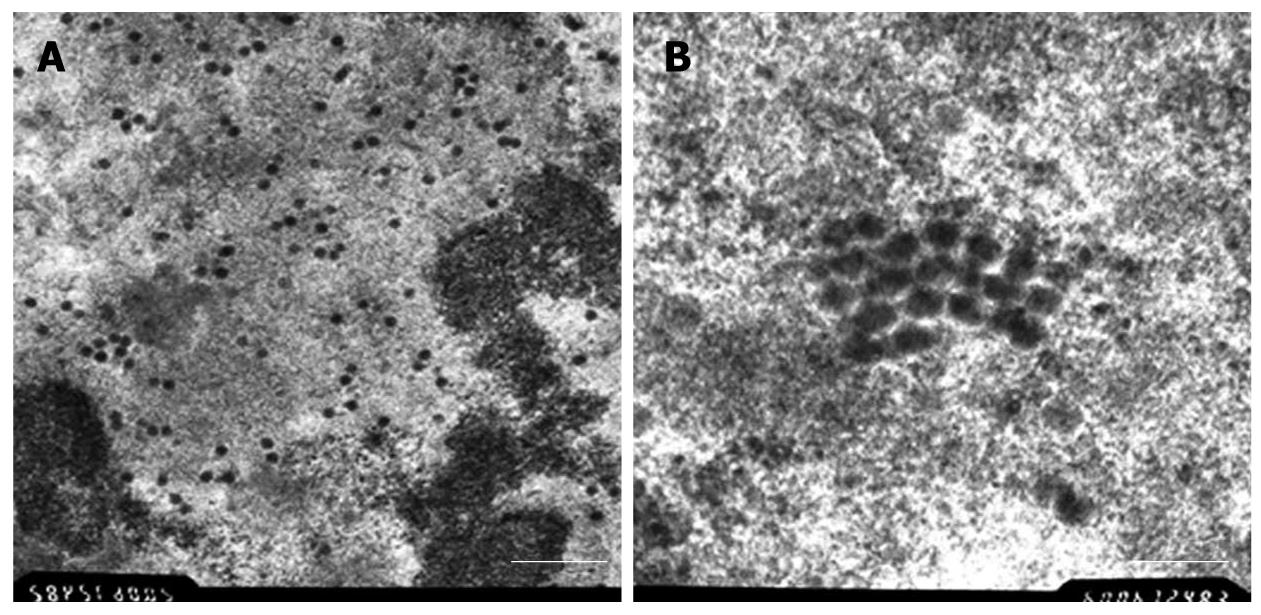
Two days after infection, we were able to find characteristic morphological changes. There were lots of scattered virus capsids in the nucleus, next to the nuclear membrane cytoplasm with few virus capsids (Figure 1A). At highest magnifications (× 60 000), the capsids showed no connection to each other. Virus capsids were 50-70 nm with an almost round appearance (Figure 1B).
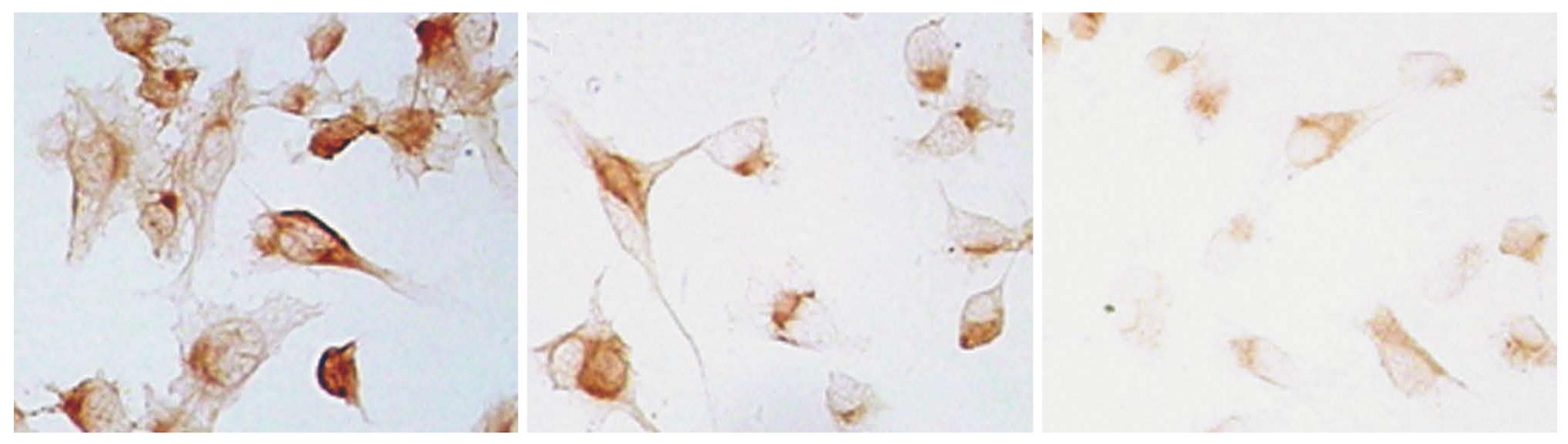
Using immunocytochemistry, we found a strong staining pattern for PTN in the cytoplasm of normal BxPC-3 cells (control, Figure 2A). The silencing rate was determined in comparison with the control alone. The expression rate of the PTN protein on the membrane was decreased by 30% (3 d, Figure 2B) and 70% (5 d, Figure 2C).
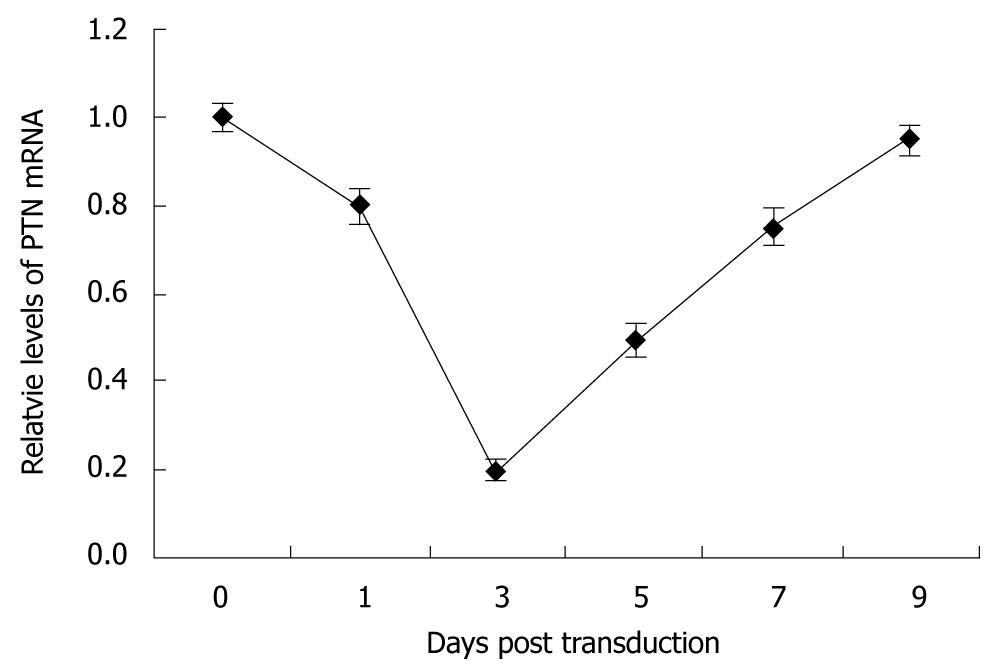
The efficacy of pAd-shRNA-PTN with regard to knockdown of PTN mRNA was confirmed through real-time quantitative PCR analysis. The melting temperature range of PTN was 86.5-87.5°C, and the melting temperature range of GAPDH was 89.5-90.5°C. No nonspecific amplification was observed. High expression levels of PTN mRNA were observed in the control. The inhibition rates of PTN mRNA expression in the BxPC-3 cells were 20%, 80%, 50%, and 25% on the 1st, 3rd, 5th and 7th d after infection. The PTN mRNA level was decreased on the 1st d, and down-regulation of PTN mRNA expression was most obvious on the 3rd post-infection day. On the 5th d, the PTN mRNA level increased gradually, and the PTN mRNA expression approximately returned to the level of normal BxPC-3 cells on the 9th post-infection day (Figure 3).
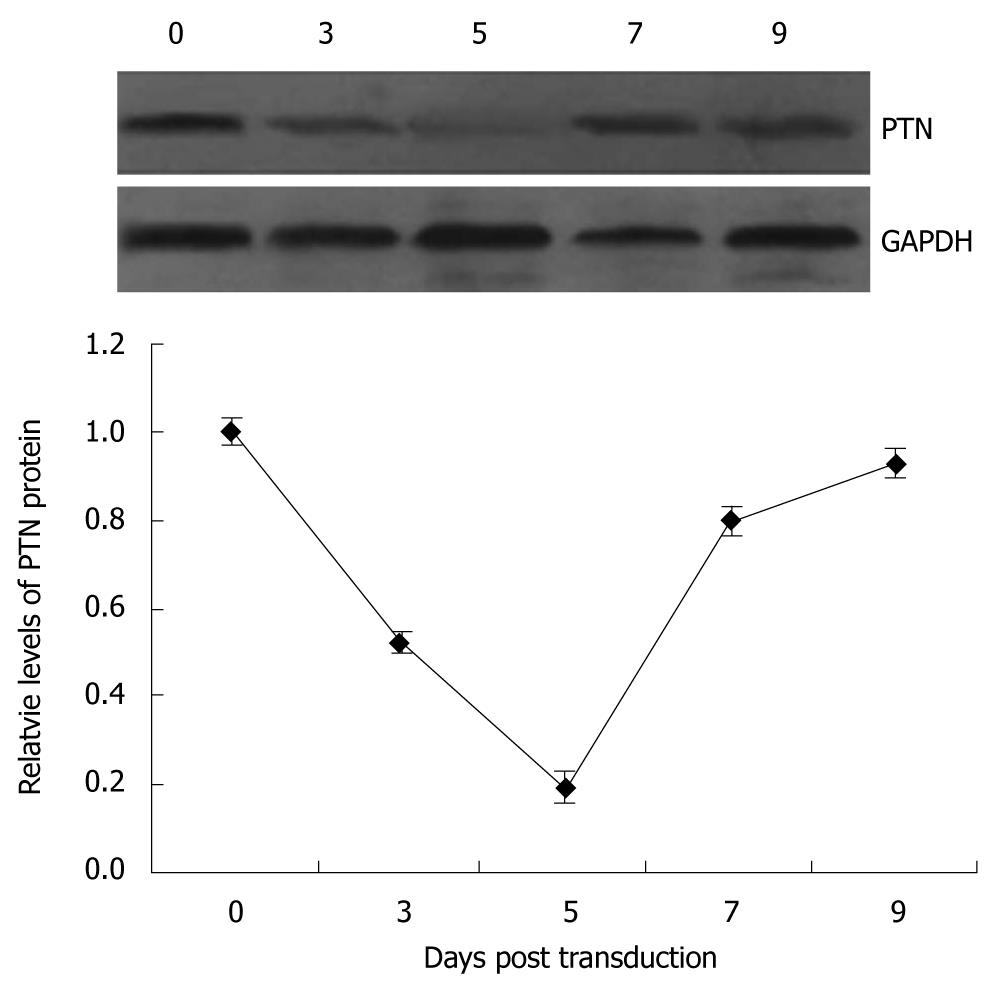
Western blotting analysis of the anti-PTN-specific antibodies revealed that PTN protein expression in BxPC-3 cells infected with pAd-shRNA-PTN was markedly suppressed on days 3-7 compared with the control (0 d). The inhibition rates of PTN protein expression were 47.5%, 80.5% and 20%, respectively, on the 3rd, 5th and 7th d following pAd-shRNA-PTN infection. The maximal knockdown level was observed on the 5th post-infection day. The PTN protein levels began to rise on the 7th post-infection day (Figure 4).
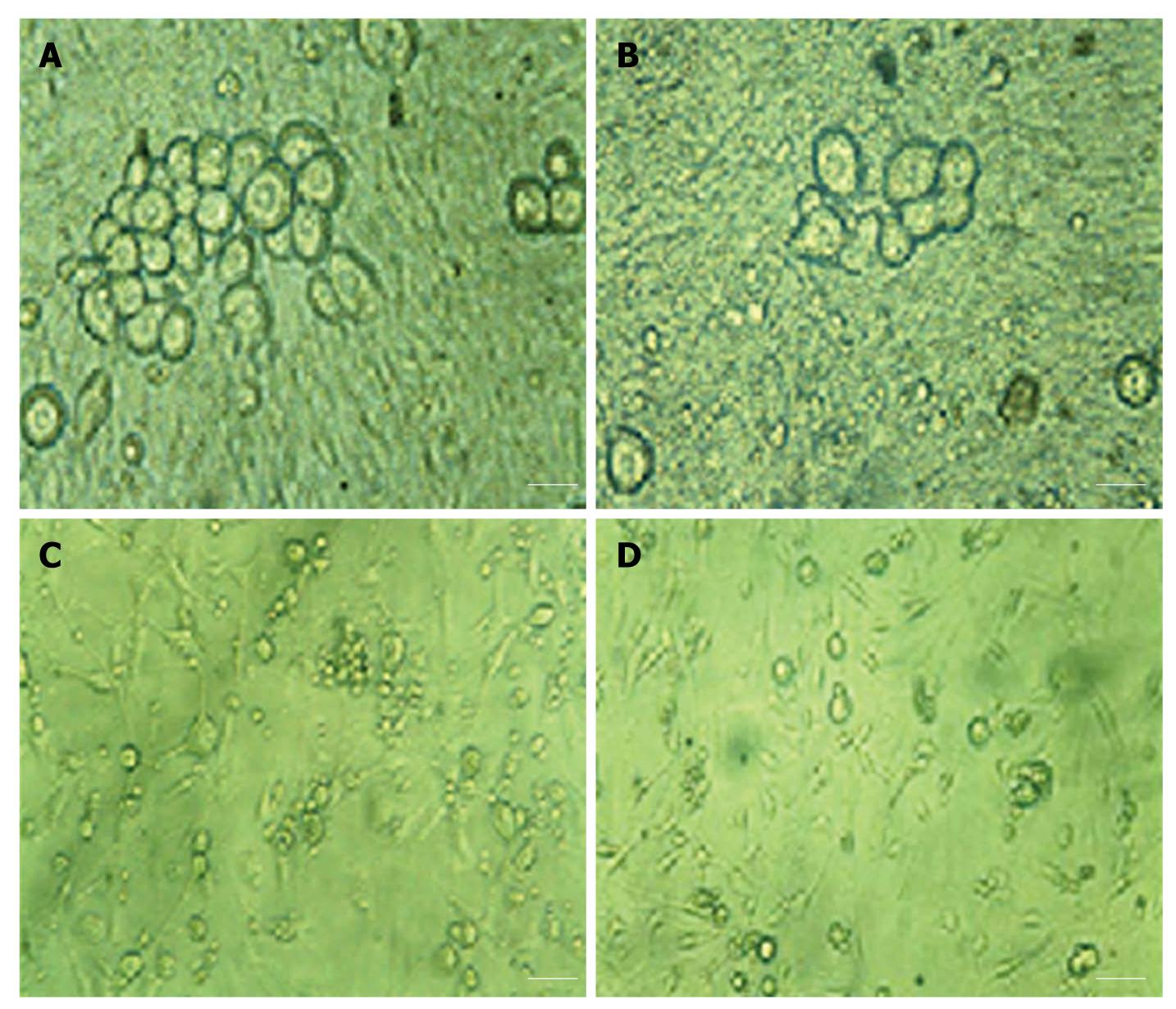
Healthy DRG neurons began to adhere and extend their processes within 3 h of plating. Within 24 h, the neurons were mainly adherent. On the 1st d, the DRG neurons of the monoculture were mostly adherent, and were round or elliptical in shape and approximately 25 μm in diameter. Decades of them gathered together, and no neurite outgrowth was observed from DRG neurons; glial and Schwann cells were also not observed (Figure 5A). During cultivation, morphological changes in the DRG neurons were not obvious. On the 7th d, the number of neurons decreased, and very few neurons extended neurites (Figure 5B);
these neurites were significantly shorter than those extending from the neurons co-cultured with normal BxPC-3 cells (Figure 5D). On the 7th d after co-culture with BxPC-3 cells, the control (co-cultured with normal BxPC-3 cells) DRG neurons were larger in size and irregular in shape. Robust neurite outgrowth was observed, with extensive outgrowth forming reticulate links and neural networks (Figure 5C). In contrast to the control, the DRG neurons co-cultured with the infected BxPC-3 cells shrunk; the number of neurons that extended neurites was significantly decreased, as was the length of the neurites. No neural networks were formed (Figure 5D).
Pancreatic cancer is characterized by perineural invasion[21], early lymph node metastasis, and poor prognosis[22,23]. Perineural invasion is an important cause of local recurrence, but little is known about its mechanism. The perineural invasion originates from not only the extrapancreatic nerve but also the intrapancreatic nerve in pancreatic carcinoma[24]. It is likely that as a neurite growth-promoting factor, PTN acts synergistically to promote the development of perineural invasion in pancreatic cancer. Weber et al[9] showed that PTN is expressed in gastrointestinal and, particularly, in pancreatic cancer cells. Using immunocytochemistry, 10 different human pancreatic cancer cell lines were analyzed for PTN expression. Six cell lines were positively stained for PTN: A816-4, BxPC-3, Panc-Tu1, SW850, Panc89, and Colo357[9]. In this study, we also found that PTN was clearly expressed in BxPC-3 cells.
In cancer gene therapy, the success of a strategy depends on its cancer specificity and efficient delivery into mammalian cells. In this study, the RNAi technique and recombinant adenovirus were used to achieve this. The availability of a high virus titer and infection of a broad spectrum of cell types makes adenovirus the vector of choice for siRNA delivery. For example, oncogenic K-ras and other genes could be specifically and stably inactivated in human pancreatic cancer cells using a viral RNA interference (RNAi) vector, leading to loss of tumorigenicity[25]. The construction of recombinant adenovirus was confirmed via electron microscopic observation. The pAd/BLOCK-iT™ adenoviral construct is replication-incompetent and does not integrate into the host genome. Therefore, once transduced into mammalian cells, the shRNA of interest will be expressed as long as the viral genome is present. In the actively dividing cells, the adenovirus genome is gradually diluted as cell division occurs, resulting in an overall decrease in shRNA expression over time, i.e. the target protein levels generally return to background levels within 1-2 wk following transduction. For reducing the dilution, we cultured BxPC-3 cells in DMEM/F12 medium containing 2% FCS to reduce cell division. Besides, we used an MOI of 50 to obtain the optimal degree of target gene knockdown. In this study, we found that PTN gene knockdown in BxPC-3 cells might be detectable (20%) by real-time quantitative PCR on the 1st post-infection day, with maximal knockdown levels (80%) observed on the 3rd post-infection day. On the 5th d, as the adenovirus genome was diluted, the level of PTN mRNA was increased gradually and the PTN mRNA expression returned to the level observed in control BxPC-3 cells on the 9th post-infection day. Immunocytochemistry and Western blotting analysis also revealed the same tendency, except that the maximal knockdown levels of PTN protein were observed two days later; this may be related to the translation process of the protein.
In the nervous system, PTN has been shown to induce neurite outgrowth from dopaminergic neurons[8,26]. PTN and its receptor, N-syndecan, are important in promoting neurite outgrowth. N-syndecan expression was stronger in both the brain and spinal cord during the later developmental period (days 14-16 of gestation); N-syndecan isolated from a 16-d-old rat fetus bound strongly to PTN[27]. Anti-N-syndecan antibodies bound to the surface of the neurites and also perturbed neurite growth[28]. PTN was also involved in the peripheral nerve regeneration following nerve injury and functional recovery following neural transplantation in rats[29,30]. Taken together, the available evidence suggests that PTN bound to N-syndecan can promote neurite outgrowth from DRG neurons. In this study, we selected DRG neurons for investigation because they may play a role in the neural invasion of pancreatic cancer[31]. We dissociated DRG neurons from a 16-d-old rat fetus for monoculture or co-culture with BxPC-3 cells. The results indicated that pAd-shRNA-PTN could also be used to infect the BxPC-3 cells and suppress PTN gene expression efficiently. In contrast to the number of control cells, the number of DRG neurons co-cultured with infected BxPC-3 cells and extended neuritis significantly decreased. These results were in agreement with those of our previous studies[19].
In conclusion, the adenoviral construct pAd-shRNA-PTN showed efficient and specific knockdown of PTN in the pancreatic cancer cell line BxPC-3, and the inhibition of neurite outgrowth from DRG neurons was observed in vitro. Therefore, the PTN gene and pAd-shRNA-PTN may be very important for the research on the neural invasion of pancreatic cancer. We will set up a model of pancreatic cancer in situ in nude mice in the future studies. By injecting pAd-shRNA-PTN stock, we can investigate the effects of the PTN gene and adenoviral construct pAd-shRNA-PTN on the neural invasion of pancreatic cancer.
Pancreatic cancer is still one of the most aggressive and intractable human malignant tumors. Perineural invasion extending into the pancreatic nerve plexus is a histopathologic characteristic of pancreatic cancer. However, the mechanisms contributing to the invasion of intrapancreatic nerves and spread of cancer cells along extrapancreatic nerves in the course of pancreatic cancer are still poorly understood. As a neurite growth-promoting factor, pleiotrophin (PTN) and its receptor, N-syndecan, may play a very important role in tumor growth and neural invasion of pancreatic cancer. Therefore, the authors of this study used recombinant adenovirus pAd-shRNA-PTN to investigate the silencing effects of PTN in pancreatic cancer cells and to observe the inhibition of pAd-shRNA-PTN on the neurite outgrowth from dorsal root ganglion (DRG) neurons in vitro.
Perineural invasion extending into the pancreatic nerve plexus is a histopathologic characteristic of pancreatic cancer. The neural invasion of pancreatic cancer leads to local recurrence, metastasis and poor prognosis, which have been brought difficulty to diagnosis and treatment of pancreatic cancer.
In recent years, researches of pancreatic cancer have focused on the biological characteristics, especially perineural invasion. This study explored the effects of pAd-shRNA-PTN on PTN of pancreatic cancer cells and neurites outgrowth of DRG neurons, which might reveal the relative mechanism of the neural invasion.
The study indicates that PTN appears to be an attractive target for nerve infiltration of pancreatic cancer gene therapy.
PTN is a type of neurotrophic factor and is also known as a neurite growth-promoting factor. N-syndecan is a transmembrane protein and a high- affinity receptor for PTN. PTN and N-syndecan are important in promoting neurite outgrowth. RNA interference (RNAi) is a process during which double-stranded RNA induces the homology-dependent degradation of cognate mRNA. RNAi has been proven to be a powerful tool for suppressing gene expression.
The study investigated the silencing effects of recombinant adenovirus pAd-shRNA-PTN on PTN gene in pancreatic cancer cells, and observed the inhibition of pAd-shRNA-PTN on the neurite outgrowth from DRG neurons in vitro. The subject is important because very little is known about the molecular mechanism of perineural invasion in pancreatic cancer, that is to say, this study has revealed the role of PTN gene in perineural invasion using pAd-shRNA-PTN. The study is well designed.
Peer reviewer: De-Liang Fu, MD, Department of General Surgery, Pancreatic Disease Institute, 12 Wulumuqi Road (M), 200040 Shanghai, China
S- Editor Sun H L- Editor Ma JY E- Editor Ma WH
| 2. | Laheru D, Jaffee EM. Immunotherapy for pancreatic cancer - science driving clinical progress. Nat Rev Cancer. 2005;5:459-467. |
| 3. | Ceyhan GO, Giese NA, Erkan M, Kerscher AG, Wente MN, Giese T, Büchler MW, Friess H. The neurotrophic factor artemin promotes pancreatic cancer invasion. Ann Surg. 2006;244:274-281. |
| 4. | Veit C, Genze F, Menke A, Hoeffert S, Gress TM, Gierschik P, Giehl K. Activation of phosphatidylinositol 3-kinase and extracellular signal-regulated kinase is required for glial cell line-derived neurotrophic factor-induced migration and invasion of pancreatic carcinoma cells. Cancer Res. 2004;64:5291-5300. |
| 5. | Muramatsu T. Midkine and pleiotrophin: two related proteins involved in development, survival, inflammation and tumorigenesis. J Biochem. 2002;132:359-371. |
| 6. | Rauvala H, Huttunen HJ, Fages C, Kaksonen M, Kinnunen T, Imai S, Raulo E, Kilpeläinen I. Heparin-binding proteins HB-GAM (pleiotrophin) and amphoterin in the regulation of cell motility. Matrix Biol. 2000;19:377-387. |
| 7. | Takamatsu H, Itoh M, Kimura M, Gospodarowicz D, Amann E. Expression and purification of biologically active human OSF-1 in Escherichia coli. Biochem Biophys Res Commun. 1992;185:224-230. |
| 8. | Hida H, Jung CG, Wu CZ, Kim HJ, Kodama Y, Masuda T, Nishino H. Pleiotrophin exhibits a trophic effect on survival of dopaminergic neurons in vitro. Eur J Neurosci. 2003;17:2127-2134. |
| 9. | Weber D, Klomp HJ, Czubayko F, Wellstein A, Juhl H. Pleiotrophin can be rate-limiting for pancreatic cancer cell growth. Cancer Res. 2000;60:5284-5288. |
| 10. | Souttou B, Juhl H, Hackenbruck J, Röckseisen M, Klomp HJ, Raulais D, Vigny M, Wellstein A. Relationship between serum concentrations of the growth factor pleiotrophin and pleiotrophin-positive tumors. J Natl Cancer Inst. 1998;90:1468-1473. |
| 11. | Raulo E, Chernousov MA, Carey DJ, Nolo R, Rauvala H. Isolation of a neuronal cell surface receptor of heparin binding growth-associated molecule (HB-GAM). Identification as N-syndecan (syndecan-3). J Biol Chem. 1994;269:12999-13004. |
| 12. | Zamore PD, Tuschl T, Sharp PA, Bartel DP. RNAi: double-stranded RNA directs the ATP-dependent cleavage of mRNA at 21 to 23 nucleotide intervals. Cell. 2000;101:25-33. |
| 14. | Hunter T, Hunt T, Jackson RJ, Robertson HD. The characteristics of inhibition of protein synthesis by double-stranded ribonucleic acid in reticulocyte lysates. J Biol Chem. 1975;250:409-417. |
| 15. | Elbashir SM, Harborth J, Lendeckel W, Yalcin A, Weber K, Tuschl T. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature. 2001;411:494-498. |
| 17. | Kovesdi I, Brough DE, Bruder JT, Wickham TJ. Adenoviral vectors for gene transfer. Curr Opin Biotechnol. 1997;8:583-589. |
| 18. | Hitt MM, Addison CL, Graham FL. Human adenovirus vectors for gene transfer into mammalian cells. Adv Pharmacol. 1997;40:137-206. |
| 19. | Yao J, Ma QY, Wang LC, Zhang M, Shen SG. [Construction of the recombinant adenovirus mediated shRNA to silence PTN in pancreatic carcinoma and the effect of DRGn on neurite in vitro]. Xibao Yu Fenzi Mianyixue Zazhi. 2007;23:797-800. |
| 20. | LaBarre DD, Lowy RJ. Improvements in methods for calculating virus titer estimates from TCID50 and plaque assays. J Virol Methods. 2001;96:107-126. |
| 21. | Kayahara M, Nakagawara H, Kitagawa H, Ohta T. The nature of neural invasion by pancreatic cancer. Pancreas. 2007;35:218-223. |
| 22. | Ozaki H, Hiraoka T, Mizumoto R, Matsuno S, Matsumoto Y, Nakayama T, Tsunoda T, Suzuki T, Monden M, Saitoh Y. The prognostic significance of lymph node metastasis and intrapancreatic perineural invasion in pancreatic cancer after curative resection. Surg Today. 1999;29:16-22. |
| 23. | Nakao A, Harada A, Nonami T, Kaneko T, Takagi H. Clinical significance of carcinoma invasion of the extrapancreatic nerve plexus in pancreatic cancer. Pancreas. 1996;12:357-361. |
| 24. | Yi SQ, Miwa K, Ohta T, Kayahara M, Kitagawa H, Tanaka A, Shimokawa T, Akita K, Tanaka S. Innervation of the pancreas from the perspective of perineural invasion of pancreatic cancer. Pancreas. 2003;27:225-229. |
| 25. | Chen LM, Le HY, Qin RY, Kumar M, Du ZY, Xia RJ, Deng J. Reversal of the phenotype by K-rasval12 silencing mediated by adenovirus-delivered siRNA in human pancreatic cancer cell line Panc-1. World J Gastroenterol. 2005;11:831-838. |
| 26. | Mourlevat S, Debeir T, Ferrario JE, Delbe J, Caruelle D, Lejeune O, Depienne C, Courty J, Raisman-Vozari R, Ruberg M. Pleiotrophin mediates the neurotrophic effect of cyclic AMP on dopaminergic neurons: analysis of suppression-subtracted cDNA libraries and confirmation in vitro. Exp Neurol. 2005;194:243-254. |
| 27. | Nakanishi T, Kadomatsu K, Okamoto T, Ichihara-Tanaka K, Kojima T, Saito H, Tomoda Y, Muramatsu T. Expression of syndecan-1 and -3 during embryogenesis of the central nervous system in relation to binding with midkine. J Biochem. 1997;121:197-205. |
| 28. | Nolo R, Kaksonen M, Raulo E, Rauvala H. Co-expression of heparin-binding growth-associated molecule (HB-GAM) and N-syndecan (syndecan-3) in developing rat brain. Neurosci Lett. 1995;191:39-42. |
| 29. | Blondet B, Carpentier G, Lafdil F, Courty J. Pleiotrophin cellular localization in nerve regeneration after peripheral nerve injury. J Histochem Cytochem. 2005;53:971-977. |
| 30. | Hida H, Masuda T, Sato T, Kim TS, Misumi S, Nishino H. Pleiotrophin promotes functional recovery after neural transplantation in rats. Neuroreport. 2007;18:179-183. |
| 31. | Dai H, Li R, Wheeler T, Ozen M, Ittmann M, Anderson M, Wang Y, Rowley D, Younes M, Ayala GE. Enhanced survival in perineural invasion of pancreatic cancer: an in vitro approach. Hum Pathol. 2007;38:299-307. |